Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
2024-02-18JieWuYuleiHuangHanruiYuKaixiuLiShifengZhangGuoqingQiaoXiaoLiuHongmeiDuanYifeiHuangKwokFaiSo0ZhaoyangYangXiaoguangLiLiqiangWang
Jie Wu ,Yulei Huang ,Hanrui Yu,Kaixiu LiShifeng ZhangGuoqing QiaoXiao Liu,Hongmei Duan,Yifei HuangKwok-Fai So0,Zhaoyang Yang,,Xiaoguang Li,,Liqiang Wang
Abstract Neurotrophic keratopathy is a persistent defect of the corneal epithelium,with or without stromal ulceration,due to corneal nerve deficiency caused by a variety of etiologies.The treatment options for neurotrophic keratopathy are limited.In this study,an ophthalmic solution was constructed from a chitosanbased thermosensitive hydrogel with long-term release of murine nerve growth factor (CTH-mNGF).Its effectiveness was evaluated in corneal denervation(CD) mice and patients with neurotrophic keratopathy.In the preclinical setting,CTH-mNGF was assessed in a murine corneal denervation model.CTH-mNGF was transparent,thermosensitive,and ensured sustained release of mNGF for over 20 hours on the ocular surface,maintaining the local mNGF concentration around 1300 pg/mL in vivo.Corneal denervation mice treated with CTH-mNGF for 10 days showed a significant increase in corneal nerve area and total corneal nerve length compared with non-treated and CTH treated mice.A subsequent clinical trial of CTH-mNGF was conducted in patients with stage 2 or 3 neurotrophic keratopathy.Patients received topical CTH-mNGF twice daily for 8 weeks.Fluorescein sodium images,Schirmer’s test,intraocular pressure,Cochet-Bonnet corneal perception test,and best corrected visual acuity were evaluated.In total,six patients (total of seven eyes) diagnosed with neurotrophic keratopathy were enrolled.After 8 weeks of CTH-mNGF treatment,all participants showed a decreased area of corneal epithelial defect,as stained by fluorescence.Overall,six out of seven eyes had fluorescence staining scores < 5.Moreover,best corrected visual acuity,intraocular pressure,Schirmer’s test and Cochet-Bonnet corneal perception test results showed no significant improvement.An increase in corneal nerve density was observed by in vivo confocal microscopy after 8 weeks of CTH-mNGF treatment in three out of seven eyes.This study demonstrates that CTH-mNGF is transparent,thermosensitive,and has sustained-release properties.Its effectiveness in healing corneal epithelial defects in all eyes with neurotrophic keratopathy suggests CTH-mNGF has promising application prospects in the treatment of neurotrophic keratopathy,being convenient and cost effective.
Key Words: chitosan;corneal reinnervation;murine nerve growth factor;neurotrophic keratopathy;thermosensitive hydrogel
From the Contents
Introduction 680
Methods 681
Results 682
Discussion 685
Introduction
The cornea is one of the most innervated structures in the human body,being densely supplied by sensory and autonomic nerve fibers (Muller et al.,2003).The sensory nerve density of the cornea is 400-fold greater than the skin(Bonini et al.,2003),ensuring high sensitivity to any ocular irritation.Normal blink reflexes and tear secretion require normal corneal perception.The sensory nerves of the cornea primarily originate from the ophthalmic division of the trigeminal nerve.Damage at any region from the trigeminal ganglion to the corneal nerve terminals may therefore lead to loss of corneal perception,and consequent degenerative changes to the corneal epithelium.This can ultimately lead to neurotrophic keratopathy (NK) (Dua et al.,2018).Many systemic and ocular conditions are associated with NK,including trigeminal nerve palsy,herpes virus infection,chemical burns,kerato-refractive surgery,abuse of local anesthetics,and diabetes mellitus (Goins,2005).NK can be mild (epithelium and tear film changes),moderate (non-healing epithelial defect),or severe (stromal melting and perforation).Moderate and severe NK can profoundly affect vision and adversely impact upon quality of life (Dua et al.,2018).
Therapeutic options for NK are limited and often receive a minimal response;these include preservative-free artificial tears,soft bandage contact lens,amniotic membrane transplantation,palpebral fissure reduction,and corneal transplantation.Since the 1990s,topical nerve growth factor (NGF) treatment has been used to treat NK.Treatments initially used murine NGF (Lambiase et al.,1998;Bonini et al.,2000),and were followed by a commercialized humanderived recombinant NGF (cenegermin) (Bonini et al.,2018a,b).Cenegermin shows significant therapeutic efficacy on corneal epithelial defects of NK(Bonini et al.,2018a,b;Deeks and Lamb,2020).As the major component of cenegermin,NGF is an important neurotrophin in corneal tissue and plays a role in both corneal nerve regeneration and promotion of corneal epithelial wound healing (Diamond et al.,1992;Lambiase et al.,2000).However,as the first drug to be approved by the United States Food and Drug Administration(FDA) for the treatment of NK,cenegermin has certain limitations that restrict its widespread clinical use,including strict preservation conditions,high frequency of administration (6-times daily),and high price.
