Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
2024-02-17JieLiWenJiangYuefangCaiZhenqiuNingYingyingZhouChengyiWangSookjaKiChungYanHuangJingboSunMinzhenDengLihuaZhouXiaoCheng
Jie Li ,Wen Jiang ,Yuefang Cai,Zhenqiu NingYingying Zhou,Chengyi WangSookja Ki Chung,Yan HuangJingbo SunMinzhen DengLihua Zhou,Xiao Cheng
Abstract Vascular etiology is the second most prevalent cause of cognitive impairment globally.Endothelin-1,which is produced and secreted by endothelial cells and astrocytes,is implicated in the pathogenesis of stroke.However,the way in which changes in astrocytic endothelin-1 lead to poststroke cognitive deficits following transient middle cerebral artery occlusion is not well understood.Here,using mice in which astrocytic endothelin-1 was overexpressed,we found that the selective overexpression of endothelin-1 by astrocytic cells led to ischemic stroke-related dementia (1 hour of ischemia;7 days,28 days,or 3 months of reperfusion).We also revealed that astrocytic endothelin-1 overexpression contributed to the role of neural stem cell proliferation but impaired neurogenesis in the dentate gyrus of the hippocampus after middle cerebral artery occlusion.Comprehensive proteome profiles and western blot analysis confirmed that levels of glial fibrillary acidic protein and peroxiredoxin 6,which were differentially expressed in the brain,were significantly increased in mice with astrocytic endothelin-1 overexpression in comparison with wild-type mice 28 days after ischemic stroke.Moreover,the levels of the enriched differentially expressed proteins were closely related to lipid metabolism,as indicated by Kyoto Encyclopedia of Genes and Genomes pathway analysis.Liquid chromatography-mass spectrometry nontargeted metabolite profiling of brain tissues showed that astrocytic endothelin-1 overexpression altered lipid metabolism products such as glycerol phosphatidylcholine,sphingomyelin,and phosphatidic acid.Overall,this study demonstrates that astrocytic endothelin-1 overexpression can impair hippocampal neurogenesis and that it is correlated with lipid metabolism in poststroke cognitive dysfunction.
Key Words: astrocytic endothelin-1;dentate gyrus;differentially expressed proteins;hippocampus;ischemic stroke;learning and memory deficits;lipid metabolism;neural stem cells;neurogenesis;proliferation
From the Contents
Introduction 650
Methods 651
Results 652
Discussion 654
Introduction
Stroke is a primary cause of mortality and morbidity globally (Murala et al.,2022).Of all incidences of stroke,87% are ischemic (Virani et al.,2021).Poststroke cognitive impairment (PSCI) is common condition in stroke survivors (Mijajlović et al.,2017),and generally occurs within 3 months of stroke (Nys et al.,2005).PSCI and post-stroke dementia are major causes of global morbidity and mortality (Rost et al.,2022).However,the underlying mechanisms of cognitive dysfunction after stroke remain poorly understood.Endothelin-1 (ET-1) is a 21-residue peptide that has a powerful vasoconstrictor function (Yanagisawa et al.,1988).It has been found to play very important roles in vascular injury response mechanisms,such as vascular remodeling,cell growth,and proliferation (Luscher and Barton,2000;Kedzierski and Yanagisawa,2001).For instance,patients with stroke and multi-infarct dementia were found to have higher levels of ET-1 in their cerebrospinal fluid than patients with no neurological disease (Nakajima et al.,1994;Lampl et al.,1997).At the same time,Alzheimer’s disease (AD) patients have been reported to have increased ET-1 immunostaining in the frontal,temporal,parietal,and occipital cortices (Minami et al.,1995).After ischemic stroke,ET-1 is mainly released by astroglial cells and vascular endothelial cells (Tsang et al.,2001).In our previous study,we found that after transient ischemia and long-term reperfusion,endothelial ET-1 overexpression increased anxiety and cognitive impairment in mice (Zhang et al.,2013).Astrocytic ET-1 overexpression (GET-1) also increased the severity of ischemic brain damage while exacerbating ischemic insult-induced learning and memory impairments in a spatial task (Hung et al.,2015).However,the underlying mechanism is not well understood.
Adult hippocampal neurogenesis has been implicated in various cognitive functions (Anacker and Hen,2017).Two regions in the mammalian brain,the subventricular zone (SVZ) of the lateral ventricles and the subgranular zone (SGZ) of the dentate gyrus (DG) of the hippocampus,have been found to generate new neurons (McMillan et al.,2022;Malik et al.,2023).Many studies have shown that transient middle cerebral artery occlusion (MCAO)results in a reactive increase in neurogenesis in discrete regions of the adult mammalian brain,including the SVZ and SGZ regions (Marlier et al.,2015).Adult neurogenesis is the process by which progenitor cells differentiate into new mature neurons across four stages: cell proliferation,neuronal fate specification,maturation of neuronal progeny,and functional integration into neuronal circuits (Ribeiro and Xapelli,2021).Accordingly,the proliferation of neural stem cells (NSCs) is one step of neurogenesis.In our previous study,we found that GET-1 mice exhibited reinforced progenitor stem cell proliferation and astrocytic differentiation in the SVZ region via the Jak2/Stat3 pathway,which led to greater neurologic impairment and a larger infarction area after MCAO injury (Cheng et al.,2019).Learning,memory,and mood regulation are attributed to adult hippocampal neurogenesis (Zhao et al.,2008).Thus,we further investigated whether the astrocytic ET-1 overexpression involved in MCAO-induced cognitive deficits was related to hippocampal neurogenesis.To beter understand the function and mechanism of astrocytic ET-1 in cognitive deficits after cerebral ischemic injury,we established MCAO/R models in GET-1 and wild-type (WT) mice.We investigated the effects of astrocytic ET-1 on neurogenesis in the DG of the hippocampus and conducted comprehensive proteome profiling after MCAO.
