Long-term follow-up of intravesical abobotulinumtoxinA(Dysport®)injections in women with idiopathic detrusor overactivity
2024-02-25MohmmSjjRhnmAminBghriElhmJhntiHnihSlhiPourmhrHiMostiBrigittShurhAiJvnBlghMrnSkinhHjrhimi
Mohmm Sjj Rhnm’i,Amin Bghri,Elhm Jhnti,Hnih Slhi-Pourmhr,Hi Mosti,Brigitt Shurh,Ai Jvn Blgh Mrn,Skinh Hjrhimi,*
a Department of Urology,St.Elisabeth-Tweesteden Hospital Tilburg,the Netherlands
b Research Center for Evidence-based Medicine,Iranian EBM Centre: A Joanna Briggs Institute (JBI)Center of Excellence,Tabriz University of Medical Sciences,Tabriz,Iran
c Urology Department,Faculty of Medicine,Tabriz University of Medical Sciences,Tabriz,Iran
d Department of Urology,Comprehensive Cancer Center,Medical University of Vienna,Vienna,Austria
e Service de Neuroréhabilitation,CHUV,Lausanne,Switzerland
f Society of Urological Research and Education (SURE),Heerlen,the Netherlands
KEYWORDS AbobotulinumtoxinA;Dysport®;Intravesical;Idiopathic;Detrusor overactivity
Abstract Objective: Only a few numbers of studies have been published on the use of abobotulinumtoxinA (Dysport®) in idiopathic detrusor overactivity (IDO).This study reported the long-term follow-up of women with IDO who were treated with intravesical Dysport® injections.Methods: Two hundred and thirty-six patients with IDO who had failed first-line conservative and antimuscarinic therapy received 500-900 units of Dysport® between April 2014 and July 2015.All patients were followed up for 5 years after their initial injection and interviewed on the phone.Results: A total of 236 women with IDO aged from 18 years to 84 years (mean±standard deviation: 49.6±15.9 years) were included in our study.The median follow-up time for patients was 36.5 (range: 10-70) months,and the median recovery time after injection was 18.5(range: 0-70)months.A total of 83(35.2%)patients stated that they had subjective improvement of their symptoms whereas 84(35.6%)patients did not report any improvement in symptoms.The initial International Consultation on Incontinence Questionnaire Overactive Bladder mean score was 6.9 (standard deviation 3.4).There was a positive association between the median recovery time and the components of the International Consultation on Incontinence Questionnaire Overactive Bladder questionnaire.Conclusion: In a sub-population of overactive bladder patients with IDO who have failed first-line therapy,a single intravesical Dysport® injection can resolve patient symptoms completely or reduce the symptoms to an acceptable level that can be controlled with antimuscarinics or re-injection on demands.
1.Introduction
Overactive bladder syndrome (OAB) is a chronic medical condition that has a tremendous impact on the quality of life in both men and women [1,2].Patients with OAB can have urinary incontinence,frequency,and urgency symptoms,as well as nocturia.The urodynamic findings of these patients may include changes in bladder capacity,compliance,and detrusor overactivity [3,4].
The first-line medical treatment of OAB is oral pharmacotherapy with antimuscarinics or β3 receptor agonists.However,long-term antimuscarinic treatment is often unsuccessful due to side effects and lack of effectiveness[5-7].For drug-refractory OAB,intravesical injection of botulinum toxin has emerged as a second-line minimally invasive treatment [8,9].
There are seven botulinum toxin types (A-G) with different tertiary structures and sequence differences.The A,B,and E serotypes cause human botulism,with the activities of types A and B enduring the longestin vivo(from several weeks to months) [10].Type B has a shorter duration of action,thought to be 8-10 weeks.Botulinum toxin type A (BoNT-A) is a potent neurotoxin produced byClostridium botulinum[10].There are three different types of BoNT-A: onabotulinumtoxinA (Onabot-A,Botox®,Allergan,Irvine,CA,USA),abobotulinumtoxinA(Abobot-A,Dysport®,Ipsen,Paris,France),and incobotulinumtoxinA (Incobot-A,Xeomin®,Merz Pharmaceuticals GmbH,Frankfurt,Germany) [11].
