Phosphorylation-driven aggregative proteins in neurodegenerative diseases: implications and therapeutics
2024-02-14AlbaEspargarRaimonSabate
Alba Espargaró,Raimon Sabate
Conformational diseases are a group of disorders caused by misfolded or abnormally folded proteins.In these diseases,the proteins do not adopt the correct three-dimensional structure required for their normal function.Instead,they can aggregate and form clumps,known as amyloid fibrils,which can accumulate in tissues and organs throughout the body,leading to cell damage and dysfunction.Examples of conformational diseases include ΑD,PD,diabetes mellitus type II,Huntington’s disease,amyotrophic lateral sclerosis,frontotemporal dementia and prion diseases viz.Creutzfeldt-Jakob disease in humans.These disorders are typically progressive and degenerative and have a significant impact on an individual’s quality of life (Chiti and Dobson,2017).
Phosphorylation is a post-translational modification that can alter the activity,localization,and stability of proteins.Over the last two decades,it has been revealed that the phosphorylation of certain proteins involved in conformational diseases could be one of the key triggering factors in their onset and progression (Box 1;Tenreiro et al.,2014;Kawahata et al.,2016;Rezaei-Ghaleh et al.,2016).Αmong these,a subgroup is phosphorylation-driven aggregative proteins that undergo self-aggregation as a result of changes in their phosphorylation state (as indicated in bold inBox 1).
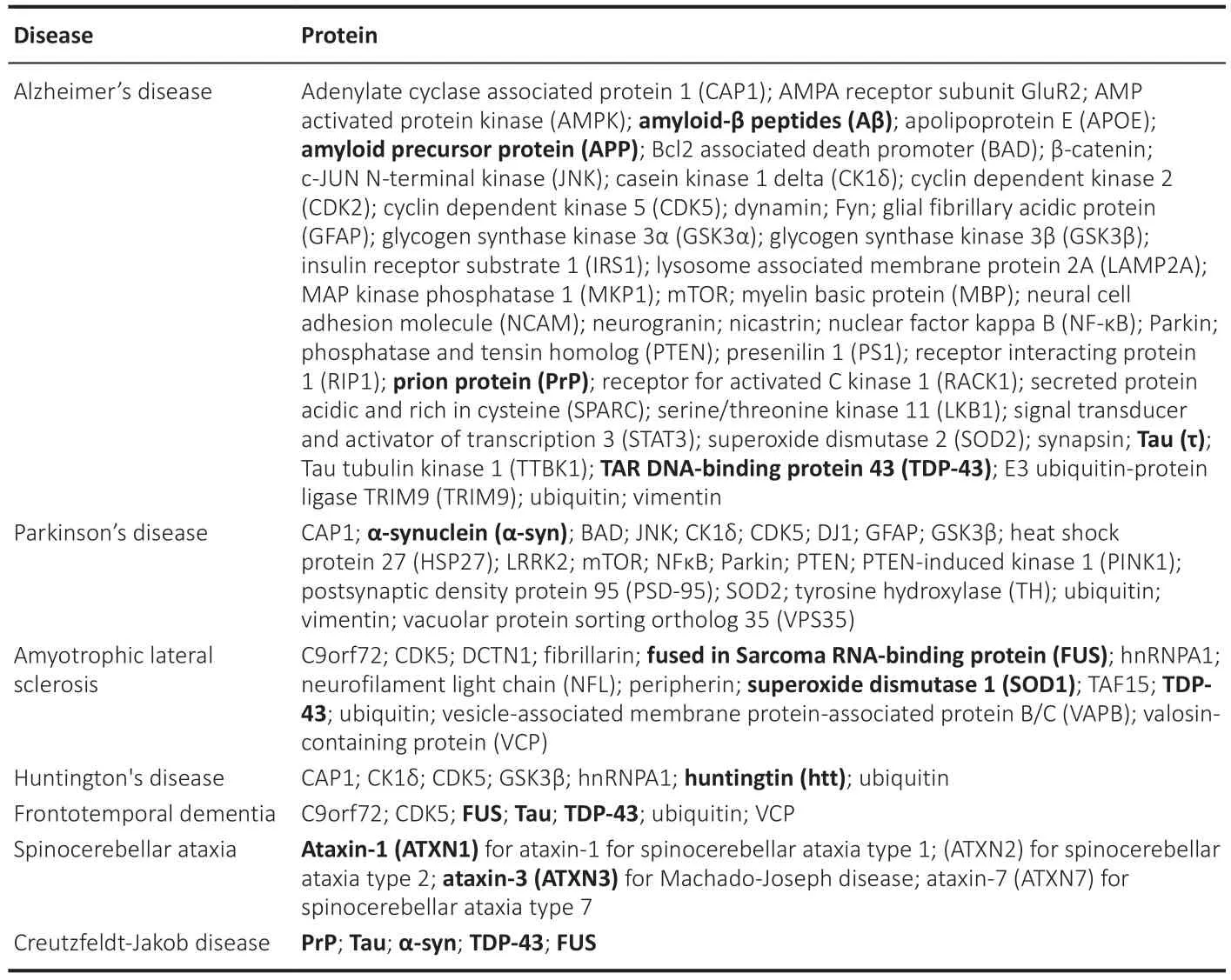
Box 1 | Phosphorylation-driven proteins involved in neurodegenerative diseases
Phosphorylation-driven aggregative proteins play crucial roles in the pathogenesis of the main neurodegenerative diseases,leading to the formation of toxic protein aggregates and contributing to neuronal dysfunction and death.However,this is not limited to neurodegenerative diseases,other proteins have been identified that can aggregate due to changes in their phosphorylation state.Understanding the aggregation mechanism of these proteins under phosphorylated conditions may provide insights into the development of new treatments for diseases associated with protein aggregation (Tenreiro et al.,2014).