Methods
Animals
We used WT mice (n=60) and GET-1 transgenic mice (n=60) in the present study.Because the sex hormones of female mice can inhibit the expression of ET-1 mRNA,and the expression level of ET-1 is higher in male mice than in female mice,we selected male mice for the experiment (Gillis et al.,2016).The male mice were aged 8 to 12 weeks,and their weights were controlled at 20–25 g.The control WT mice were obtained from the Guangdong Medical Experimental Animal Center (SYXK (Yue) 2018-0094,specific-pathogen-free grade) and raised in the Guangdong Traditional Chinese Medicine Hospital’s Experimental Animal Center.We obtained GET-1 mice from The University of Hong Kong,China.We have previously published articles about the production of GET-1 transgenic mice (Lo et al.,2005).Before we began the experiment,we had the GET-1 mice genotyped,using the method sourced from The University of Hong Kong.The genetic validation data for the mouse strains are shown inAdditional Figure 1A.The GET-1 mice appeared healthy and normal,and showed no significant physiological,gross anatomical,or behavioral abnormalities compared with the WT mice (Lo et al.,2005;Hung et al.,2015).They were housed with a fixed temperature and humidity,a 12-hour day and night cycle,and ad-lib access to water and food.Each cage held up to five mice.The Guangdong Provincial Hospital of Traditional Chinese Medicine’s Experimental Animal Ethics Committee approved the animal experiments on November 16,2017 (approval No.2017048).All experimental procedures were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8thed.;National Research Council,2011).This study is reported in accordance with the ARRIVE 2.0 guidelines (Animal Research: Reporting ofIn VivoExperiments) (Percie du Sert et al.,2020).Mice in the different categories were labeled and then grouped via the random number method.The WT mice and GET-1 transgenic mice were randomized to the sham (n=30) and MCAO groups (n=30),respectively.
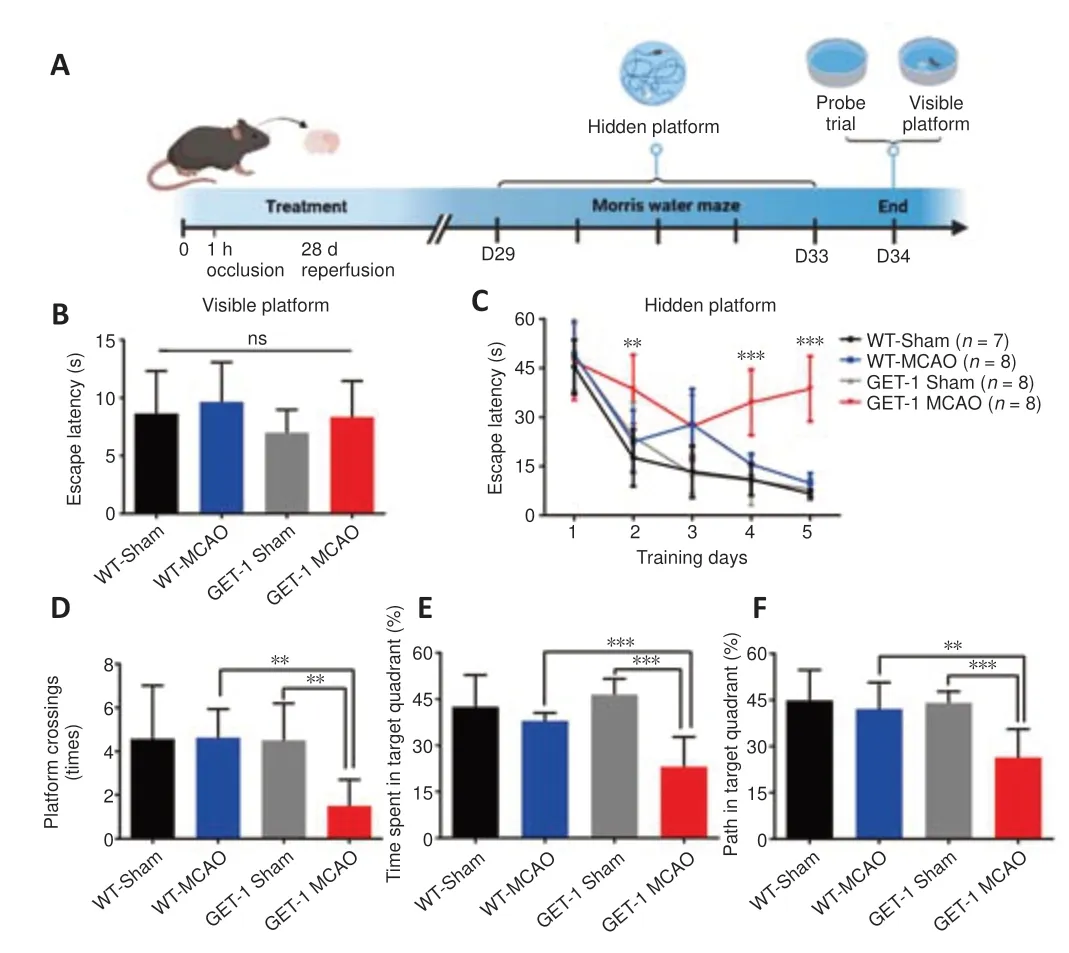
Figure 1|GET-1 mice show impaired spatial learning at 28 days after stroke.