Onabot-A (Botox®) is the only one with Food and Drug administration (FDA) approval for OAB and neurogenic detrusor overactivity (NDO).Its particles are coated with protein buffers in order to slow releasing of the neurotoxin.Abobot-A (Dysport®) received FDA approval in 2009.Like Onabot-A (Botox®),it affects nerve impulses.However,its formula is slightly different and contains smaller particles.Unlike Onabot-A (Botox®),it has fewer protein buffers.The safety,efficacy,and quality of both products are similar[12].Both Onabot-A(Botox®)and Abobot-A[Dysport®]are type A serotype despite differences in derived bacterial strains,manufactured processes of isolation,purification,and extraction.This leads to the need for different dosing units(the dose conversion ratio of Onabot-A[Botox®]to Abobot-A[Dysport®]is between 2:1 and 3:1,but this is not validated in urology yet).In addition,many patients experience beneficial outcomes more quickly with Dysport®(4 days with Abobot-A[Dysport®]vs.7-10 days with Onabot-A[Botox®]).It can be injected more deeply and spread easily.Thus,a broad area can be treated with fewer disport injections at certain times.Conditionally,some patients just like their results better with Onabot-A (Botox®) than Abobot-A (Dysport®),and vice-versa.The standard dose of Abobot-A(Dysport®)was first determined based on the previously suggested conversion ratio of 2.5:1 for the two toxins,and then after this dose was reduced to 300 units(U).Most published studies on BoNT-A in OAB treatment have focused on Onabot-A(Botox®).However,Abobot-A(Dysport®)effectiveness has been shown on patients with NDO [13],and the use of it in the bladder should be considered off-label.There are only a small number of published articles on the use of Abobot-A (Dysport®) in idiopathic detrusor overactivity (IDO).Intravesical application of Abobot-A(Dysport®)has been shown to be a safe and highly effective treatment option for patients with refractory OAB symptoms[14].
This study reported the first long-term follow-up results of women with IDO who were treated with intravesical Abobot-A (Dysport®) injections.
2.Patients and methods
2.1.Eligibility criteria
In this retrospective study,the data of all adult female patients with urodynamically proven IDO,who were refractory to anticholinergic therapy (for at least 3 months)and discontinued the use of them,and visited the female urology clinic of our teaching hospital between April 2014 and July 2015 were collected.
The most used anticholinergics were solifenacin(5 mg/daily),tolterodine (4 mg/daily),and oxybutynin(5 mg/daily extended-release),for at least 4 weeks,and the refractory cases to treatment were candidates for combination anticholinergic therapy.The prescribed dosage was titrated,too.During the study period,we did not have access to any β3 agonists in our country,and the second line of treatment was considered Abobot-A (Dysport®),the only available brand in our country,in refractory cases to anticholinergic therapy for at least 3 months.All patients were free of urinary tract infection symptoms (negative urine culture before the intravesical injection).
The exclusion criteria were NDO,history of sacral neuromodulation,pelvic organ prolapse (using Pelvic Organ Prolapse Quantification system),or congenital lower urinary tract anomalies.All pregnant women or patients with symptoms of stress urinary incontinence,history of abdominal and pelvic surgery,radiation therapy,and previous Onabot-A(BOTOX®)injection were excluded from the study.Our cut-off value for incontinence episodes per 24 h in the bladder diary for recruitment was two or more times.
All patients gave informed consents and were able and willing to do clean intermittent catheterization (CIC) in case of increased post-void residual volume or retention after Abobot-A (Dysport®) injections.
2.2.Outcome measure
The study’s primary outcome was a decrease in the number of urinary incontinence episodes per day according to the bladder diary and the International Consultation on Incontinence Questionnaire Overactive Bladder (ICIQ-OAB)questionnaire score after the first injection.Hence,we only included the patient who experienced one-time intravesical Abobot-A (Dysport®) injection.
