Role of CD36 in central nervous system diseases
2024-02-13MinFengQiangZhouHuiminXieChangLiuMengruZhengShuyuZhangSonglinZhouJianZhao
Min Feng ,Qiang Zhou ,Huimin Xie ,Chang Liu ,Mengru ZhengShuyu Zhang,Songlin Zhou,Jian Zhao
Abstract CD36 is a highly glycosylated integral membrane protein that belongs to the scavenger receptor class B family and regulates the pathological progress of metabolic diseases.CD36 was recently found to be widely expressed in various cell types in the nervous system,including endothelial cells,pericytes,astrocytes,and microglia.CD36 mediates a number of regulatory processes,such as endothelial dysfunction,oxidative stress,mitochondrial dysfunction,and inflammatory responses,which are involved in many central nervous system diseases,such as stroke,Alzheimer’s disease,Parkinson’s disease,and spinal cord injury.CD36 antagonists can suppress CD36 expression or prevent CD36 binding to its ligand,thereby achieving inhibition of CD36-mediated pathways or functions.Here,we reviewed the mechanisms of action of CD36 antagonists,such as Salvianolic acid B,tanshinone IIA,curcumin,sulfosuccinimidyl oleate,antioxidants,and small-molecule compounds.Moreover,we predicted the structures of binding sites between CD36 and antagonists.These sites can provide targets for more efficient and safer CD36 antagonists for the treatment of central nervous system diseases.
Key Words: animal experiments;antagonists;CD36 antagonist;central nervous system diseases;clinical trial;curcumin;microRNA;salvianolic acid B;small-molecule drugs;sulfosuccinimidyl oleate
From the Contents
Introduction 512
Search Strategy 512
CD36 Structure and Functions 512
Role of CD36 Inhibitors in Central Nervous System Diseases 512
Conclusions 516
Introduction
Cluster of differentiation 36 (CD36),which belongs to the scavenger receptor class B protein family,is a highly glycosylated transmembrane glycoprotein also known as platelet membrane glycoprotein IV,fatty acid translocase,and glycoprotein 88.It is expressed in various cell types,including perivascular macrophages,platelets,endothelial cells,microglia,astrocytes,and adipocytes.Recent research has shown that CD36 dysregulation is related to Parkinson’s disease (PD),stroke (Balkaya et al.,2021),Alzheimer’s disease(AD) (Moore et al.,2002),and other neurological diseases.CD36 is involved in mediating endothelial dysfunction,oxidative stress,mitochondrial dysfunction,and inflammatory responses,which can accelerate neurodegenerative processes and cognitive decline (Ioghen et al.,2021).Using CD36 inhibitors to target CD36 expression and improve neurological function has been effective in the treatment of many neurological disorders.Therefore,developing and mining molecules or antagonists that can target CD36 to inhibit its function are expected to provide new strategies to treat clinical diseases.
Search Strategy
The PubMed database was searched for articles involving CD36 and central nervous system (CNS) diseases published from 2017 to 2022.The results were further filtered by title and abstract,including only studies on the mechanisms of CD36 and CD36-targeting agents,and meta-articles were excluded.The search terms were as follows: “CD36”,“spinal cord injury”(SCI),“CD36 antagonist”,“salvianolic acid B”,“curcumin”,“sulfosuccinimidyl oleate”,“central nervous system diseases”.
CD36 Structure and Functions
CD36 is encoded by theCD36gene,which consists of 15 exons and is located on chromosome 7 (7q11.2).To explore CD36’s function and identify molecules that can target it,we used Phyre2 web portal (http://www.sbg.bio.ic.ac.uk/phyre2;Kelley et al.,2015) to further analyze the structure of CD36 and obtain a three-dimensional structural model the protein (Figure 1A).The CD36 protein consists of 472 amino acids that span the membrane twice,with two concise cytoplasmic structural domains (N-and C-termini) and a large glycosylated extracellular structural domain (Figure 1A).The N-and C-termini contain two palmitoylation sites that localize CD36 in membrane lipid rafts.The extracellular domain of CD36 is covered by an α-helical bundle and a vertex region containing an accumulation of cationic residues,in addition to an incomplete hydrophobic cavity spanning most of the molecule’s length,which was found to have two entry points—a fatty acid entry point in the central cavity and an opening point on the distal side of the membrane in the extracellular domain.CD36 also contains multiple modification for glycosylation,palmitoylation,acetylation,and phosphorylation.These modification sites are able to regulate the stability,translocation,and folding of the CD36 protein (Neculai et al.,2013;Hsieh et al.,2016;Cabrera et al.,2019).