MCAO model
The MCAO model was developed as previously described (Cheng et al.,2019).In brief,the mice were anesthetized via inhalation of 3% isoflurane (RIWARD,Shenzhen,China) in 70% N2O/30% O2for induction and 1% isoflurane in 70% N2O/30% O2for maintenance.Then,the three arteries of the carotid vasculature were separated and a 7-0 suture line was used to ligate the right external carotid artery at the beginning and distal ends.Standardized silicone-coated monofilaments (Guangzhou Jialing Technology Co.,Ltd.,Cat#1800AAA) were inserted into the external carotid artery on the right side and then pushed upward into the right internal carotid artery until a sense of resistance to stop was felt.At this point,transient ischemia was induced via the middle cerebral artery,which was blocked for 1 hour.One hour later,the plug was removed to initiate 7 days,28 days,or 3 months of reperfusion.To ensure that the MCAO model was successful,we continuously measured regional cerebral blood flow (rCBF) throughout the procedure by placing an optical fiber (probe 418-2) laser dropper flowmeter (Perimed,Jarfalla,Sweden) directly on the mouse skull (the laser dropper placement is 2 mm posterior,6 mm lateral to bregma).A decrease in rCBF of 70% or greater was considered to reflect success in inducing ischemia (Hung et al.,2015)(Additional Figure 1BandC).In total,60 mice were subjected to MCAO surgery.The sham operation group was set up as a control in both WT mice and GET-1 mice,and all procedures were the same except for the presence of absence of a plug.
Assessment of cognitive function
We used the Morris water maze (MWM) to assess spatial learning and memory in the experimental mice 28 days after MCAO (Barnhart et al.,2015;Zhou et al.,2019).In this test,the mice were trained to find a black escape platform hidden 1 cm below the surface of opaque water in a circular pool.The escape platform had a diameter of 10 cm.The time taken to find the platform was recorded for each mouse.If a mouse did not find the platform within 1 minute,the experimenter assisted it in finding the platform,where it was permitted to stay for 20 seconds.There were four different starting positions in the hidden platform task,the training duration was 5 days,and the frequency was four times per day.The escape latency was the averagetime taken to locate a hidden platform positioned in one of the four quadrants of the pool.Throughout the learning trials,the platform was kept in the same position.In the probe test,which took place on the 6thday after training,the platform was removed from the pool,and the mice were permitted to swim for 1 minute.The number of platform area crossings and the time spent in the target quadrant were recorded,as well as the distance traveled in the target quadrant.To assess sensorimotor ability and motivation,we conducted a visual cue test 30 minutes after the probe test.In this test,the black escape platform was positioned 1 cm above the surface of the water on the opposite side of the pool from the hidden platform,and marked with a red flag.Thus,instead of relying on extra-maze cues for spatial orientation,the mice could use these local visual stimuli to locate the platform.
5-Bromo-2'-deoxyuridine administration
We used saline containing 5-bromo-2′-deoxyuridine (BrdU,Sigma,50 mg/kg)to evaluate the proliferation and differentiation of NSCs in the DG region after ischemic injury.BrdU was administered intraperitoneally twice daily (at 8-hour intervals) for 5 consecutive days beginning 24 hours after MCAO.Using BrdU/SOX2 and BrdU/doublecortin (DCX) double immunofluorescence labeling,the proliferation and migration of neural progenitor cells (NPCs) were assessed at 7 days after MCAO.BrdU,glial fibrillary acidic protein (GFAP),and neuronspecific nuclear protein (NeuN) immunofluorescence were used to evaluate the differentiation of NPCs 28 days after MCAO.Ki67/GFAP and Ki67/NeuN double immunofluorescence labeling was performed 3 months after MCAO.