Murine NGF (mNGF) is a mature product in the current clinical market,and has a low price,is easily accessible,and has a stable effect.Moreover,it has been widely used in the clinic to treat optic nerve injury with remarkable efficacy.mNGF shares more than 90% homology with human NGF.NGF contains three subunits: α,β,γ;its main active unit is β NGF.The human β NGF gene sequence is highly homologous to mouse (Ullrich et al.,1983),and there is no apparent interspecies specificity in its biological effects.The therapeutic efficacy of mNGF for NK was demonstrated by clinical trials in 1998 and 2000 (Lambiase et al.,1998;Bonini et al.,2000).Therefore,we used mNGF in this study.
Chitosan is a polysaccharide widely used in various biomedical applications(Shariatinia,2019;El Soury et al.,2023;Zhao et al.,2023).It is obtained by partial deacetylation of chitin,the second most abundant polysaccharide,and is present in the exoskeleton of crustaceans and insects and the cell walls of fungi (Islam et al.,2020).The favorable characteristics of chitosan(such as biocompatibility,biodegradability,and antibacterial activity) make it an attractive biomaterial for many biomedical applications including tissue engineering,drug delivery,vaccine administration,and medical device production (Baranwal et al.,2018;Sultankulov et al.,2019).Chitosan-based drug delivery systems have the beneficial characteristic of release at a desired rate and specific location in the body.Thus,they are often used as carriers for small active molecules (such as proteins,polypeptides,vaccines,genes,and oligonucleotides),which are encapsulated,and covalently and/or electrostatically attached to their surface or within their matrix (Shariatinia,2018).Recently,chitosan has been designed for application as coatings in contact lenses (Akbari et al.,2021),drug delivery eye drops (Hosseini et al.,2023),and contact lenses themselves (Huang et al.,2016),for the treatment of ophthalmic diseases.Indeed,chitosan has high potential for clinical applications in ophthalmology (Shariatinia,2019).
Thermosensitive,chitosan-based hydrogel is increasingly prepared into a variety of ophthalmic formulations,and widely studied for its temperaturesensitive characteristics,and ability to carry bioactive molecules and achieve a sustained release (Tang et al.,2017,2022;Wang et al.,2020).On the ocular surface,it is used to carry different bioactive substances as eye drops (such as exosomes derived from induced pluripotent stem cell-derived mesenchymal stem cells,or recombinant human stromal cell-derived factor-1 alpha),which can effectively promote corneal epithelial and stroma regeneration (Tang et al.,2017,2022).Further,thermosensitive chitosan hydrogel containing both ciliary neurotrophic factor and FK506 helped to directly protect the optic nerve from traumatic optic nerve injury by establishing a stable release profile(Wang et al.,2020).However,there have been no studies of any sustained release drugs for the treatment of NK,with clinical trials all based upon a high dosing frequency (up to 6 times daily).This indicates that developing thermosensitive chitosan hydrogels suitable for NK therapy is a promising direction for improving the medication experience of patients.Our team has focused on chitosan-based sustained-release technology to prepare bioactive molecular agents with long-term sustained-release (Rao et al.,2018;Liu et al.,2022).In this study,we used a chitosan-based thermosensitive hydrogel with long-term release of murine NGF (CTH-mNGF).The sustained release characteristics and therapeutic effects were first verified in animal experiments,and then in a clinical trial to explore the safety and therapeutic effects of CTH-mNGF on NK.
Methods
Preparation method of mNGF chitosan carrier
Chitosan (Cat# 448869-50G,Sigma,St.Louis,MO,USA) was dissolved at a mass fraction of 3% in 1% acetic acid (Sigma) under sterile conditions;the remaining acetic acid was eluted.Genipin (TargetMol,Boston,MA,USA) was dissolved in deionized water to prepare an 8 mM solution.Next,3% chitosan solution and 8 mM genipin solution were mixed at a 1:1 ratio.After cooling to 4°C,mNGF (No.S20060051,Hiteck,Wuhan,China) was added to the mixture and slowly stirred for 6 hours at 4°C to obtain a gelatinous scaffold material(final concentration of 100 ng/mL),which was then stored at 4°C.