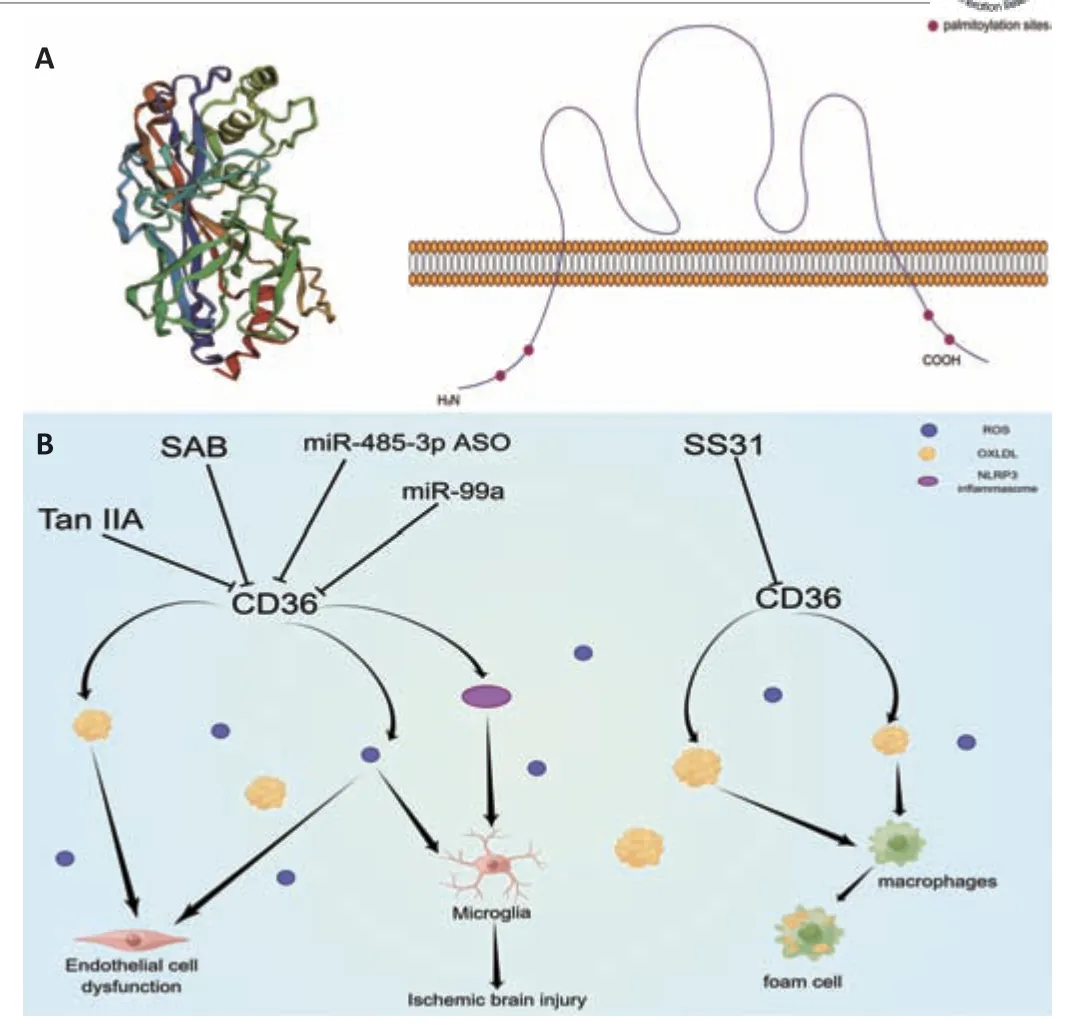
Figure 1|CD36 structure and its role in diseases of the central nervous system.
CD36 is a multi-ligand pattern recognition receptor capable of interacting with many structurally distinct ligands,including long-chain fatty acids,oxidized low-density lipoproteins (oxLDLs),advanced glycosylation end products,thrombospondin 1,and high-density lipoproteins.Studies have shown that CD36,which is mainly localized in caveolae structures on the cell surface,can deliver fatty acids to cells via endocytosis,promote fatty acid uptake,and mediate lipid metabolism (Hao et al.,2020).In addition,CD36 phosphorylation inhibits binding to thrombospondin 1 (Chu and Silverstein,2012) and induces apoptosis of endothelial cells to inhibit angiogenesis.CD36 can also initiate fibrosis by interacting with Jun,which leads to scar formation (Griffin et al.,2021).In a recent study,CD36 was found to be one of the regulators of the glial scarring response after SCI and its expression was inhibited with CD36 inhibitors to reduce fibrosis (Gong et al.,2023).There are also data from stroke models suggesting that CD36 deficiency attenuates blood-brain barrier (BBB) disruption and scar formation during the chronic phase of stroke and leads to seizure resistance (Zheng et al.,2017;Balkaya et al.,2021).
Role of CD36 Inhibitors in Central Nervous System Diseases
In the CNS,CD36 is expressed on microglia,vascular endothelial cells,brain pericytes,astrocytes,and hematogenous macrophages found in the brain in pathological conditions (Coraci et al.,2002;Bao et al.,2012b;Kim et al.,2012;Li et al.,2022).CD36 has been implicated in the pathogenesis of neurological diseases,such as stroke,AD,and PD,and its mediation of neuroinflammation,oxidative stress,and endothelial dysfunction contribute to the progression of CNS diseases (Coraci et al.,2002;Balkaya et al.,2021;Ioghen et al.,2021).In addition,CD36 was used as a treatment target for endothelial dysfunction during stroke (Cho,2012).
A recent study showed CD36 expressed in fibroblasts,one of the cellular components of spinal glial scarring (Gong et al.,2023).In particular,CD36 was one of the regulators of the glial scarring response after SCI,and SCI enhanced Jun expression and induced CD36 expression in fibroblasts,which activated fibroblasts to form scars in mice.Jun is a key factor in pathological skin scarring,while CD36 is its downstream effector (Griffin et al.,2021).In further experiments,the expression of CD36 in activated fibroblasts was significantly inhibited by its inhibitors.Therefore,we propose that CD36 may be a target for the treatment of spinal scars.Notably,Gong et al.(2023) analyzed the cellular composition and spatial and temporal distribution of spinal scars,and fibroblasts were found to occupy the center of the scar during the subacute phase,which may indicate the ideal time for the use of CD36 antagonists.Although CD36 is also expressed in macrophages and astrocytes,the researchers did not discuss whether CD36 plays a role in these two cell types in this study.Past studies have shown that scar formation after CNS injury creates a physical barrier to synaptic growth (Desclaux et al.,2009;Zhang et al.,2021;Nogueira-Rodrigues et al.,2022),and reducing scar formation to overcome the physical barrier to promote axonal growth has become a strategy for treating CNS injury (Desclaux et al.,2009).Targeted inhibition of CD36 expression to reduce scar formation is a potential treatment for CNS injury.