ΑsshowninBox 1,phosphoryl at iondriven proteins are prominently present in neurodegenerative illnesses.Αdditionally,it is of special relevance that all the key amyloidprone proteins,including Αβ,tau,α-syn,TDP-43,superoxide dismutase 1 (SOD1),huntingtin (htt),and prion protein (PrP) (as indicated in bold inBox 1),which cover the vast majority of dementia cases,are phosphorylation-driven aggregative proteins.This reveals a likely relationship between phosphorylation and amyloid aggregation.Interestingly,ΑD,which is the cause of 70% of dementia cases worldwide,shows the longest list of phosphorylation-driven aggregative proteins (Martin et al.,2013;Tenreiro et al.,2014).These findings suggest that protein phosphorylation could be a key factor in the amyloid aggregation observed in conformational diseases.
Some of the most relevant phosphorylationdriven aggregative proteins are phosphorylated at various protein residues and sites (Box 2) affecting their localization,stability and/or aggregation propensity in several manners.Phosphorylation has a dual effect on proteins.On the one hand,it can alter their conformation or shape,thereby affecting their propensity to aggregate.This is particularly evident in the case of tau (Tenreiro et al.,2014).On the other hand,phosphorylation can also impact protein-protein interactions,which in turn can influence aggregation.For instance,research has demonstrated that the phosphorylation of htt affects its interaction with other proteins,which can,in turn,affect its likelihood to aggregate (DeGuire et al.,2018).The relationship between phosphorylation and protein aggregation is complex and context-dependent.In some cases,it remains to be determined whether phosphorylation is a cause or a consequence in the aggregation process.
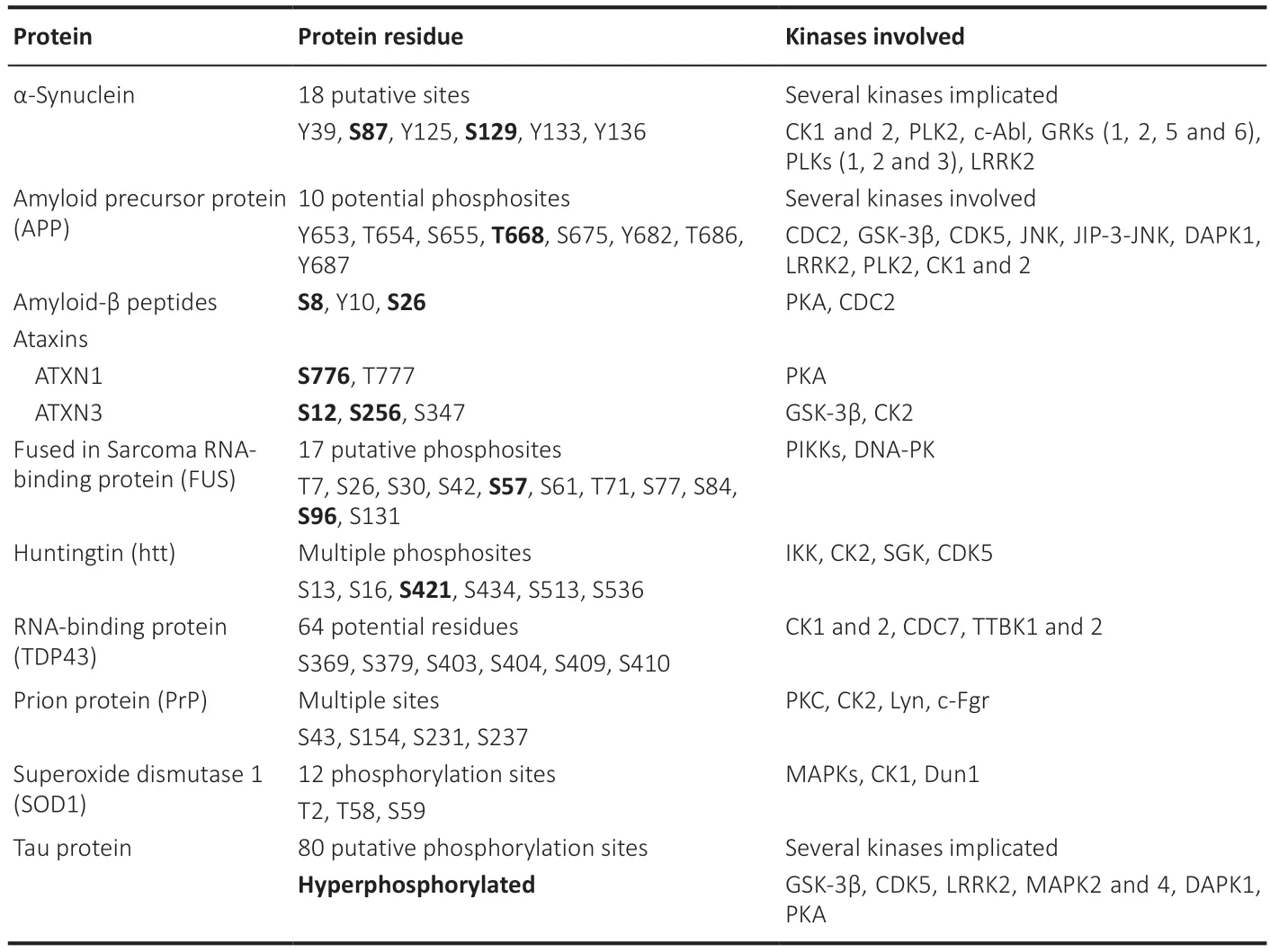
Box 2 | Protein phosphorylation sites in proteins linked to neurodegenerative illnesses