Animal maintenance
For this study,5–6-week-old male wild-type C57BL/6 mice were provided by SPF Biotechnology (Beijing,China,license No.SCXK (Jing) 2019-02010).Mice were bred and housed at the animal facility of the People’s Liberation Army(PLA) General Hospital in a temperature-controlled room with an automatic 12-hour light/dark cycle.All mice were allowed access to food and waterad libitumunder specific-pathogen-free (SPF) grade condition.All experimental procedures in animals were approved by the Institutional Animal Care and Research Advisory Committee of the PLA General Hospital (approval No.2023-X19-02) and were carried out in accordance with the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
Kinetics of mNGF release
The kinetics of mNGF release were tested using a previously described method (Liu et al.,2022),in accordance with the instructions of a NGF enzyme linked immunosorbent assay (ELISA) kit (Cat# JL12298-96T,Laibio,Shanghai,China).In animal experiments,10 μL CTH-mNGF was administered onto the ocular surface of each murine eye and then the eyelids were sutured.After 30 minutes,2,4,6,8,12,24,36,48,and 72 hours,the mice were euthanized with intraperitoneal injection of 1% pentobarbital sodium(200 mg/kg,Sigma).The corneas were harvested,ultrasonic fragmentated,and tested using the NGF ELISA kit.Each sample contained two corneas for a total of three sample replicates per time point.In another set of experiments,20 μL of CTH-mNGF gel was added to 1.5 mL tubes and incubated at 37°C for 5 minutes after solidification.Then 20 μL PBS was added.After 30 minutes,2,4,6,8,12,24,36,48,and 72 hours,the supernatant was collected and tested with the ELISA kit.Absorbance was read at 450 nm using an ELISA plate reader (Tecan,Groedig,Austria).
Animal model
The corneal denervation (CD) model was constructed as described in previous studies (Yamaguchi et al.,2013;Li et al.,2019).Briefly,after the mice were anaesthetized by intraperitoneal injection of pentobarbital sodium (100 mg/kg,Sigma),a temporal eyelid margin incision was made on the right eye,then the temporal conjunctival sac was cut and the peribulbar tissue bluntly separated to expose the optic nerve.The temporal nerve bundles accompanying the optic nerve were clamped for 30 seconds with forceps(3CSA,Ideal-tek,Ticino,Switzerland).Next,the conjunctival sac and temporal eyelid were sutured,with tobramycin ophthalmic gel covering the operated eye (Figure 1A).During the whole operation,the cornea of the operated eye was covered with saline soaked cotton.A cohort of 36 mice was randomly divided into three groups,with 12 mice in each groups after ciliary nerve clamping.The mice in the CD group were given no medication after the model was established,whereas the other two groups were given eyedrop treatments of CTH with or without mNGF: CD+CTH group and CD+CTHmNGF group.Ophthalmic medication was given twice daily over 10 days of treatment,with a 12-hour interval between treatments.A flow chart of the experimental approach is shown inAdditional Figure 1.

Figure 1|Characterization of CTHmNFG.
Corneal perception test
A Cochet-Bonnet esthesiometer (Luneau Ophtalmologie,Chartres Cedex,France) was used to test corneal perception,as in a previous clinical trial(Nosch et al.,2022).The tip of a cotton swab was elongated and touched the temporal and nasal murine cornea.The number of times the eye blinked in every 10 contacts was counted and recorded as the corneal perception.
Optical coherence tomography of the cornea
Anterior segment optical coherence tomography (OCT) of the cornea was performed in mice using an inverse spectroscopic optical coherence tomography (ISOCT,Optoprobe,Pontypridd,UK),in accordance with the machine instructions.Anterior segment OCT of the human cornea was performed by VisanteTMOCT (Zeiss,Oberkochen,Germany).
Schirmer’s test
Filter paper (Jingming,Tianjin,China) was placed between the lower eyelid and the globe at the junction between the middle and lateral thirds of the eyelid as described (Wang et al.,2022).The test lasted for 5 minutes.The tear moistened fluorescein-labeled length was read directly from the scale on the filter paper.
Epithelial debridement wounds
Epithelial wound healing was tested as previously described (Yang et al.,2014).The mice were anesthetized by intraperitoneal injection of pentobarbital sodium (100 mg/kg).A central corneal debridement injury was created on the right eye by marking a central circle using a 2-mm trephine,then the epithelium was removed using an Algerbrush II (Alger,New York,NY,USA) with a 0.5 mm rotating burr (Alger) (Puri et al.,2020).After wounding,the right eye was washed with saline to remove cell debris.Corneal fluorescein sodium staining was performed with 10 μL of 0.1% sodium fluorescein (Alcon Laboratories,Inc.,Fort Worth,TX,USA) applied onto the ocular surface.After removal of excess fluid,each murine eye was inspected and imaged.The fluorescein stained area was calculated using ImageJ software (v1.8.0;National Institutes of Health,Bethesda,MD,USA;Schneider et al.,2012) at 0 and 24 hours after corneal debridement injury.