Studies have shown that inhibition of CD36 expression can reduce the inflammatory response and oxidative stress,thereby ameliorating neurological damage and providing neuroprotective effects (Cho,2012;Kim et al.,2015).Therefore,the development of new CD36 antagonists or the discovery of compounds that can target CD36 to inhibit its expression or prevent it from binding to its ligands or interacting with other proteins may be beneficial in the treatment of many nervous system diseases.Here,we summarized the role of CD36 in CNS diseases and related studies of CD36 antagonists(Table 1).In the search for CD36 antagonists,Chinese herbal extracts such as saikosaponin A,icariin,andrographolide,resveratrol,and quercetin have been demonstrated to modulate CD36 (Jian et al.,2019).Other natural compounds that affect CD36 function include gerberin,an active ingredient derived fromPueraria lobata,and puerarin,which significantly inhibited CD36 expression in a dose-dependent manner,thereby reducing oxLDL uptake and inhibiting oxLDL-induced apoptosis in macrophages (Zhang et al.,2015).In a study on neurological disease,puerarin showed effective protective activity against cerebral ischemic stroke (Ling et al.,2018).However,whether the mechanisms behind this are related to CD36 requires investigation.
CD36 aptamers have been developed using cell-systematic evolution of ligands with exponential enrichment technology (aptamer technology using intact cell/nucleic acid libraries,enabling screens of cell surface molecule binding)and experiments have shown that the non-alcoholic fatty liver disease cellspecific aptamer NAFLD01 may target and inhibit CD36 expression (Pu et al.,2021).In addition,natural plant constituent compounds,small molecules,and aptamers have been found to exhibit antagonistic activity against CD36 and have been widely used in scientific research.We look forward to finding safer and more effective CD36 inhibitors to provide new clinical treatment strategies for neurological diseases such as AD,PD,and SCI (Figure 1B).
Small-molecule compounds
Salvianolic acid B and Tanshinone IIASalvianolic acid B (SAB) can be isolated from the plant Salvia miltiorrhiza Bge(Labiatae),and possesses potent anti-inflammatory,antioxidant,and antifibrotic properties (Xiao et al.,2020).With the chemical formula C36H30O16and a molecular weight of 718,SAB is a good prospect for preventing and treating neurological conditions (Fan et al.,2018;He et al.,2018;Tan et al.,2021).SAB has been shown to have neuroprotective effects,ameliorates neurobehavioral deficits after ischemia-reperfusion injury in the mouse brain,and attenuates the inflammatory response by reducing the production of reactive oxygen species (Fan et al.,2018).In a study on AD,SAB attenuated amyloid beta(Aβ)-induced excessive mitochondrial superoxide production and effectively protected mitochondrial function (He et al.,2018).SAB also improved cognitive impairment by reducing neuroinflammation and decreasing Aβ levels inPorphyromonas gingivalis-infected mice (Taha et al.,2004;Liu et al.,2020).In Aβ-induced memory impairment,SAB was found to directly inhibit Aβ production,and,therefore,it is seen as a promising drug for AD treatment(Tang et al.,2016).
SAB has been previously reported to target CD36,and surface plasmon resonance analysis showed that SAB directly bound to CD36.For this review,we performed molecular docking prediction for SAB and CD36 using Autodock Vina software (http://vina.scripps.edu;Eberhardt et al.,2021),and the results showed that SAB does have a binding spatial structure with CD36,which further explained the basis of its function (Figure 2A).One study found that SAB not only reduced oxLDL-induced CD36 expression inin vitroexperiments but also inhibited CD36 inin vivoexperiments (Bao et al.,2012a).Further experimental studies were conducted to determine whether SAB could inhibit CD36 in lipid metabolism-related neurological disorders and thus provide therapeutic benefits.Hyperlipidemia can exacerbate poststroke injury with severe brain swelling,and researchers further investigated the role of CD36 inhibition in reducing infarct size and swelling in patients with hyperlipidemic stroke (Kim et al.,2020).Unfortunately,the mechanisms of action were not clarified in this study.However,it has been shown that SAB can improve metabolic function by inhibiting CD36 expression,thereby reducing visceral fat accumulation and improving insulin resistance in a wildtype mouse model of obesity (Yang et al.,2018).Thus,inhibition of CD36 by SAB may normalize metabolic function in patients with hyperlipidemia and reduce brain swelling after stroke.Furthermore,the loss of vascular tonus and BBB integrity underpins the pathology of stroke and AD,and stroke pathology also involves multiple factors that contribute to the death response,including inflammation and oxidative stress.CD36 has been used as a therapeutic target for endothelial dysfunction in stroke,where CD36-mediated pathways are activated by different ligands that will lead to inflammatory responses and endothelial dysfunction (Richards et al.,1994;Cho,2012).SAB,as a CD36 antagonist,has been shown to inhibit the production of mitochondrial reactive oxygen species in endothelial cells and reduce oxLDL to achieve a protective effect in endothelial cell dysfunction (Ko et al.,2020).Therefore,the use of SAB to improve endothelial dysfunction and the inflammatory response is beneficial for alleviating stroke progression,but more experiments are needed to elucidate the exact mechanisms behind the inhibition of CD36 by SAB in neurological diseases.Notably,in neurological diseases not associated with lipid metabolism such as SCI,targeted inhibition of CD36 expression using SAB has been shown to attenuate CD36-mediated scar formation after SCI to reduce fibrosis,providing theoretical data for the treatment of spinal scars (Gong et al.,2023).In conclusion,SAB as an effective CD36 antagonist has potential for the treatment of neurodegenerative diseases and related complications by inhibiting CD36 function.