Double immunofluorescence labeling and cell counting
We performed double immunofluorescence labeling after cerebral ischemia/reperfusion.The mice were euthanized under 3% isoflurane in 70% N2O/30% O2anesthesia with cervical dislocation.Each brain was fixed with 10% formalin,serially dehydrated in a gradient of 75%,85%,90%,95%,and 100% ethanol,incubated with chloroform,and finally embedded in paraffin.A microtome (Microm International GmbH,Walldorf,Germany) was used to cut 7-μm-thick coronal slices.The slices were treated with 2 M HCl for 20 minutes at 30°C,washed with PBS,and incubated with 0.1 M borate buffer (pH 8.5)for 10 minutes at ambient temperature.After that,the slices were washed again,and then placed in citrate buffer (pH 6) for thermal antigen repair.The slices were then incubated with blocking solution for 1 hour,followed by primary antibody incubation using rat anti-BrdU (1:500,Abcam,Boston,MA,USA,Cat# ab6326,RRID: AB_305426),mouse anti-SOX2 (1:200,Santa Cruz Biotechnology,Santa Cruz,CA,USA,Cat# sc365823,RRID: AB_10842165),rabbit anti-DCX (1:400,Abcam,Cat# ab18723,RRID: AB_732011),rabbit anti-NeuN (1:500,Abcam,Cat# ab177487,RRID: AB_2532109),rabbit anti-GFAP(1:1500,Dako,Copenhagen,Denmark,Cat# Z0334,RRID: AB_10013382),mouse anti-Ki67 (1:200,Abcam,Cat# ab279653,RRID: AB_2934265),and mouse anti-ET-1 (1:200,Abcam,Cat# ab2786,RRID: AB_303299) at 4°C overnight.The slices were then washed three times for 10 minutes followed by secondary antibody incubation using goat anti-rat IgG labeled with Alex Fluor 488 (1:1000,Thermo Fisher Scientific,Waltham,MA,USA,Cat# A-11006,RRID: AB_2534074),goat anti-mouse IgG labeled with Alex Fluor 488 (1:1000,Thermo Fisher Scientific,Cat# A-11001,RRID: AB_2534069),goat antimouse IgG labeled with Alex Fluor 568 (1:1000,Thermo Fisher Scientific,Cat#A-11004,RRID: AB_2534072),and goat anti-rabbit IgG labeled with TRITC(1:1000,Thermo Fisher Scientific,Cat# T-2769,RRID: AB_2556777) at an ambient temperature for 2 hours.
BrdU-positive cells in the DG in the 7-μm immunofluorescence-stained coronal slices (5 to 10 slices per mouse) were quantified by two investigators who were blinded to the experimental conditions and groups.The slices were mounted with VectaShield Mounting Medium H-1000 (Vector Laboratories,Burlingame,CA,USA).The cells with fluorescence signals were counted using a Zeiss Axioplan microscope (Zeiss,Oberkochen,Germany).Adobe Photoshop CC 2018 (Adobe Systems,San Jose,CA,USA) was used to acquire and manipulate the images.
Label-free quantitative proteomic analysis
We examined differential protein expression in the GET-1 and WT mice with cerebral ischemia.We used label-free quantitative (LFQ) proteomic analysis to compare the differences in protein expression between the four groups of mice at 7 and 28 days.In brief,three samples of fresh right-hemisphere brain tissue were selected from each group.Radioimmunoprecipitation assay lysis buffer was added to the samples,which were placed on ice,to enable full lysis,and the supernatant was collected after centrifugation.After bicinchoninic acid (BCA) quantification,100 μg of protein from the BCAquantified sample tissue was used for acetone precipitation;the precipitated protein was resuspended for tryptic digestion,and the peptides were cleaned up using sodium deoxycholate and desalted for base reversed phase (RP)fractionation.An average of 2 μg of peptides was collected from each sample before conducting Q-Exactive mass spectrometry coupled with nano ultra performance liquid chromatography (UPLC) (EASY-nLC1200,Thermo Fisher Scientific) for analysis.Separation was achieved using a reversed-phase column (100 μm,ID × 15 cm,Reprosil-Pur 120 C18-AQ,1.9 μm).Following liquid chromatography-mass spectroscopy/mass spectroscopy (LC-MS/MS),data-dependent acquisition was carried out using an Orbitrap analyzer in both positive and profile modes.The mass spectrometry parameters were as follows: 70,000 resolution at @ 200 m/z for mass spectroscopy 1 (MS1)and 17,500 resolution at @ 200 m/z for mass spectroscopy 2 (MS2);3E+6 automatic gain control(AGC) for MS1 and 1E+5 AGC for MS2;maximum ion injection time: 50 ms for MS1 and 45 ms for MS2;normalized collision energy of 28%;isolation window of 2 m/z;and dynamic exclusion time of 40 s.Finally,MaxQuant analysis and LFQ analysis were performed,and the standardized quantitative results were statistically analyzed to determine the differentially expressed proteins (DEPs).
Western blot assay
A manual grinder was used to homogenize the brain samples immersed in radioimmunoprecipitation assay buffer with protease inhibitors (Roche,Basel,Switzerland,Cat# 5892791001) and phosphatase inhibitors (Roche,Cat# 04906837001).The supernatants were collected,and the BCA method was used to measure the protein concentrations (Smith et al.,1985).Then,loading buffer was mixed in,and the samples were heated for 10 minutes at 100°C.After taking 5-μL protein samples from each group,the samples were separated using 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Bio-Rad,Hercules,CA,USA),followed by transfer of the proteins to a polyvinylidene fluoride (PVDF) membrane,which was blocked with 5% bovine serum albumin in tris buffer solution Tween (TBST)buffer for 1.5 hours.The membrane was incubated overnight at 4°C with diluted primary antibodies,including rabbit anit-GFAP (1:10,000,Abcam,Cat#ab7260,RRID: AB_305808),rabbit anti-Prdx6 (1:1000,Abcam,Cat# ab73350,RRID: AB_1658907),and rabbit anti-GAPDH (1:1000,Cell Signaling Technology,Boston,MA,USA,Cat# 2118,RRID: AB_561053) antibodies.The membranes were washed three times with TBST for 10 minutes each time.After incubation with the corresponding diluted anti-rabbit IgG,followed by HRPlinked secondary antibody (1:2000,Cell Signaling Technology,Cat# 7074,RRID:AB_2099233) in TBST for 1 hour at ambient temperature,the membrane was washed with TBST,and finally,an enhanced chemiluminescence kit (Millipore,Boston,MA,USA) was used to visualize the signal.After obtaining the image results,the gray density value was measured with Image Lab software-5.2.1(Bio-Rad) relative protein expression was normalized to GAPDH.