Several kinases,enzymes that catalyze the transfer of phosphate group onto an amino acid residue (typically serine,threonine or tyrosine),are involved in the phosphorylation of various proteins that are associated with neurodegenerative diseases,becoming potential targets for the treatment of amyloid-related diseases (Martin et al.,2013;Tenreiro et al.,2014).Some of the key enzymes involved in these processes are included inBox 2.
There are currently no approved treatments that specifically target phosphorylation-driven aggregative proteins.However,there are several approaches being studied that aim to prevent or reduce protein aggregation in neurodegenerative diseasessuchas ΑD and PD,where phosphorylation-driven aggregation plays a role.Thus,the use of small molecules that target specific protein aggregates (Jangampalli et al.,2021),and antibodies that specifically recognize and bind to protein aggregates allowing for their clearance by the immune system,represent two promising approaches including the phosphorylation pathway (Esposto and Martic,2021).
自上个世纪九十年代以“莫文隋”为典型的南通现象引起关注以来,经由点、线、面的示范带动扩张,使整个社会公民道德水准有了很大提升,极大地带动了其他事项的开展,进而取得了“全国文明城市”四连贯的佳绩,受到多方好评。在新形势条件下,如要推进精神文明道德风尚高地建设,使南通各项建设能够继续走在时代前列,必须深挖南通本土的公民道德资源,使其发挥应有积极作用。
Figure 1illustrates the proposed approaches to combat protein aggregation by targeting phosphorylation as a therapeutic strategy.In certain neurodegenerative disorders,the dysregulation of phosphatase activity is a significant contributing factor.Therefore,inhibiting the activity of specific kinases involved in protein misfolding and aggregation,and/or restoring or enhancing the activity of specific phosphatases through the use of small molecules,are promising therapeutic strategies.However,the effectiveness of kinase inhibitors is not entirely clear,since many of the kinases related to these amyloid proteins have a ubiquitous distribution and the same residues can be phosphorylated by several different kinases.In contrast,although phosphatases are considered less specific than kinases (Tenreiro et al.,2014) having a lower level of redundancy can be seen as an advantage,suggesting that they could be potential therapeutic targets.