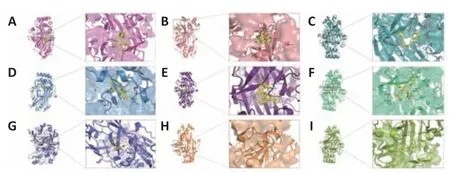
Figure 2|3D structure of binding sites predicted by Phyre2 web tool.
Tanshinone IIA (Tan IIA) is a lipid-soluble component of S.miltiorrhiza with various neuroprotective mechanisms,including anti-inflammatory,antioxidant,anti-apoptotic,and vascular protective effects.The regulation of Tan IIA involves multiple targets such as Nrf2,AMP-activated protein kinase,heme oxygenase,NADPH oxidase 4,tumor necrosis factor-α,caspase-3,B-cell lymphoma-2 protein,and CD36,which directly or indirectly affect disease processes (Zhong et al.,2021).Tan IIA has been beneficial in models of CNS disease.Li et al.(2017) found that Tan IIA reduced glutamate-induced reactive oxygen species accumulation,inhibited lipid and protein peroxidation,and reduced glutamate-related neurotoxicity.
Neurotoxicity can contribute to the progression of AD and secondary brain damage,and systemic and CNS inflammation is a key factor in neurotoxicity(Subedi and Gaire,2021;Matamoros-Angles et al.,2023;Si et al.,2023).CD36 is regarded as a new molecular target in the neurovascular unit and a potentially important actor in CNS diseases such as AD-related vascular dysfunction,oxidative stress,and neuroinflammatory responses in stroke(Ioghen et al.,2021).In an AD model,perivascular macrophages induced CD36-mediated oxidative stress.CD36 is expressed in macrophages around the brain and reacts with Aβ to produce reactive oxygen species that contribute to neurovascular dysfunction.Additionally,CD36 binding to Aβ activates inflammasome NOD-like receptor family pyrin domain containing 3 (NLRP3),which promotes a pro-inflammatory response resulting from the release of pro-inflammatory cytokines (interleukin-1β,-1α and -18).This will exacerbate neuroinflammation,leading to progressive deterioration of AD (Park et al.,2017;Ioghen et al.,2021).Similarly,in stroke models,CD36 is involved in the pathophysiology of stroke.It has been shown that CD36 is expressed on microgliamacrophages and mediates reactive oxygen species production as a mechanism of ischemic brain injury.In addition,microglia-released NLRP3 inflammasomes are considered a major cause of neuroinflammation,and inhibition of NLRP3 inflammasome activation may help to reduce neuroinflammation and prevent BBB damage (Cho et al.,2005;Luo et al.,2019;Chen et al.,2020).
In a study on the role of Tan IIA in regulating CD36,researchers found that oxLDL mediated NLRP3 inflammasome activation via CD36.The use of Tan IIA reduced the expression of CD36 in macrophages and limited mitochondrial and lysosomal damage,eventually inhibiting the activation of NLRP3 inflammasomes and mitigating the inflammatory response (Zlowodzki et al.,2007;Wen et al.,2020).Therefore,inhibition of CD36 expression using Tan IIA to reduce NLRP3 inflammasome activation,systemic inflammation,and neuroinflammation is important to improve the symptoms of AD and stroke.Further analysis of the mechanisms by which Tan IIA inhibitsCD36expression was performed by Tang et al.(2011),who found downregulation of CD36 mRNA by Tan IIA (Figure 1B).We performed molecular docking prediction for Tan IIA with CD36 using Autodock Vina software,and the findings showed that Tan IIA has a spatial structure that links to CD36,which further explained the basis of its function (Figure 2B).
In the search for CD36 antagonists,Gao et al.(2016) also found that danshenin,extracted fromS.miltiorrhiza,also significantly reduced CD36 levels.Wang et al.(2010) used an enzyme-linked immunosorbent assay-like high-throughput screening assay for the discovery of CD36 antagonists.They found that,in addition to SAB,salvianolic acid sodium and rosmarinic acid were small-molecule antagonists of CD36 (Wang et al.,2010).In conclusion,SAB or its derivatives/analogues,as well as active ingredients ofS.miltiorrhizawith antagonistic effects on CD36,may improve neurovascular dysfunction and reduce neuroinflammation,providing benefits in the treatment of CNS disorders;however,more experiments are needed to further elucidate the exact mechanisms of action.
Curcumin
Curcumin is the main active polyphenol derived from the turmeric rhizome with pleiotropic neuroprotective mechanisms,including antioxidant,anti-inflammatory,anti-apoptotic,autophagy modulation,and direct neuroprotective functions (Subedi and Gaire,2021;Cao et al.,2022).Studies have shown that curcumin reduces neurotoxicity,attenuates AlCl3-induced neuroinflammation,and exerts neuroprotective effects in AD animal models(ELBini-Dhouib et al.,2021).In an animal model of SCI,curcumin treatment reduced neuronal apoptosis,inhibited inflammatory responses,and enhanced autophagy to promote functional recovery (Li et al.,2021).In addition,curcumin also exhibited neuroprotective effects in a PD model (Jin et al.,2022).Although curcumin has been found to have biomechanical effects on a variety of diseasesin vivo,its poor bioavailability has limited the results of research and clinical utilization,so researchers have been focusing on the use of analogues of curcumin and its nanomedicines.The most common targets of curcumin analogues and derivatives are nuclear factor-κB (NFκB),microtubulin,proteasomes,epidermal growth factor receptor,cysteine proteases,and signal transducers and activators of transcription proteins(Chainoglou and Hadjipavlou-Litina,2019).Bisceglia et al.(2019) obtained a set of analogues in their experiments and found them to have good anti-AD effects with anti-inflammatory and antioxidant properties.In another study,Yang et al.(2021) experimentally encapsulated curcumin in polymer-based nanoparticles and showed its ability to inhibit ferroptosis with good benefits for the treatment of cerebral hemorrhage.Thus,curcumin and its analogues can be used as therapeutic agents for neurological disorders.