LC-MS nontargeted metabolome analysis
To examine changes in the entire metabolite profile between the four groups of mice at 28 days,we performed LC-MS nontargeted metabolome analysis.Briefly,five fresh brain tissue samples were selected from each group,and a 30 mg sample was weighed.To each sample,we added 20 μL of L-2-chlorophenylalanine (0.06 mg/mL) dissolved in methanol as the internal standard and 400 μL of methanol and water (4/1,v/v).The samples were ground,ultrasonically extracted while submerged in ice water,and centrifuged,and the supernatant was transferred to LC-MS injection vials and dried.A 300 μL mixture of methanol and water (1/4,v/v) was added to each sample for redissolution,and the supernatants were collected and transferred to LC vials.To prepare quality control (QC) samples,we pooled an aliquot of all samples.For metabolic profiling,we employed a Nexera UPLC system (Shimadzu Corporation,Kyoto,Japan) together with a Q-Exactive Quadrupole-Orbitrap mass spectrometer equipped with heated electrospray ionization sources (Thermo Fisher Scientific).Each injection volume was 2 μL,and gradient elution was performed on an ACQUITY UPLC HSS T3 column(1.8 μm,2.1 × 100 mm) with (A) water (containing 0.1% formic acid,v/v) and(B) acetonitrile (containing 0.1% formic acid,v/v) as elution materials.We used positive and negative ion scanning modes for mass spectrometry signal acquisition.Masses were scanned from 125 to 1000 m/z.The resolution was 70,000 for all MS scans and 17,500 for HCD MS/MS scans.The collision energies were 10,20,and 40 eV.Regular QC injections (for each sample) were performed during the analysis process to assess repeatability.Finally,LC-MS data processing and analysis were performed.
Statistical analysis
Data are presented as the mean ± standard deviation.SPSS 25.0 (IBM Corp,Armonk,NY,USA) was used to conduct all statistical analyses.We used a Student’st-test,and multiple comparisons were performed using a one-way analysis of variance followed by the least significant differencepost hoctest.P< 0.05 was considered statistically significant.
Results
Astrocytic endothelin-1 overexpression leads to the deterioration of learning and memory after ischemic stroke
Behavioral tests of learning and memory were performed 28 days after stroke.Previously,we reported that GET-1 mice exhibited severe neurological deficits and larger infarct areas compared with WT mice 1 and 7 days after cerebral ischemia/reperfusion injury,as determined by neurological assessment scores and 2,3,5-triphenyltetrazolium chloride (TTC) staining.However,after 60 minutes of ischemia and 28 days of reperfusion,sensorimotor function,as measured by neurological scores,was recovered in both genotypes (Cheng et al.,2019).This indicated that the motor function of those mice at 28 days after MCAO did not affect subsequent water maze performance.The mortality rate was not significantly different between the GET-1 and WT mice:4 out of the 30 WT mice died,and 5 out of the 30 GET-1 mice died before sacrifice at the planned time points.No significant differences in rCBF were found between the WT and GET-1 mice during the whole MCAO procedure,which was consistent with our previous study (Cheng et al.,2019).The results are shown inAdditional Figure 1.
We used the MWM to test hippocampus-dependent spatial learning and memory in mice 28 days after stroke.The visual platform test was used to assess sensorimotor function and motivation,which was expected to influence MWM performance (Vorhees and Williams,2006).The visual platform test was performed for all experimental mice immediately after the probe test on day 34 (Figure 1A).There were no sensorimotor deficits in the visible platform session,and the mice in the different groups performed equally well (Figure 1B).In contrast,in the hidden platform session,there was significant retardation in the GET-1 MCAO group on days 2,4,and 5 of the learning process when compared with the GET-1 sham group and the WT counterparts (P=0.003 for GET-1 MCAOvs.WT-MCAO on day 2,P< 0.001 for GET-1 MCAOvs.WT-MCAO on day 4,P< 0.001 for GET-1 MCAOvs.WT-MCAO on day 5;Figure 1C).The results of the probe trial were similar to those of the hidden platform session,which was administered on training day 6.Thetime and path length percentages for the target quadrant were significantly reduced in the GET-1 MCAO mice compared with the other groups,and we also observed a reduction in platform crossing time (platform cross:P=0.001 for GET-1 MCAOvs.WT-MCAO,P=0.001 for GET-1 MCAOvs.GET-1 Sham,time in target quadrant:P< 0.001 for GET-1 MCAOvs.WT-MCAO,P< 0.001 for GET-1 MCAOvs.GET-1 Sham,path in target quadrant:P=0.001 for GET-1 MCAOvs.WT-MCAO,P< 0.001 for GET-1 MCAOvs.GET-1 Sham;Figure 1D–F).These results suggest that overexpression of astrocytic ET-1 contributed to ischemic stroke-induced deficits in spatial learning and memory.