Immunofluorescence of murine cornea
Immunofluorescence was performed as described previously (Wu et al.,2021).After the mice were euthanized by intraperitoneal injection of pentobarbital sodium (100 mg/kg),the eyeballs were removed and immersed in Zamboni’s fixative solution (Cat# GT7821,Waryong,Beijing,China) for 1 hour at 4°C.The corneas were dissected and immersed in Zamboni’s fixative solution for another hour at 4°C.Then the corneas were blocked with blocking buffer for Immunol staining (Cat# P0260,QuickBlockTM,Beyotime Institute of Biotechnology,Shanghai,China) for 1 hour at room temperature.The corneas were then stained with Alexa Fluor® 488 anti-Tubulin β 3 (TUBB3,1:100,host: mouse,Cat# 657404,BioLegend,San Diego,CA,USA) overnight at 4°C.All staining was observed using a fluorescence microscope (Leica DMi8,Leica,Weztlar,Germany).The Angio Tool (NIH software,National Institutes of Health,Bethesda,MD,USA) was used for quantitative analysis of neural networks as described in previous studies (Zudaire et al.,2011;Christ and Jakus,2023;Schrenk et al.,2023).
Clinical trial
This was an open-label,non-randomized controlled study.A total of seven eyes from six patients diagnosed with NK were included.This study complies with theDeclaration of Helsinkiand was approved by the Ethics Committee of the Chinese PLA General Hospital (approval No.S2022-013-01).NK was diagnosed and staged by two cornea specialists (the authors YH &LW) based on medical history,clinical findings (slit-lamp biomicroscopy,fluorescein staining,and corneal perception),andin vivoconfocal microscopy (IVCM).This is in agreement with the diagnosis and staging criteria reported in a previous study (Dua et al.,2018).Written informed consent was obtained from all patients.
Patients with stage 2 and 3 NK caused by different causes were enrolled,and whose corneal nerves were obviously reduced or missing (as confirmed by IVCM).Patients satisfying any of the following criteria were excluded: 1,patients too young to cooperate with the IVCM examination;2,patients with keratoprosthesis who could not cooperate with the corneal perception test and IVCM;3,patients with corneal perforation;and 4,patients who were pregnant or with other serious systemic diseases.The patients received one drop (approximately 50 μL) of CTH-mNGF on the ocular surface of the affected eye twice a day for 8 weeks.
All patients were evaluated by ophthalmic examination (slit-lamp photography,tonometry,photography,fluorescence staining image,corneal perception test,estimation of best corrected visual acuity [BCVA],and Schirmer’s test) at baseline and every 2 weeks during 8 weeks of treatment.IVCM examination was performed at baseline and every 4 weeks during 8 weeks of treatment.A general history was obtained and physical examination performed to exclude serious systemic disease.
Statistical analysis
The data were analyzed with SPSS 26.0 software (IBM Corp.,Armonk,NY,USA) and GraphPad Prism 6 (GraphPad Software,San Diego,CA,USA,www.graphpad.com).Comparisons between two groups were performed by Student’st-test,and comparisons among three or more groups were performed by one-way analysis of variance with Bonferroni ‘spost hoctest.P< 0.05 was considered statistically significant.
Results
CTH-mNGF is transparent,thermosensitive,and shows sustained release of mNGF
As shown inFigure 1A,CTH-mNGF was in liquid form at 4°C and solid form at 32°C.Transparence of CTH-mNGF on the murine ocular surface was shown by a low OCT reflection signal (Figure 1B).CTH-mNGF on the human cornea was observed by OCT scan,with 20 μm thickness over the epithelium (Figure 1C).The kinetics of mNGF release were calculated from the concentration of mNGF in the murine cornea after CTH-mNGF administration,along with the concentration of mNGF in the supernatant of solidified CTH-mNGF within 72 hours.As shown inFigure 1D,mNGF was maintained over 1300 pg/mL for more than 20 hoursin vivo.Accumulative release of NGF was 39.1% at 24 hours and 77.8% at 72 hoursin vivo(Figure 1E).mNGF was maintained at about 1500 pg/mL for more than 36 hours in the supernatant (Figure 1F),with the accumulative NGF release rate being 41.2% at 24 hours and 73.9% at 72 hours (Figure 1G).The sustained-release system was used to reduce the dosing frequency,and may also help maintain NGF concentration on the ocular surface during sleep.CTH-mNGF was applied twice a day for 10 days in the subsequent animal study,and twice a day for 8 weeks in the clinical study.