In a study on the regulation ofCD36expression by curcumin,Min et al.(2013)found that curcumin significantly inhibitedCD36expression in macrophages and that the expression and activity of peroxisome proliferator-activated receptor-γ (PPAR-γ),which is involved in the expression of CD36,was also inhibited in cells treated with curcumin.We also performed a molecular docking prediction of curcumin with CD36 using Autodock Vina software,and the results showed that curcumin does have a binding spatial structure with CD36,which further explains the basis of curcumin’s action (Figure 2C).It has been previously shown that CD36-mediated activation of NLRP3 inflammasomes leads to the accumulation of pro-inflammatory factors and that inhibition of CD36 expression attenuates NF-κB activation and prevents subsequent activation of the NLRP3 inflammasome,thereby reducing the inflammatory response (Jiang et al.,2021).Notably,in a model of ischemic stroke,curcumin was shown to improve functional outcomes by inhibiting NFκB pathway activation and NLRP3 inflammasomes,ameliorating post-stroke white matter and brain tissue damage,and attenuating microglia apoptosis(Ran et al.,2021).Unfortunately,the mechanisms of CD36 involvement were not explored in this study,and more experiments are required to further elucidate the specific mechanisms by which curcumin binding to CD36 affects the NF-κB/NLRP3 pathway.
Sulfosuccinimidyl oleate
Sulfosuccinimidyl oleate (SSO) is a long-chain fatty acid with the molecular formula C22H36NNaO7S that has the ability to inhibit the oxidation of mitochondrial long-chain fatty acids,is an inhibitor of the mitochondrial respiratory chain,and is an antagonist of CD36 (Drahota et al.,2010).We also performed molecular docking prediction for SSO and CD36 using Autodock Vina software,and the findings showed that SSO has a binding spatial structure with CD36 (Figure 2D).Studies found that the target of SSO is located at lysine 164 on CD36 (Harmon and Abumrad,1993;Kuda et al.,2013).In stroke,the post-ischemic inflammatory response is highly detrimental to recovery.Microglia and astrocytes are involved in this response,and microglia are rapidly activated and release pro-inflammatory mediators and cytotoxic substances after cerebral ischemia.Data have shown that CD36 is expressed on microglia and is involved in ischemic brain injury(Cho et al.,2005;Vidale et al.,2017;Qin et al.,2019).
CD36 is involved in mediating multiple signaling pathways that play an essential part in the pro-inflammatory response,including the NF-κB signaling pathway.One study found that NF-κB activity was diminished in CD36-deficient mice,suppressing the inflammatory response induced by cerebral ischemia and thereby reducing brain injury (Kunz et al.,2008).In another study,inhibition of CD36 expression using SSO blocked the activation of NF-κB and was able to prevent subsequent activation of inflammatory vesicles (Jiang et al.,2021).Thus,SSO may exert neuroprotective effects by binding to CD36.Interestingly,SSO was found to inhibit inflammation independently of CD36 in ischemic stroke.The experimental results showed that SSO could directly reduce lipopolysaccharide/interferon-γ-induced pro-inflammatory mediators produced by microglia,such as interleukin-6 and tumor necrosis factor-α,and could prevent inflammation-induced neuronal death and attenuate ischemic brain injury with neuroprotective effects in a mouse model of ischemic stroke(Dhungana et al.,2017).In conclusion,SSO has an anti-inflammatory profile that could make it a candidate molecule for further drug development.However,more studies are required to determine the exact mechanisms behind the neuroprotective effects of SSO inhibition of CD36 expression after ischemic stroke.
CD36 ligands
In addition to the development of new synthetic compounds as high-affinity scavenger receptor inhibitors,it has been shown that synthetic ligands of CD36 also show competitive binding properties with CD36.Hexarelin is a synthetic peptide with growth hormone-releasing activity that binds to the CD36 receptor.Demers et al.(2004) found that the binding domain of hexarelin on CD36 overlaps with the binding domain of oxLDL on CD36,and hexarelin may thus interfere with CD36-mediated uptake of oxLDL.We also performed molecular docking predictions for hexarelin with CD36 using Autodock Vina software,and the results showed that hexarelin has a binding spatial structure with CD36 (Figure 2E).In addition,hexarelin has shown neuroprotective and anti-apoptotic effects;hexarelin could protect Neuro-2a cells from H2O2-induced injury via a molecular pathway involving mitogen-activated protein kinase (MAPK).The experimental use of hexarelin attenuated H2O2stimulation-induced MAPK upregulation (Meanti et al.,2021).Data showed that CD36 plays a key role in activating the MAPK pathway and that inhibition of MAPK signaling reduces apoptosis and contributes to neuroprotection (Kwon et al.,2011;Sini et al.,2017).Oxidative stress is involved in the pathogenesis of neurological diseases such as stroke,AD,and PD,and oxLDL levels influence stroke prognosis.Therefore,inhibition of CD36-mediated pathways using hexarelin has potential therapeutic relevance in the treatment of CNS diseases (Cho,2012;Wang et al.,2017).