Astrocytic endothelin-1 overexpression promotes the proliferation of NSCs after ischemic stroke
Neurogenesis in the SGZ of the hippocampal DG is very important for cognitive function.We used BrdU as a measure of cell proliferation to confirm the influence of ET-1 overexpression on cell proliferation in both GET-1 and WT mice after ischemic stroke (Han et al.,2015;Bowers et al.,2020).We administered an intraperitoneal injection of BrdU to investigate DG cell proliferation at 7 days after ischemic stroke (Figure 2A).As shown inFigure 2B,in both the GET-1 and WT mice,the ipsilateral side (injured)expressed more BrdU+cells than the contralateral side.Compared with the WT mice,the BrdU+cells in the ipsilateral DG region of the GET-1 mice were significantly increased 7 days after ischemic injury (P=0.026 for GET-1 ipsvs.WT ips,P< 0.001 for GET-1 ipsvs.GET-1 contra,Figure 2B).We conducted double-labeling with BrdU and SOX2 (a known phenotypic marker for NSCs) to identify the proliferating cells (Penisson et al.,2019).We found a conspicuous increase in SOX2+BrdU+cells in the ipsilateral SGZ of GET-1 mice compared with the contralateral side 7 days after ischemic stroke.Ipsilateral SOX2+BrdU+expression in the GET-1 mice was also higher than that in the WT mice (P=0.013 for GET-1 ipsvs.WT ips,P< 0.001 for GET-1 ipsvs.GET-1 contra;Figure 2C).DCX is often used as a marker of migrating neuroblasts and early differentiated (immature) neurons (Brown et al.,2003).To further investigate whether overexpression of ET-1 in astrocytes was associated with neurogenesis in the DG region,including the regulation of neurogenesis,we performed BrdU and DCX double immunofluorescence labeling.At 7 days post-MCAO,there were more DCX+BrdU+cells on the ipsilateral side than on the contralateral side in both GET-1 mice and WT mice,and this difference was significant in the GET-1 mice.Additionally,more DCX+BrdU+cells were observed on the ipsilateral side in GET-1 mice than on the corresponding side in WT mice (P< 0.001 for GET-1 ipsvs.WT ips,P< 0.001 for GET-1 ipsvs.GET-1 contra;Figure 2D).Consequently,astrocytic ET-1 overexpression reinforced ischemia-induced NSC proliferation in the DG region of the hippocampus,as verified by our results.
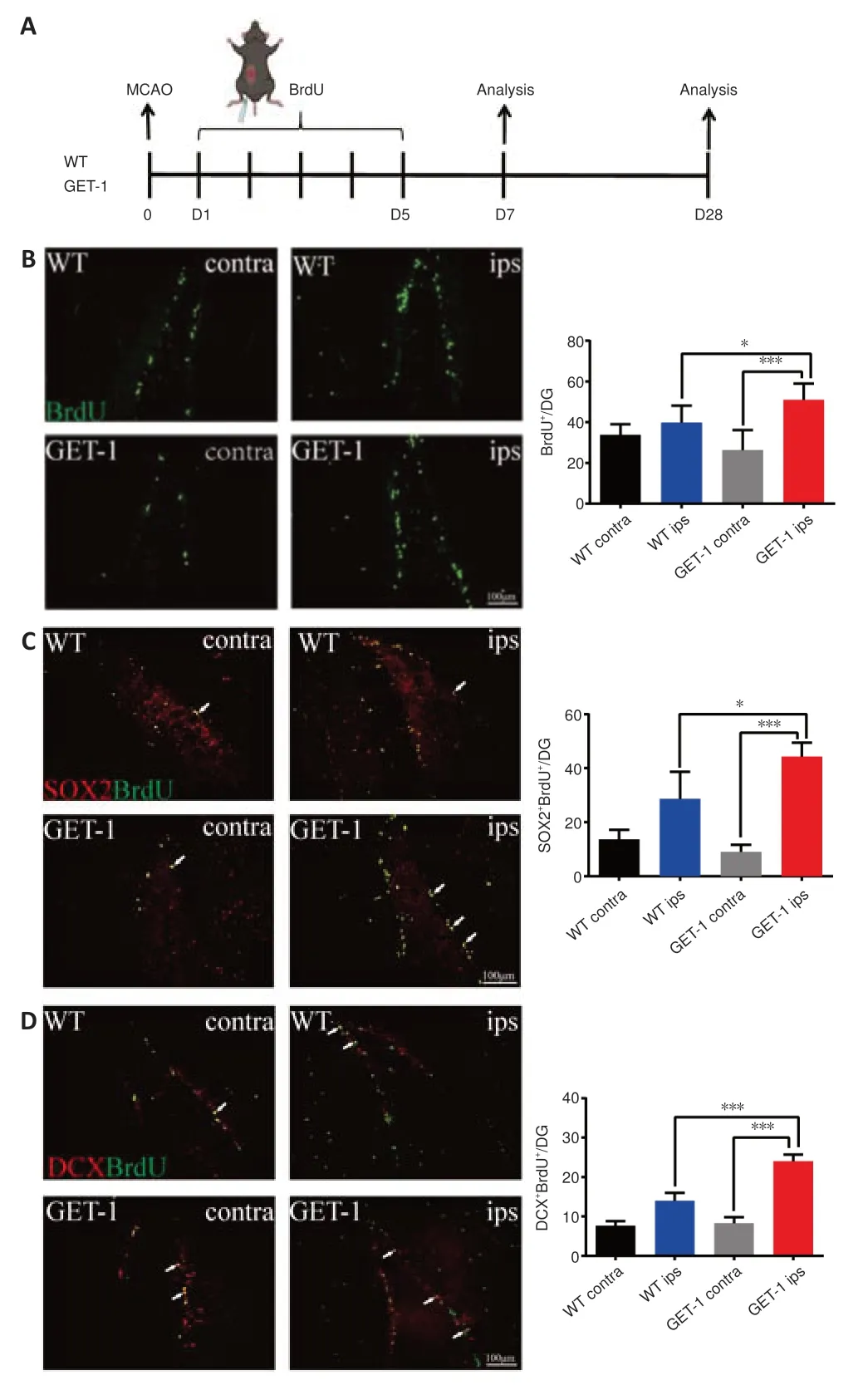
Figure 2|Astrocytic ET-1 overexpression after MCAO promotes NSC proliferation in the DG region of the mouse hippocampus.