CTH-mNGF promotes corneal reinnervation in CD mice
To determine the effect of CTH-mNGF on corneal reinnervation,we used a murine model of NK.To induce CD,the mice underwent clamping of the ciliary nerve of the trigeminal nerve.The experimental procedures are summarized inFigure 2Aand were confirmed by whole-mount corneal nerve staining and the corneal perception test.Sham mice underwent the same ciliary nerve exposure process but without clamping,with unoperated mice as negative controls (NC).Whole-mount corneal nerve staining showed that most of the corneal nerves had disappeared by 3 days after clamping,except for the nasal quarter in CD mice (Figure 2CandF).Two weeks after operation,the corneal nerves of mice had regenerated,but at a lower density than in the control group.The corneal perception test showed a loss of temporal perception in the cornea of CD mice on the third day after operation (Figure 2B).Schirmer’s test results showed decreased tear secretion in the CD group,while the epithelial debridement wound area showed less corneal healing area in the CD group on the third day after operation (Figure 2DandE).The intervention started on the fourth day in the follow-up study.

Figure 2|Corneal denervation model.
On the fourth day of the CD model,the postoperative mice were randomly divided into three groups,in which the CD group received no eye drops and the other two groups received 10 μL of CTH or CTH-mNGF eye drops twice a day for 10 days.The mice were observed after 2 weeks.As shown inFigure 3A,the nasal corneal perception was 8–10 blinks per 10 stimuli across the three groups.Temporal corneal perception was significantly higher in the CTH-mNGF treated group compared with the CD and CTH treated groups (P<0.05).Schirmer’s test and epithelial debridement wound healing area showed a trend of improvement in the CTH-mNGF treated group,albeit without statistical significance (Figure 3B).Whole-mount corneal nerve staining showed a higher nerve density in the central and peripheral cornea in the CTH-mNGF treated group (Figure 3C).Quantitative statistical results showed that the corneal nerve area and total nerve length were significantly higher in the CTH-mNGF treated mice compared with the other two groups (P< 0.05;Figure 3DandE).
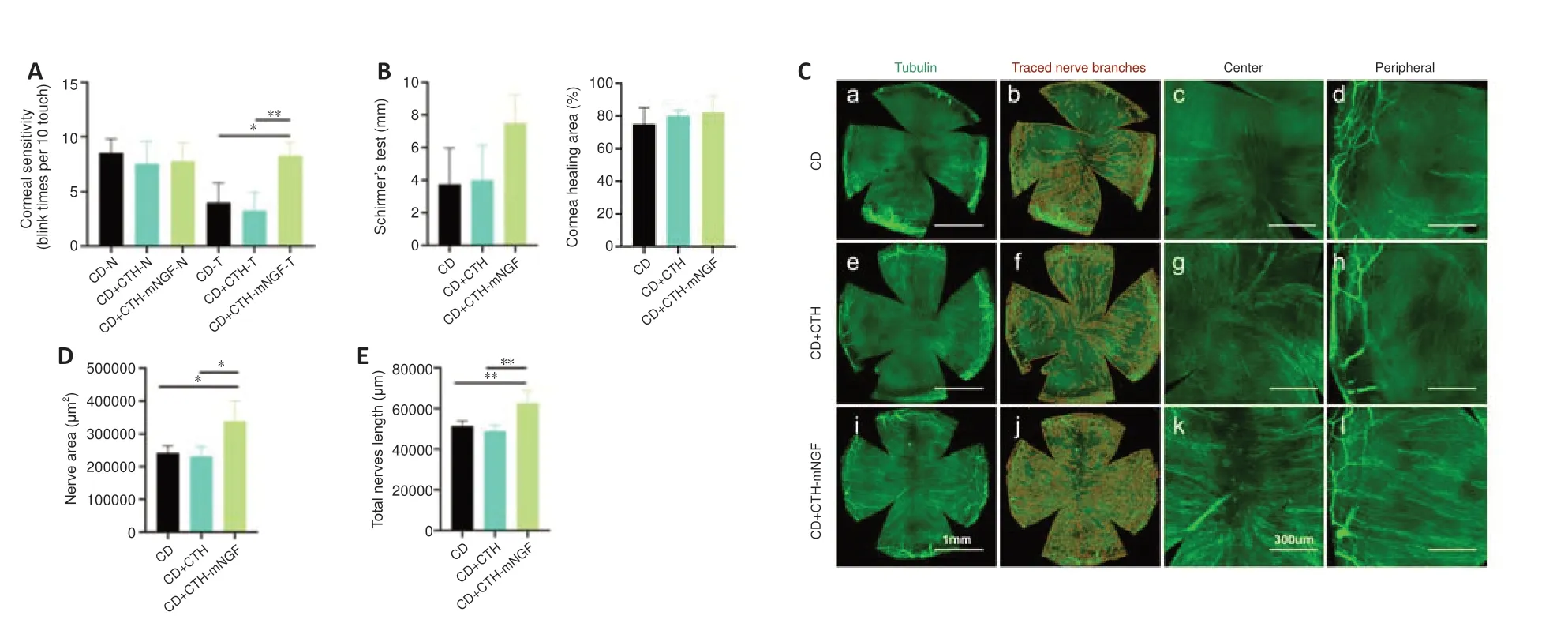
Figure 3|Corneal perception,corneal nerve attribution,Schirmer’s test,and corneal epithelial wound healing of CD mice 10 days after CTH-mNGF treatment.