EP 80317 is a novel peptide-derived CD36-selective ligand without any growth hormone-releasing activity and also reduces CD36-mediated oxLDL internalization (Marleau et al.,2005).Hexarelin and EP 80317 were able to prevent seizures and exhibit anticonvulsant effects in rats (Bisceglia et al.,2019).Notably,the involvement of CD36 in the activation of PPAR-γ and in mediating cellular secretion of inflammatory mediators,such as interleukin-1β,was shown to play a key role in seizures (Balosso et al.,2008;Lucchi et al.,2017).However,the exact mechanisms of neuroprotection involving CD36 induced by hexarelin and EP 80317 still need further study.In addition to this,the growth hormone-releasing peptide-6 analogues of azapeptides as well as cyclic azapeptides showed affinity for and modulation of CD36 (Proulx et al.,2012;Zhang et al.,2017a).In another study,Rawji et al.(2020) found that niacin (vitamin B3) upregulatedCD36expression and promoted the clearance of myelin debris from lesions by microglia and macrophages,which could be used to treat chronic demyelinating diseases such as multiple sclerosis.
Apolipoprotein A-I mimetic peptides
Apolipoprotein A-I (apoA-I) is a high-density lipoprotein component that has anti-inflammatory and anti-apoptotic properties.However,the preparation of pharmaceutical grade apoA-I is expensive,and apoA-I mimetic peptides have been developed in studies to mediate some functions instead of apoA-I.In the study of apoA-I mimetic peptides targeting CD36,we found that several mimetic peptide types regulate CD36 expression.Researchers analyzed apoA-I mimetic peptides L37pA,5A,and ELK,which were all found to competitively bind to CD36,a multiclass receptor for apoA-I,in the experiments.Among them,ELK-B peptides mainly acts through CD36,and,similarly,L37pA can bind to CD36 and block interleukin-8 secretion to reduce the inflammatory response (Bocharov et al.,2016).Notably,apoA-I mimetic peptides have similar anti-inflammatory properties to high-density lipoprotein and can block the pro-inflammatory signaling cascade,which includes CD36 ligand-induced activation of NF-κB (Xia et al.,1999;Di Bartolo et al.,2011).It has been shown that the CD36-mediated NF-κB pathway plays a key role in neuroinflammation in stroke (Kunz et al.,2008).Therefore,blocking NF-κB activation by inhibiting CD36 expression using an apoA-I mimetic peptide could be beneficial in reducing neuroinflammation and treating neurological disorders.
In the treatment of CNS diseases,recent studies (Swaminathan et al.,2020;Jiang and Bai,2022;Zhou et al.,2022) have found that apoA-I mimetic peptides have an anti-inflammatory activity that reduces inflammatory factor secretion,decreases oxidative stress in PD mice,and improves brain neurotransmitters by affecting dopamine and 5-hydroxytryptamine.apoA-I mimetic peptides can have a neuroprotective effect and improve motor deficits and coordination in PD mice,which can be beneficial in controlling the development of PD (Jiang and Bai,2022).In another study,apoA-I mimetic peptide L-4F crossed the BBB and was able to reduce neurovascular and white matter damage and improve neurological function in mice with stroke (Zhou et al.,2022).D-4F treatment in stroke rats significantly reduced BBB leakage and white matter damage,decreased neuroinflammation,and improved functional prognosis (Ning et al.,2017).Notably,Swaminathan et al.(2020) found that 4F reduced the accumulation of toxic amyloid beta in a mouse model of AD.Whereas CD36 has been shown to be involved in the uptake of Aβ,Aβ1-40 was found to exacerbate BBB damage by binding to CD36 on the surface of pericytes in experiments (Li et al.,2022).However,more research is needed to determine whether CD36 is involved in the process of Aβ reduction by apolipoprotein mimetic peptides.
Although there is a growing body of data showing that apoA-I mimetic peptides exhibit promising benefits in the treatment of neurological disorders,unfortunately,there are fewer applications regarding the use of apoA-I mimetic peptides to target the inhibition of CD36 expression in the treatment of CNS diseases,and we still need to conduct more experiments to determine the exact mechanism.
SS-31
SS-31 is a new class of mitochondria-targeting antioxidant Szeto-Schiller peptides with promising therapeutic effects in neurodegenerative diseases.Zhao et al.(2019) showed that treatment with SS-31 could reduce lipopolysaccharide-induced memory impairment and had protective effects against mitochondrial dysfunction and oxidative stress.In an animal model of sepsis-associated encephalopathy,administration of SS-31 led to a reduction in inflammation and neurological damage and improved cognitive deficits(Wu et al.,2015).In a model of Friedreich’s ataxia,SS-31 alleviated not only oxidative stress but also mitochondrialFXNdeficiency (Zhao et al.,2017).In a mouse model of AD,SS-31 significantly reduced soluble Aβ levels and attenuated Aβ-induced mitochondrial dysfunction (Reddy et al.,2017).Thus,SS-31 may have therapeutic potential in preventing damage caused by oxidative stress and neuroinflammation.