2.2.1.Symptom’s improvement
In this study,patient satisfaction was divided into three categories: high,medium,and low.Treatment success was defined as improvement in symptoms such as urinary incontinence,urgency,and nocturia to the extent that was acceptable for the patient.For this purpose,patients were asked to record a score between 0 and 100 to improve their symptoms following the treatment,and then were categorized into three parts:high,intermediate,and low.If the patients stated that the symptoms were recovered more than 50%,they were categorized as a high improvement.The score between 30% and 50% improvements in symptoms was deemed intermediate,and less than 30% improvement was considered low.We assessed the patients’ percent of improvement using the visual analogue scale.In addition,we asked the patients’ quality of life satisfaction after the procedure using 0-100 points scoring.
2.2.2.Recovery time
Recovery time was defined as a median duration of a single treatment cycle with Abobot-A (Dysport®) without any need for more therapeutic methods.
2.3.Procedure
Due to the availability of Abobot-A (Dysport®) in our country,we used this type of botulinum toxin in urological cases if indicated.The procedure was applied under general or local anesthesia.Cystoscopy was performed using a 21 French rigid cystoscope in the lithotomy position.After filling the bladder with 150 mL of irrigation fluid,500-900 U (or 10-15 U/Kg) of Abobot-A (Dysport®) was injected to 30 to 50 intradetrusor injection sites in each patient.Abobot-A (Dysport®) (500 U) was reconstituted with 5 mL distilled water.The intradetrusor injections were performed using a 27 Gauge disposable needle.The injection was carried out in the bladder wall,away from the trigon.All patients were discharged from the hospital on the same day after the injection.
2.4.Follow-up
All patients were routinely visited at 2 weeks for symptomatic urinary infection and retention assessment,and then at 3 months to assess response.We conducted the follow-up using telephone interviews.
2.5.Statistical analysis
Statistical analyses were performed using SPSS version 19.0 statistical software (SPSS Inc.,Chicago,IL,USA).A univariate descriptive statistics analysis was conducted using a non-parametric procedure,Kaplan-Meier method to estimate overall survival.A log-rank test was used to compare the survival rates between the groups.Thep-values less than 0.05 were considered statistically significant.
2.6.Ethical consideration
The local ethical committee of Tabriz University of Medical Sciences,Tabriz,Iran approved the study proposal(IR.TBZMED.REC.1401.007).
3.Results
The total number of patients in the mentioned time points were 385 cases.Of these,122 who had IDO were eliminated from the study.In addition,27 cases either did not answer their phone or were not available or did not show any interest in collaborating in the research.Finally a total of 236 women with IDO,aged from 18 to 84 years(mean±standard deviation [SD]: 49.6±15.9 years),were included in our study.Of them,193 (82%) had wet OAB,and the remained cases had dry OAB.We recorded 95 (40.3%) patients who had a history of concomitant diseases,with hypertension being the most prevalent condition (32.6%) as shown in Table 1.