Baseline characteristics of patients included in the clinical study
We enrolled six patients (total of seven eyes) who were diagnosed with NK.The cause of NK varied,and included LASIK surgery (two eyes),nerve injury after pan-retinal photocoagulation in diabetes (one eye),multiple lamellar keratoplasty after alkali burn (one eye),lamellar keratoplasty for corneal dystrophy (one eye),postoperative trigeminal nerve injury due to right acoustic neuroma (one eye),and left trigeminal schwannoma (one eye) (Table 1).The eyes included had all experienced recurrent or persistent epithelial defects and showed a poor response to multiple traditional therapies including artificial tears,soft bandage contact lens,calf blood,or amniotic membrane transplantation.

Table 1|Patient demographics and clinical characteristics
The corneal lesion recovered in all participants after 8 weeks of CTH-mNGF treatment
The patients received one drop (approximately 50 μL) of CTH-mNGF on the ocular surface of the affected eye twice a day for 8 weeks.After 8 weeks of treatment with CTH-mNGF,all participants showed a decreased corneal epithelial defect area,as stained by fluorescence (Figure 4A–C).Cases 2 and 3 reached full recovery,while six out of seven eyes had a fluorescence staining score < 5.Schirmer’s test results showed improved tear secretion at 4 weeks but this effect was not prominent at 8 weeks (Figure 4DandE).The corneal perception test (Figure 4FandG) and BCVA results (Figure 4I) showed a trend of improvement but without statistical significance.Intraocular pressure showed no obvious alterations (Figure 4H).
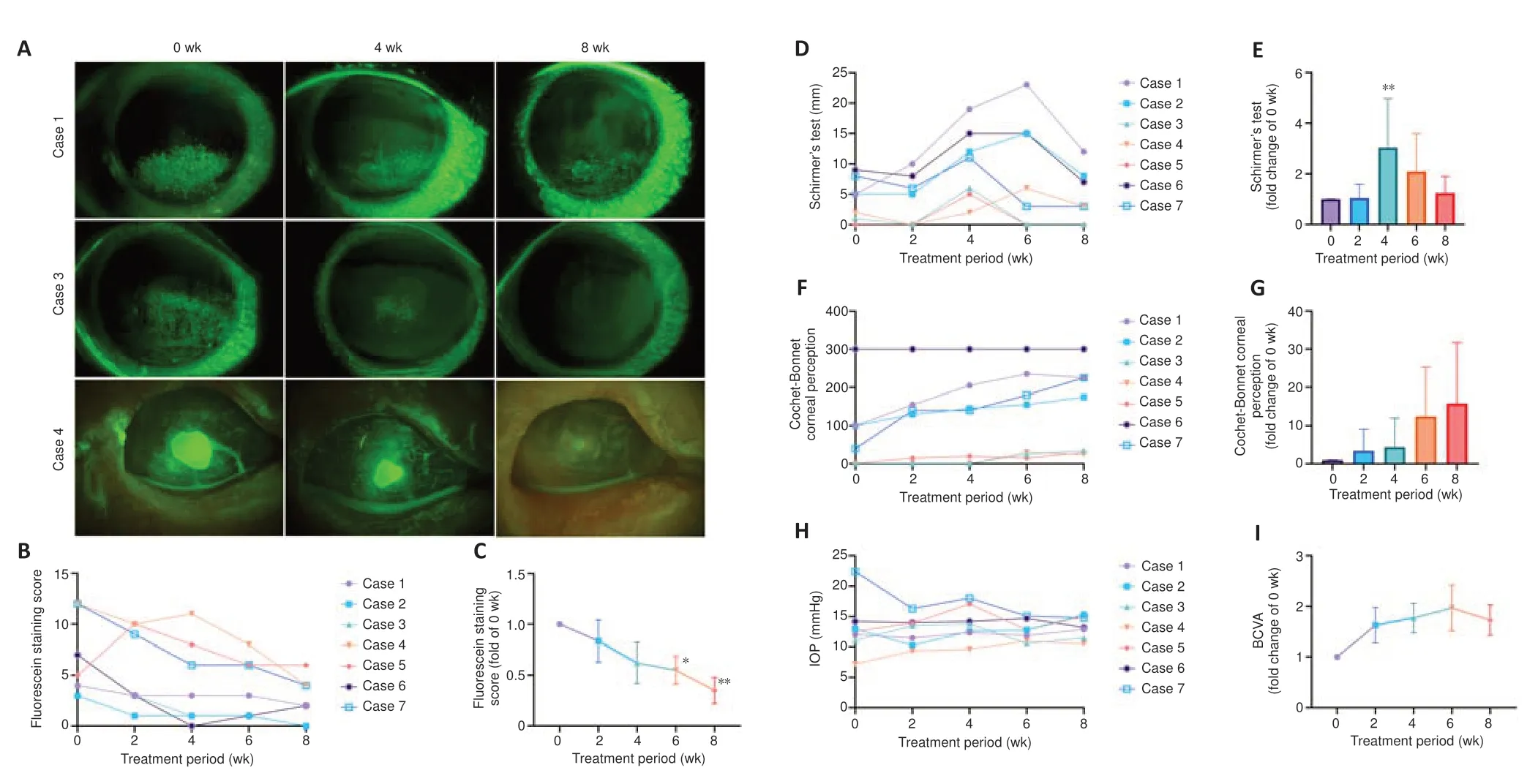
Figure 4|Clinical results following 8 weeks of CTH-mNGF treatment in seven eyes (from six patients) with neurotrophic keratopathy.
The adverse events during treatment were recorded.A total of three adverse events were collected during medication,including orbit pain in 1 case for 1 day,unusual feeling during blinks in 1 case for 1 week,and corneal epithelial exfoliation in 1 case for 1 week (managed with bandage contact lens).These minimal adverse events indicate the safety of the CTH-mNGF application.
Increased corneal nerve density was found in 3 out of 7 cases after 8 weeks of CTH-mNGF treatment