CD36 has recently been identified as a multimodal target for reducing oxidative stress and inflammation in ischemic stroke.In a study on the targeted inhibition of CD36 by SS-31,Zhang et al.(2017b) found that injection of SS-31 downregulatedCD36expression,inhibited oxidative stress,and improved systemic inflammation in mice.We performed molecular docking prediction for SS-31 and CD36 using Autodock Vina software,and the results showed a binding spatial structure between SS-31 and CD36 (Figure 2F).Cho et al.(2007) showed that treatment with SS-31 in a C57BL/6 mouse model of cerebral artery occlusion inhibitedCD36expression,reduced inflammatory cells and infarct size,and had neuroprotective effects.Notably,use of the SS-31 to target CD36 in the treatment of ischemic stroke was effective in a transient stroke model but virtually ineffective in a permanent ischemic stroke model (Kim et al.,2015).Therefore,targeted inhibition of CD36 by SS-31,a novel antioxidant,may be a useful therapeutic strategy to reduce acute stroke-induced injury and has potential therapeutic relevance in neurodegenerative diseases (Figure 1B).
Antioxidants
CD36 mediates oxLDL absorption,and oxLDL is a standalone predictor of poor functional outcome in individuals with mild stroke and transient ischemic attack (Wang et al.,2019).In a multicenter study,oxLDL levels were associated with stroke prognosis;higher oxLDL levels were significantly associated with higher risk of death and adverse outcomes within 1 year after stroke onset (Wang et al.,2017).OxLDL levels may be reduced by suppressing CD36 with antioxidants,which may be more effective in treating stroke and enhancing prognosis,as studies have shown that antioxidants can regulateCD36expression and,hence,decrease oxLDL uptake.
Vitamin E is one of the most prominent antioxidants and is an essential nutrient for neurodevelopment and cognitive function;vitamin E deficiency leads to neurodegenerative diseases such as ataxia and cognitive decline(Traber,2021).α-Tocopherol is the most active form of vitamin E and was found to have a regulatory function on CD36.Ricciarelli et al.(2000) found inin vitroexperiments that α-tocopherol could downregulate CD36 mRNA expression and protein translation by reducing CD36 promoter activity,thereby inhibiting oxLDL uptake.We performed molecular docking prediction for α-tocopherol and CD36 using Autodock Vina software,and the results showed a binding spatial structure between α-tocopherol and CD36 (Figure 2G).In a recent study,Zingg et al.(2022) used vitamin E analogues and derivatives,and found that both α-tocopherol phosphate (αTP) and an αTP/β-cyclodextrin nanocarrier complex inhibited CD36 surface exposure in human acute monocytic leukemia cells,leading to decreased oxLDL uptake.Similarly,interferon-γ was reported to significantly inhibit cell surface CD36 expression in a dose-dependent manner and to stimulate macrophages,leading to the synthesis of 7,8-dihydroneopterin,a potent antioxidant that inhibits cellular oxidative damage (Nakagawa et al.,1998).The reason for this was investigated by Gieseg et al.(2010),who showed that the reduction in oxLDL uptake was due to a significant downregulation of CD36 levels.Studies found that,similar to α-tocopherol,7,8-dihydroneopterin inhibited CD36 expression via a cellular signaling pathway that is also associated with PPAR-γ (Munteanu et al.,2006;Ghodsian et al.,2022).In addition,another antioxidant,N-acetylcysteine,has also shown an ability to inhibit CD36 expression (Ding et al.,2022).Notably,antioxidants that inhibit CD36 expression can be used to reduce oxLDL levels as well as attenuate oxidative stress to have neuroprotective effects and,thus,improve prognosis in stroke(Murad et al.,2014).However,data suggest that excessive antioxidant levels can exacerbate the neuroinflammatory response and oxidative stress in acute ischemic stroke,ultimately leading to increased brain damage (Khanna et al.,2015).Therefore,antioxidant dosages should be carefully considered in the treatment of people at high risk of stroke.
Pharmacological agents
Recent studies have found that some lipid-lowering drugs have also shown good benefits in the treatment of neurological diseases (Zeng et al.,2019;Yu et al.,2020;Sharma et al.,2021).Metformin is effective in the treatment of AD,PD,multiple sclerosis,spinal cord injury,and ischemic stroke (Sharma et al.,2021;Zhao et al.,2023).Researchers have found that metformin is neuroprotective,significantly reduces cerebral ischemia-reperfusion injury,and has a powerful modulating effect on stroke-induced oxidative stress (Zeng et al.,2019).In another experiment,the powerful anti-inflammatory effects of metformin reduced neuroinflammation in mice,and metformin treatment also prevented neuronal death and reduced Aβ levels,which led to improved neurological deficits and provided a new basis for the prevention and treatment of AD (Ou et al.,2018).It has been shown that berberine similarly exhibits neuroprotective functions in cerebral ischemia-reperfusion injury,possibly by inhibiting neuronal apoptosis through downregulation of the canopy fibroblast growth factor signaling regulator 2 signaling pathway (Zhao et al.,2021).Ezetimibe and statins have also shown beneficial effects in the treatment of neurological disorders in related studies (Vitturi and Gagliardi,2020;Yu et al.,2020).