Astrocytic endothelin-1 overexpression impairs neurogenesis in the hippocampus after ischemic stroke
To determine whether NSCs in the WT and GET-1 mice differentiated into astrocytes or neurons after ischemic stroke,we conducted GFAP/BrdU and NeuN/BrdU costaining 28 days after MCAO.We found no significant differences between NeuN+BrdU+and GFAP+BrdU+costained cells on the contralateral (normal) side in GET-1 and WT mice (data not shown).However,at 28 days after MCAO,WT mice had more NeuN+BrdU+cells in the ipsilateral DG region than GET-1 mice (P=0.034 for GET-1 ipsvs.WT ips;Figure 3AandC).In contrast,we observed slightly more GFAP+BrdU+cells in GET-1 mice than in WT mice,but this difference was not significant (Figure 3BandD).This result suggests that ET-1 overexpression impairs neurogenesis in the DG region after cerebral ischemic injury (Figure 3E).In addition,Ki67 is a protein that labels cells that are actively dividing but not cells that have divided and have exited the cell cycle.We conducted Ki67/NeuN and Ki67/GFAP double immunofluorescence labeling at 3 months after ischemic stroke,and observed similar expression trends in Ki67+NeuN+and Ki67+GFAP+cells on the ipsilateral sides in WT and GET-1 mice (Additional Figure 2).

Figure 3|Astrocytic ET-1 overexpression after MCAO impairs neurogenesis in the DG region.
Comprehensive brain proteome profiles from GET-1 and WT mice after ischemic stroke
To determine the potential targets of astrocytic endothelin-1 overexpression with respect to cognitive impairment after MCAO-induced ischemic stroke injury,we used LFQ proteomic analysis to assess brain tissue from WT and GET-1 mice at 7 and 28 days after MCAO via LC-MS/MS detection.If a DEP had aP-value < 0.05 and fold change > 1.5,it was filtered out.In total,we identified 223 DEPs (153 upregulated and 70 downregulated) that differed between the MCAO groups of WT and GET-1 mice at 7 days after stroke(Figure 4A).Furthermore,there were 243 DEPs (166 upregulated and 77 downregulated) that differed between the MCAO groups of WT and GET-1 mice at 28 days after stroke (Figure 4B).The expression patterns of some DEPs were visualized via hierarchical clustering inFigure 4CandD.
Next,we applied Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway annotation to obtain a deeper understanding of DEP molecular and functional distinctions between GET-1 and WT mice at 7 and 28 days after ischemic stroke.Interestingly,the results showed that DEP enrichment was closely related to lipid metabolism and the negative regulation of neurogenesis in GET-1 mice compared with WT mice at both 7 and 28 days after stroke (Figure 5AandB).We identified some interesting DEPs,as shown inFigure 4CandD,such as Prdx6,GFAP,Apoe,Lgals1,Vim,Apoa1,and Apoa2,which may be connected with the negative regulation of neuron projection development and lipid metabolism.We selected Prdx6,GFAP,Apoe,Lgals1,Vim,Apoa1,and Apoa2 for verification by western blotting.Among these,GFAP and Prdx6 showed statistically significant differences in the final results (the others are not shown).The levels of GFAP and Prdx6 proteins were upregulated on the ipsilateral side in the GET-1 mice compared with the WT mice at 28 days after MCAO (GFAP:P=0.001 for GET-1 MCAOvs.WT-MCAO,Prdx6:P=0.007 for GET-1 MCAOvs.WT-MCAO;Figure 5C),and their levels were the same as those shown in the results of the label-free analysis.
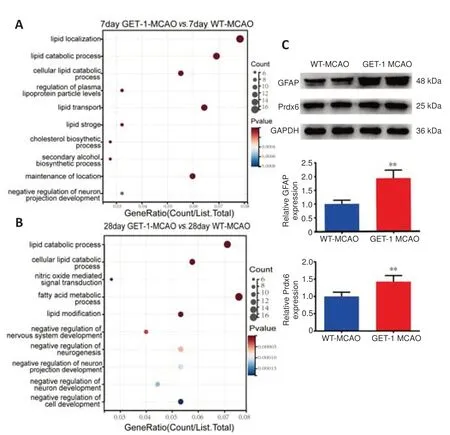
Figure 5|The pathway and validation of WT and GET-1 mice.