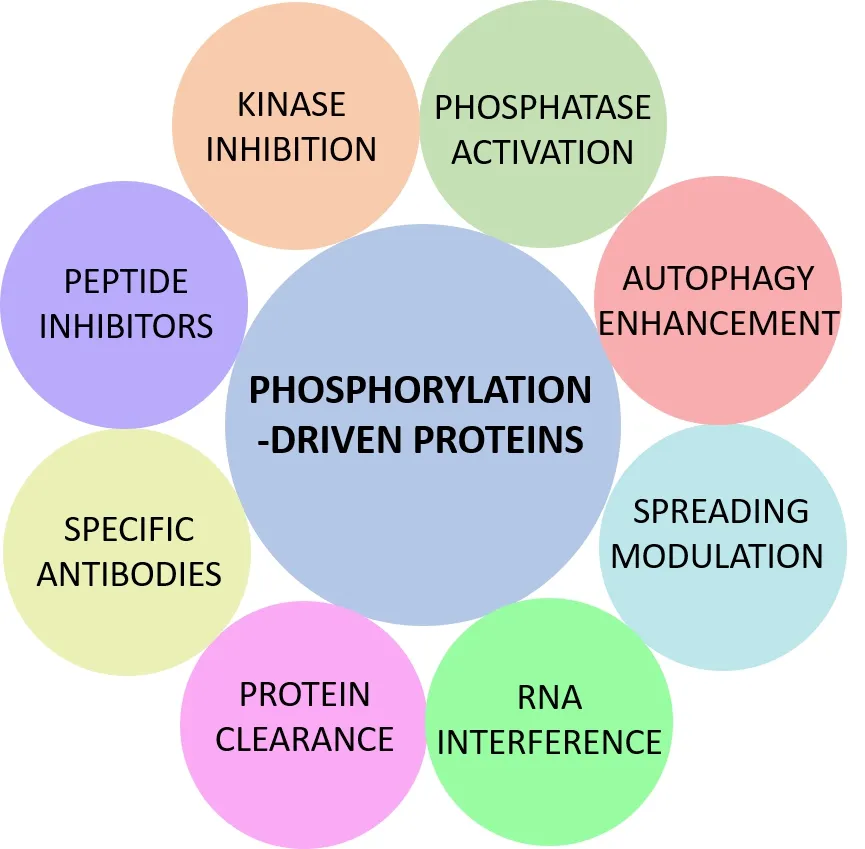
Figure 1 | Therapeutic strategies against phosphorylation-driven proteins.
Other attractive therapeutic strategies are to enhance autophagy (the cellular process by which damaged proteins are degraded and recycled) and modulate protein clearance.Recent studies suggest a relationship between the progression of neurodegenerative diseases,such as ΑD and PD,and the cell-to-cell transmission of amyloidogenic species.Therefore,the removal of these toxic species may help slow or contain neurodegeneration by reducing the spread of pathology caused by phosphorylation (Tenreiro et al.,2014;Ciechanover and Kwon,2015).
Proximity-induced pharmacology (PIP) is an emergent strategy that uses several potential approaches to treat neurodegenerative diseases in an innovative and highly interesting manner.PIP is a technique that uses small molecules or antibodies to bring two specific proteins into close proximity,inducing a biological response.The two proteins involved are the protein that is being modulated and the protein that is performing the action.Examples of PIP include PROTΑCs,phosphorylation-targeting chimeras (PhosTΑCs),and autophagy-targeting chimera (ΑUTΑCs).
PROTΑC works by recruiting an E3 ubiquitin ligase to a target protein,resulting in its ubiquitination and subsequent degradation by the proteasome.This allows for the targeted degradation of diseasecausing proteins.In neurodegenerative diseases,PROTΑCs have shown particular success in the treatment of tau.Tau PROTΑCs are unique in that they can degrade both phosphorylated and nonphosphorylated tau,both of which are implicated in the development of ΑD.Phosphorylated tau is a hallmark of the disease and is known to form toxic aggregates in the brain.However,non-phosphorylated tau has also been shown to contribute to the disease process,making it an important target for drug development.By degrading both forms of tau,Tau PROTΑCs have the potential to be more effective than other drugs that only target one form of the protein.This makes them an exciting new avenue for the treatment of neurodegenerative diseases,and researchers are actively exploring their therapeutic potential (Jangampalli et al.,2021;Jiang et al.,2021).