An increase in corneal nerve density after 8 weeks of CTH-mNGF treatment was observed in three out of seven eyes (in cases 1,2,and 6).The IVCM results of Case 1 showed a significant increase in nerve density compared with baseline at week 8 (P< 0.05;Figure 5AandC),and a significant increase in the number of branches of corneal nerves compared with baseline at week 4 (P< 0.05;Figure 5D).There was no statistical difference in the length of the nerve among the three time points (Figure 5B).
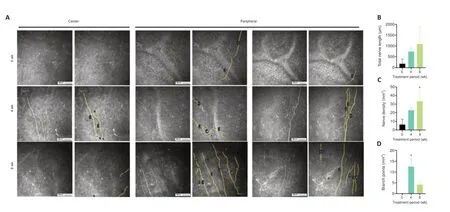
Figure 5|IVCM results of Case 1.
Discussion
This study aimed to create a chitosan-based thermosensitive hydrogel with long-term release of mNGF for the treatment of NK,and to observe its efficacy and safety.The use of NGF to treat corneal epithelial defects was first reported in the 1990s (Lambiase et al.,1998).Since 2014,a series of clinical trials have been reported on the use of recombinant human (rh)NGF for NK (Bonini et al.,2018a,b;Pflugfelder et al.,2020).Topical ophthalmic rhNGF (cenegermin,Italy) is instructed to be used 6-times daily for up to 8 weeks,and has proven effective for cases of non-responding NK in all three stages (Bonini et al.,2018b;Pflugfelder et al.,2020).However,limitations of cenegermin include its strict preservation conditions,high frequency of administration,and high price.Therefore,it is necessary to come up with an efficient,effective,and accessible medication for NK patients.In this study,a chitosan-based thermosensitive hydrogel with long-term release of mNGF (i.e.,CTH-mNGF) was constructed.
The chitosan-based sustained-release technology was used to create sustained release of mNGF.In our previous studies,this system could release mNGF for up to 8 weeks in a closed structure such as the spinal cord or sciatic nerve trunk (Rao et al.,2018;Liu et al.,2022).The hydrogel is in liquid form at 4–8°C and undergoes rapid solidification at 30–38°C.Since tears and blinking on the ocular surface can washout and dilute the drugs in the conjunctival sac,the eye drops were administered twice a day.The concentration of released mNGF was approximately 1500 pg/mLin vitroand 1300 pg/mL in the murine cornea within 24 hours.Therefore,sustained release of mNGF can maintain the NGF concentration on the ocular surface during the night,but also resist clearance of the drug by blinking and tears,thereby reducing the dosing frequency.