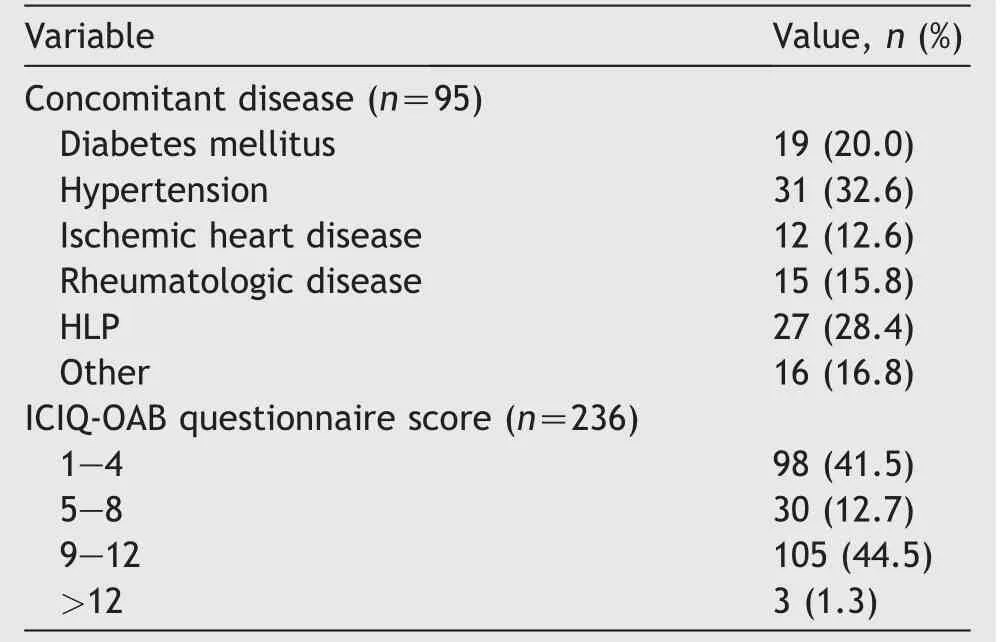
Table 1 Clinical characteristics and ICIQ-OAB questionnaire scores for all patients (n=236).
The median follow-up time of the study was 36.5(range:10-70) months (Fig.1),and the median duration of recovery time after injection was 18.5 (range: 0-70)months (Fig.2).Our results of the long-term follow-up of the included 236 women showed that 148 (62.7%) of the patients reported having improved their OAB symptoms.From the included 236 patients,83 (35.2%) patients stated that they had noticed a significant subjective improvement of their symptoms (reduced urgency and frequency episodes and/or incontinence episodes with more than 50%);84(35.6%)patients did not mention any improvement at all or less than 30%;69 (29.2%) patients reported having noticed some degree of improvement of their symptoms,but the improvement was between 30% and 50%.
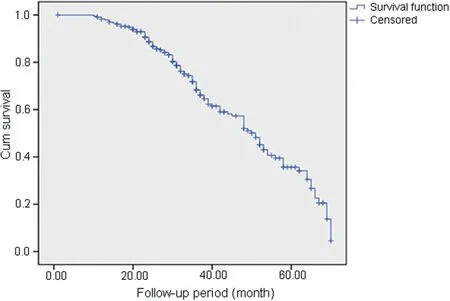
Figure 1 The Kaplan-Meier survival curve of the follow-up period (n=236).

Figure 2 The Kaplan-Meier plot survival curve of the duration of recovery in patient (n=236).
In our study,the mean initial ICIQ-OAB score was 6.9(SD 3.4).A total of 105 (44.5%) patients had an ICIQ-OAB score between 9 and 12.A positive association was observed in ICIQ-OAB score and patient satisfaction (p<0.001)(Table 2).About 128 (54.2%) had an ICIQ-OAB score of ≤8 after injection.
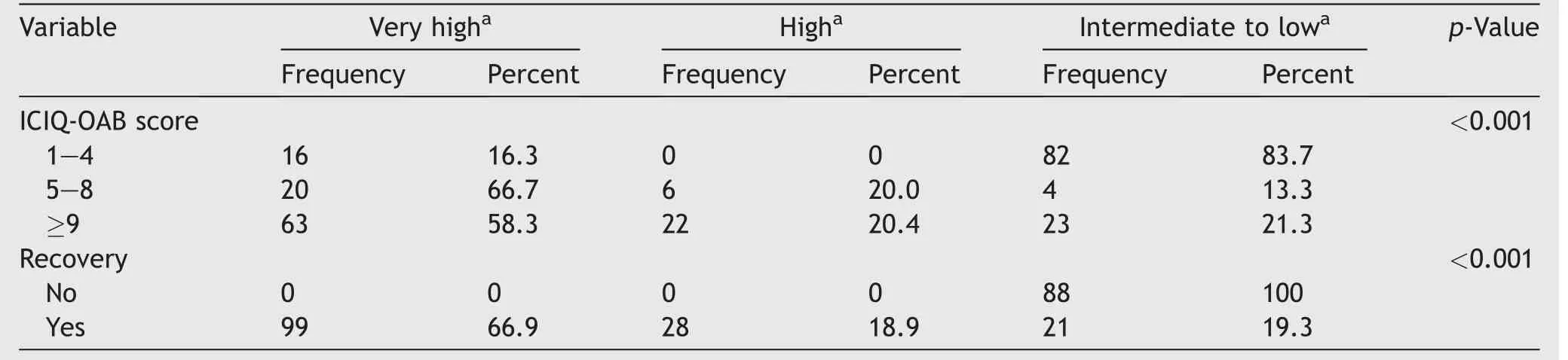
Table 2 Association between patient ICIQ-OAB scores and recovery with patient satisfaction (n=236).