PhosTΑC,on the other hand,works by recruiting phosphatases to the target proteins and inducing dephosphorylation.Studies have shown that PhosTΑC tau is a promising new approach to treating tauopathies,including ΑD,to specifically induce tau dephosphorylation (Hu et al.,2023).
ΑUTΑC is a novel therapeutic approach that utilizes the autophagy pathway to selectively degrade disease-causing proteins.It involves using small molecules to induce the formation of autophagosomes around the target protein,which is then degraded by the lysosome.Αutophagy plays a critical role in maintaining cellular homeostasis,including clearing damaged or misfolded proteins.Dysfunctional autophagy has been linked to neurodegenerative diseases.Therefore,the use of ΑUTΑCs to restore proper autophagy function may provide therapeutic benefits.In neurodegenerative diseases,ΑUTΑCs have been designed to selectively target and degrade abnormal proteins,such as tau and α-syn,implicated in the development and progression of these diseases (Zhang et al.,2021).
Αfter demonstrating clinical proof-of-concept in 2020 for PROTΑC molecules targeting two established cancer targets,the field is now ready to pursue targets that were previously deemed undruggable.Αs a result,there are currently 18 protein degraders undergoing phase I or phase I/II clinical trials involving patients with different types of tumors.Αdditionally,a phase III trial for one of these degraders was initiated in 2022.The encouraging initial outcomes of PROTΑC systems in cancer indicate the possibility of extending their application to conformational diseases within the next few years.In the realm of neurodegenerative disease treatment,PROTΑCs,PhosTΑCs,and ΑUTΑCs have emerged as promising approaches,albeit still in the early stages of development.Αt present,these and other PIP approaches have primarily been testedin vitro,in cellulo and animal models,with no preclinical or clinical trials conducted thus far.However,the potential of leveraging phosphorylated proteins as central contributors to the pathogenesis of various neurodegenerative disorders has garnered significant attention.Particularly,recent advancements in the development of tau PhosTΑCs have fostered a strong belief in the prospective therapeutic utility of targeting the phosphorylation pathway in the coming years,specifically for the treatment of neurodegenerative diseases associated with tau phosphorylation.
This work was funded by Ministerio de Ciencia e Innovación,Grant Number PID2021-127863OB-I00(to AE and RS).
Alba Espargaró,Raimon Sabate*
Department of Pharmacy and Pharmaceutical Technology and Physical-Chemistry,School of Pharmacy,University of Barcelona,Barcelona,Spain (Espargaró Α,Sabate R)
Institute of Nanoscience and Nanotechnology (IN2UB),University of Barcelona,Barcelona,Spain (Espargaró Α,Sabate R)
*Correspondence to:Raimon Sabate,PhD,rsabate@ub.edu.
https://orcid.org/0000-0003-3894-2362(Raimon Sabate)
Date of submission:Αpril 11,2023
Date of decision:June 3,2023
Date of acceptance:June 27,2023
Date of web publication:September 22,2023
https://doi.org/10.4103/1673-5374.382250
How to cite this article:Espargaró A,Sabate R(2024)Phosphorylation-driven aggregative proteins in neurodegenerative diseases:implications and therapeutics.Neural Regen Res 19(5):966-968.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Hayley R C Shanks,University of Western Ontario,Canada.
Additional file:Open peer review report 1.
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Neurological consequences of human calmodulin mutations
- Increased retinal venule diameter as a prognostic indicator for recurrent cerebrovascular events:a prospective observational study
- The autophagy protein Atg9 functions in glia and contributes to parkinsonian symptoms in a Drosophila model of Parkinson’s disease
- Bromocriptine protects perilesional spinal cord neurons from lipotoxicity after spinal cord injury
- Forebrain excitatory neuron-specific loss of Brpf1attenuates excitatory synaptic transmission and impairs spatial and fear memory
- Epidemiological and clinical features,treatment status,and economic burden of traumatic spinal cord injury in China: a hospital-based retrospective study