We used murine NGF instead of human NGF mainly because of its cost,availability,and effectiveness.One difficulty of the clinic application of cenegermin in China is its high price,which is a problem that cannot be ignored.NGF is found in almost all vertebrates,and is abundant in the murine submandibular gland,especially of male mice (Shooter,2001).Among the two types of NGF protein isolated from gland extracts,the low molecular weight protein mediates all known biological functions of NGF,and was termed mNGF (Shooter,2001).Studies have shown that mNGF is 90% homologous to human NGF (Ullrich et al.,1983).However,the process of mNGF extraction from murine submandibular glands is relatively simple and the product (mNGF)is highly active,making mNGF the first NGF on the market.Consequently,the use of mNGF can significantly reduce costs.mNGF has been widely used in the clinic to treat optic nerve injury as well as promote the repair of other nerve injuries with remarkable efficacy (Keefe et al.,2017).Topical mNGF administration in clinical studies was first reported in 1998 and 2000 with satisfactory results (Lambiase et al.,1998;Bonini et al.,2000).Patients were administered mNGF (200 mg/mL) every 2 hours for 2 days followed by one drop six times daily until the ulcers had healed.Treatment continued for 2 weeks with one drop of mNGF (100 mg/mL) administered four times daily for 2 weeks (Bonini et al.,2000).All patients showed complete healing of their corneal ulcers after 10 days to 6 weeks of treatment,indicating strong therapeutic potential of mNGF for the management of NK.The sustainedrelease system we adopted could effectively reduce the dosing frequency.
This study can be used to compare the efficacy of mNGF eyedrops to rhNGF(cenegermin) eye drops in NK management.Clinical trials have reported that topical rhNGF treatment was effective in corneal epithelial healing,in which up to 58.3–74.5% of patients showed complete corneal healing (Bonini et al.,2018b;Pflugfelder et al.,2020).However,rhNGF did not show statistically significant improvements in BCVA and corneal sensitivity (Bonini et al.,2018b;Pflugfelder et al.,2020).This is also the case in our study.Significant reductions of the corneal epithelial lesion and epithelial fluorescence staining scores were observed in all patients over the 8 weeks of treatment,but corneal nerve regeneration varied greatly among patients.Even in responsive patients with obvious improvements in corneal innervation,this occurred significantly later than healing of the epithelial lesion,with no significant improvement in corneal perception over the 8 weeks of treatment.This suggests that the effects of NGF treatment on NK may be divided into two phases: NGF on the epithelium and NGF on nerve regeneration.The two effects are temporally distinct,with significantly earlier repair in the epithelium,indicating different mechanisms.
Corneal nerve regeneration in NK patients after topical rhNGF treatment was found at 2–8 months of follow-up,which we also observed,indicating that the mechanism by which NGF promotes corneal nerve regeneration might be indirect.For the epithelium,NGF can directly activate two specific receptors,tropomyosin receptor kinase A receptor (TrkA,Trk family of tyrosine kinase receptors with high affinity) and p75 neurotrophic receptor (p75NTR,tumor necrosis factor receptor family with low affinity).This promotes limbal stem cell proliferation (Kolli et al.,2019),increases corneal integrity (Lambiase et al.,2000),and promotes corneal epithelial migration (Blanco-Mezquita et al.,2013).However,it remains to be elucidated how NGF acts to enable nerve regeneration in the absence of nerve terminals on the cornea of patients with NK.It may act indirectly by improving epithelial cell function (Okada et al.,2020),attenuating local inflammatory status (Park et al.,2016),or enhancing Schwann cell function (Li et al.,2020).
There are certain limitations to our study.The number of patients enrolled was small with a variety NK causes,resulting in large variation in the nerve regeneration results.Moreover,the frequency of CTH-mNGF usage needs further optimization.Encouraged by the results of this study,we will further recruit patients and conduct long-term follow-up observation of these patients.
In conclusion,in this study,we have demonstrated that CTH-mNGF is transparent,thermosensitive,and shows sustained-release properties.Its effectiveness in healing corneal epithelial defects in NK eyes by twice daily application for 8 weeks indicates its promising prospects for NK treatment,being convenient and cost effective.
Author contributions:Methodology,animal experiment,formal analysis,and original draft: JW.Patient enrollment,clinic investigation,and original draft:YH.Animal experiment and data curation: HY.Investigation: KL,SZ,and GQ.Methodology: XL and HD.Conceptualization,project administration,resources,supervision,writing—review and editing: YH and KFS.Funding acquisition,validation,project administration,resources,supervision,writing—review and editing: ZY,XL,and LW.All authors approved the final version of the paper.
Conflicts of interest:The authors declare no conflicts of interest.
Data availability statement:All relevant data are within the paper and its Additional file.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Additional file:
Additional Figure 1:Flow chart of the animal experiment.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
- Treatment with β-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload,endoplasmic reticulum stress,and apoptosis