In addition,we observed a significant association between ICIQ-OAB score and patient recovery (p<0.001).Log rank test results showed that the ICIQ-OAB score was higher in those who recovered than in those who did not (Fig.3).Patients’ satisfaction and their recovery from the disease also showed a significant relationship (p<0.001) (Table 2).Furthermore,the recovery was higher in patients with high satisfaction than in other patients (Table 2).In the current study,30 (12.7%) patients experienced voiding symptoms with post-void residual urine between 110 mL and 260 mL.These patients were treated conservatively (time voiding and double voiding) or by CIC.We did not have a reliable database for recording post-operative urinary infections.The main reasons that the patients discontinued the follow-up after failed first injection or symptom relapse were turning back to medical treatment (46%),loss of the efficacy of the first injection(20%),the cost of re-injection(10%),and personal or no particular reason (24%).

Figure 3 Log rank test between ICIQ-OAB score and recovery duration (n=236).ICIQ-OAB,International Consultation on Incontinence Questionnaire Overactive Bladder.
4.Discussion
BoNT-A was the only botulinum toxin that was FDA-approved to treat patients with neurogenic bladder dysfunction[15,16].Currently,Abobot-A (Dysport®) also becomes FDA-approved for the treatment of idiopathic OAB symptoms.This study assessed the long-term results of Abobot-A(Dysport®).Our results of the long-term follow-up of the included 236 women with IDO showed that after a mean follow-up of 36.5 months,148 (62.7%) of the patients reported having improved their OAB symptoms in various degree.In addition,we found an association between the median recovery time and the components of the ICIQ-OAB questionnaire.In a long-term follow-up study of 128 patients treated with 200 U BoNT-A in a single institution,almost 70%(90 cases)of all patients abandoned the treatment[17].Those patients who abandoned BoNT-A therapy reported insufficient effect (37.0%),the need for CIC (13.0%),and urinary tract infections(9.0%)as the main reasons for abandoning BoNT-A treatment[18].In another study reported in 2019,there was no statistically significant difference in choosing Abobot-A(Dysport®) re-injection or reconstructive surgery in terms of the previous anticholinergic treatment and urinary tract infection episodes[19].Hence,the reported 37.0% failure of BoNT-A was quite similar to the 35.6% failure rate of Abobot-A(Dysport®)in our study.
Onabot-A (Botox®) to Abobot-A (Dysport®) conversion factor is reported 1:3 in thepreviousstudies.Inthis regard,all studies that used the conversion factor less than or equal 1:3 observed the clinical equivalence[20-24].In cases that this factor was closer or higher than 1:3,the higher efficacy was observed besides its more adverse events[25-32].Although OAB has been treated with 100-300 U of Onabot-A(Botox®)in most studies,dose-ranging studies have been performed and 100 U was determined as the optimal dose to treat OAB.The most commonly used neurotoxin serotype to treat lower urinary tract dysfunction is Onabot-A(Botox®);it is available in the United States and Europe.Whereas Abobot-A(Dysport®)is the serotype that is the only available brand in our country and since one unit of Onabot-A (Botox®) is equivalent to approximately 3-5 U of Abobot-A (Dysport®),Onabot-A(Botox®) is available in a 100 U or 200 U vial,whereas Abobot-A(Dysport®)comes in a 300 U or 500 U vial.