Some drugs,such as AP5055,AP5258,ezetimibe,metformin,and statins,have regulatory effects on CD36 expression (Geloen et al.,2012;Zeng et al.,2019;Yu et al.,2020).Rekhi et al.(2021) further showed that AP5055 could target CD36 and reduce NF-κB activation,thereby reducing interleukin-1β production and ultimately reducing the inflammatory response.Ezetimibe is a selective inhibitor of cholesterol uptake and is commonly used in clinical practice for lipid-lowering therapy.Qin et al.(2016) showed that ezetimibe also has reduced CD36 expression.Further,metformin also has inhibitory effects on CD36 (Moon et al.,2017).Metformin inhibits NF-κB translocation in macrophages,which lowers the synthesis of pro-inflammatory cytokines as well as the expression of the scavenger receptor CD36 in macrophages,both of which work together to lower inflammation (Hyun et al.,2013).We used Autodock Vina software to conduct molecular docking prediction for ezetimibe and metformin with CD36,and the results showed that ezetimibe and metformin both had a binding spatial structure with CD36 (Figure 2HandI).Studies have also shown that statins downregulated CD36 expression inin vivoexperiments and reduced inflammatory factors such as interleukin-6 and tumor necrosis factor-α (Yin et al.,2017).Regarding the mechanisms of CD36 downregulation by simvastatin,experiments showed that inhibition of calpain-1 may mediate the downregulation of PPAR-γ by simvastatin,thereby inhibiting CD36 mRNA expression (Yang et al.,2016).Berberine is a novel lipid-lowering drug,and it has been reported that berberine can reduce the expression of CD36 mRNA (Sun et al.,2017).The main active metabolite of berberine,berberrubine,can also regulate CD36 expression to prevent lipid accumulation (Yang et al.,2022).Interestingly,during the synthesis of berberine analogues,researchers also accidentally discovered the generation of an N-(2-arylethyl) isoquinoline derivative and screened its activity.It was also found to have good activity in inhibiting CD36 and was considered as a promising new CD36 antagonist (Wang et al.,2011).A new strategy based on a family of “nanoblockers” was also examined by Chnari et al.(2006).They created nanoblockers that targeted the scavenger receptors SR-A and CD36 and showed that doing so efficiently prevented the uptake of oxLDL.Doens et al.(2017) reported the development of a colorimetric assay for screening molecules that interfere with CD36-Aβ interactions.Using this assay,seven compounds were experimentally identified that interfered with the binding of Aβ to CD36.By utilizing pharmacological inhibitors of CD36-Aβ interactions,new potential therapeutic agents for the treatment of AD may be obtained.
There is evidence from metabolic diseases that these drugs can directly modulate CD36 expression,thereby affecting the inflammatory response and oxLDL uptake and,ultimately,leading to treatment (Rekhi et al.,2021).Unfortunately,there is no evidence that the mechanisms of action of these drugs in neurological diseases are directly related to CD36;however,considering the obstacle of the BBB,not all CD36 inhibitors can be used in the CNS and still require further study.
MicroRNAs
MicroRNAs (miRNAs) have been implicated in the pathogenesis of neurological diseases like PD,AD,and amyotrophic lateral sclerosis (Rizzuti et al.,2018;Goh et al.,2019;Su et al.,2022).Hu et al.(2022) found that overexpression of miR-99a in the hippocampus regulated the expression of various genes,leading to cognitive impairment.Another study found that miR-99a levels were reduced in the plasma of patients with stroke and that miR-99a levels correlated with clinical parameters related to stroke diagnosis and prognosis(Tao et al.,2015).In a mouse model of cerebral artery occlusion,miR-99a attenuated oxidative stress-induced brain damage and promoted neurological survival after stroke.This suggests that miR-99a could be a new therapeutic agent for stroke,but its target was not elucidated in these experiments (Tao et al.,2015).While the apparent downregulation of CD36 levels has been shown to be accompanied by an increase in miR-99 levels,further studies are needed to determine whether there is a correlation between CD36 and miR-99 (Li et al.,2020).Notably,in a recent study,experimental results demonstrated that miR-99a could target and block palmitate-induced CD36 expression and inhibit mitochondrial dysfunction (Hulse et al.,2022).Additionally,Koh et al.(2021) discovered that miR-485-3p was overexpressed in patients with AD and that it prevented microglia from phagocytosing Aβ by directly interacting with CD36 in a study using an AD model.In one investigation,researchers created antisense oligonucleotides for miR-485-3p and injected them into animals of an AD model to ameliorate cognitive impairment and lessen Aβ buildup and neuroinflammation.In the process of exploring its mechanisms,miR-485-3p antisense oligonucleotide was found to affect Aβ phagocytosis by regulating CD36 expression (Koh et al.,2021).Therefore,the search for miRNAs that can target binding to CD36 or its derivatives to inhibit CD36 expression is important for the treatment of neurological diseases and provides a new strategy for clinical treatment (Figure 1B).
Conclusions
CD36 has been mostly studied in metabolic disease models,but we found that it mediates a variety of risk factors associated with CNS disease.In monocytes/macrophages,CD36 can bind and endocytose oxLDL,and the level of uptake of oxLDL correlates with a poor prognosis in stroke (Wang et al.,2017).The binding of platelet CD36 to oxLDL promotes platelet activation and thrombosis by promoting the production of reactive oxygen species,and arterial thrombosis is a risk factor for stroke (Yang et al.,2020).In particular,studies have indicated that CD36 is involved in the pathogenesis of various CNS diseases.Therefore,we believe that more experimental research is required to investigate the exact mechanisms of CD36 action in diverse disorders,as well as the types of CD36 antagonists and their ideal administration times,because CD36 is widely distributed and functionally complicated.Of course,inhibiting the presence or function of CD36 includes the possibility of introducing undesired side effects,which should always be considered and evaluated.The limitations of this review are that more neurological disease trials are needed to elucidate the exact mechanisms of CD36 in CNS disease,and that most of the studies cited in this review are from animal models,while clinical trials are not abundant.Further comprehensive clinical trials are needed to elucidate the role of CD36 in CNS disorders.
Author contributions:Review conception and design,and manuscript dfart:MF,SZ,JZ;review analysis: QZ,HX,CL,MZ,SZ.All authors have read and approved the final version of the manuscript.
Conflicts of interest:The authors declare that they have no conflicts of interest.
Data availability statement:The data are available from the corresponding author on reasonable request.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