Previous studies which reported the outcomes for Abobot-A were smaller cohorts with fewer repeat injections and shorter follow-up.They are spread across a range of doses for Abobot-A from 250 U to 750 U.The most extensive study was a prospective case series including 33 women who had more than three injections (Dysport®) of 500 U or 750 U.This study found a significant and sustained improvement in urinary frequency and quality of life scores across three treatment cycles.The mean duration between the first and second injections was 15.2 (SD 7.2) months,whereas between the second and third was 19.2 (SD 10)months (p=0.025) [33].
Irwin et al.[34]evaluated the medium and long-term results of Abobot-A injection treatment in the management of refractory OAB symptoms owing to IDO.Seventythree patients received 93 Abobot-A injection treatments over 5 years.In patients undergoing repeat injection treatment,the mean duration of symptomatic relief (until the resumption of antimuscarinic therapy)was 12.3(SD 9.8)months.In comparison,the mean interval between injection treatments was 26.7 (SD 14.3) months,and this also tended to increase with subsequent injections [34].
A limitation of our study is its retrospective nature that reviewed the available data and we do not had more sociodemographic information.In addition,some data could not be accurately evaluated.For instance,we did not record the number of urinary tract infections,as the exact number could not be reliably retrieved from the patient records.Moreover,many infections were treated with antibiotics by general practitioners or other office-based urologists and gynecologists,making adequate assessment even more difficult.
Another drawback of the study is that patients were treated with various doses of Abobot-A (Dysport®).This affects the homogeneity of the study population and the robustness of the results.Although most guidelines recommend 100 U of Onabot-A (Botox®),in point of the clinic,it is recommended that the dose of it can be individualized based on the results of urodynamics,the severity of symptoms,patient condition,bladder capacity,and the clinicians choose.In addition,in some patients who discontinued Abobot-A (Dysport®),the exact reason for stopping treatment is uncertain.Given the limited data and uncertainties around dosage of Abobot-A,it appears that more studies are needed to confirm the long-term efficacy and safety profile of Abobot-A.
5.Conclusion
In a subpopulation of OAB patients with detrusor overactivity who had failed first-line therapy,a single injection of intravesical Dysport®can resolve patient symptoms either completely or reduce the symptoms to an acceptable level that can be controlled with antimuscarinics or re-injection on demands.
Author contributions
Study concept and design: Sakineh Hajebrahimi,Mohammad Sajjad Rahnama’i.
Data acquisition: Amin Bagheri,Elham Jahantabi,Hanieh Salehi-Pourmehr.
Data analysis: Hadi Mostafaei,Brigitte Schurch,Aida Javan Balegh Marand.
Drafting of manuscript: Amin Bagheri,Elham Jahantabi,Hanieh Salehi-Pourmehr.
Critical revision of the manuscript: Sakineh Hajebrahimi,Mohammad Sajjad Rahnama’i.
Conflicts of interest
The authors declare no conflict of interest.
杂志排行
Asian Journal of Urology的其它文章
- Transurethral resection of bladder tumor:A systematic review of simulator-based training courses and curricula
- Etiology and management of urethral calculi:A systematic review of contemporary series
- Oncologic outcomes with and without amniotic membranes in robotic-assisted radical prostatectomy: A propensity score matched analysis
- Single nucleotide polymorphism within chromosome 8q24 is associated with prostate cancer development in Saudi Arabia
- The risk of prostate cancer on incidental finding of an avid prostate uptake on 2-deoxy-2-[ 18F]fluoro-D-glucose positron emission tomography/computed tomography for non-prostate cancer-related pathology:A single centre retrospective study
- Prevention of thromboembolic events after radical prostatectomy in patients with hereditary thrombophilia due to a factor V Leiden mutation by multidisciplinary coagulation management