Astrocytic endothelin-1 overexpression alters lipid metabolism in the brain after ischemic stroke
Astrocytes are the most numerous type of glial cell in the central nervous system and are involved in a variety of processes,such as metabolic homeostasis (Lee et al.,2021).The above comprehensive proteome profiles of brain tissue indicated that the enriched DEPs in the GET-1 and WT mice were closely related to lipid metabolism in the brain.We thus performed LCMS nontargeted metabolite profiling using brain samples from GET-1 mice and WT mice collected 28 days after ischemic stroke.Partial least squares discriminant analysis verified a distinct separation between GET-1 MCAO mice and WT MCAO mice (Figure 6A),indicating a shift in the total metabolism profile.We identified 91 different metabolites and generated a heatmap showing the abundance of the top 50 differential metabolite entities in the GET-1 and WT mice with the most significant p values after MCAO injury(Figure 6B).The volcano plot revealed 43 significantly upregulated and 48 significantly downregulated differential metabolites in the GET-1 group in comparison with the WT group after MCAO (Figure 6C).To analyze the metabolic pathway enrichment of the differential metabolites discovered in the brain,we imported them into the KEGG database.We identified the top 12 pathways with significant differences (P< 0.05),and plotted the associated functional enrichment maps.The pathway results for the main enrichment of differential metabolites in the brains of the GET-1 MCAO mice and the WT MCAO mice are shown inFigure 6D,of which the glycerophospholipid metabolism pathway (P=0.0048) showed the second highest degree of enrichment.These lipid molecules were further classified,and the total abundance of major lipid classes within them was calculated.We discovered that glycerol phosphatidylcholine (GPC),sphingomyelin (SM (d18:0/18:1(9Z))),and phosphatidic acid (PA (16:0/22:2(13Z,16Z))) were upregulated,whereas the abundance of phosphatidylethanolamine (PE (18:0/0:0)) and phosphatidylserine (PS (17:2(9Z,12Z)/22:1(11Z))) was significantly reduced in GET-1 mice compared with WT mice at 28 days after MCAO injury (GPC:P<0.001 for GET-1 MCAOvs.WT-MCAO,SM:P=0.018 for GET-1 MCAOvs.WTMCAO,PA:P=0.001 for GET-1 MCAOvs.WT-MCAO,PE:P=0.014 for GET-1 MCAOvs.WT-MCAO,PS:P=0.021 for GET-1 MCAOvs.WT-MCAO,Figure 6E).These results indicate that astrocytic ET-1 overexpression affected brain lipid regulation at 28 days after ischemic stroke.
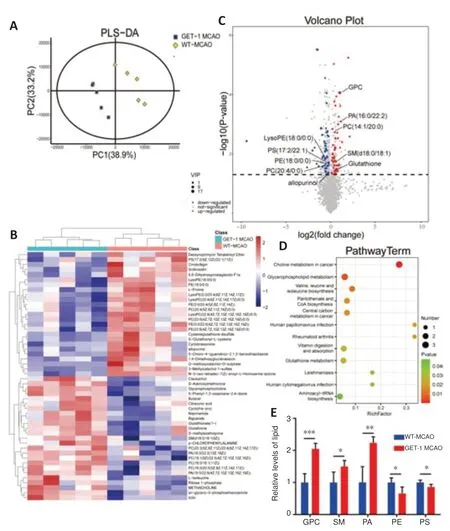
Figure 6|Differential metabolites in GET-1 mice and WT mice at 28 days after MCAO.
Discussion
This study is the first to report that astrocytic ET-1 overexpression negatively affects spatial learning and memory after ischemic stroke (1 hour of ischemia(MCAO) and 28 days of reperfusion).This phenomenon was associated with impaired hippocampal neurogenesis and lipid metabolism in the brain,thus illuminating the function of astrocyte-derived ET-1 following vascular cognitive impairment.These data can be used to identify potential targets for the development of drugs to treat cognitive impairment following cerebrovascular disease.
Neurogenesis in the SVZ and DG region persists throughout life in many mammals,including humans (Niklison-Chirou et al.,2020;Yang et al.,2023).In AD,changes in hippocampal neurogenesis affect cognitive function (Lazarov and Hollands,2016).Higher cognitive functions involve hippocampal neurogenesis,particularly memory processing,along with certain affective behaviors.It appears that adult hippocampal neurogenesis significantly contributes to hippocampal plasticity across the lifespan in humans (Kempermann et al.,2015).MWM and radial six-arm water maze task performance were impaired when the proliferation of NSCs and NPCs was switched to differentiation (Farioli-Vecchioli et al.,2008).Increased hippocampal neurogenesis is linked to better learning,whereas inhibiting adult neurogenesis leads to some behavioral impairments (Akers et al.,2014;Cameron and Glover,2015).Ben Abdallah et al.used cranial irradiation to impair hippocampal neurogenesis and found that spatial long-term memory retention was consistently altered (Ben Abdallah et al.,2013).Moreover,new neurons are very important for cognitive function (Cameron and Glover,2015).Our study showed that astrocytic ET-1 overexpression impaired neurogenesis in neuronal cells,and the proteomic results also showed that enriched DEPs were closely related to the negative regulation of neurogenesis in GET-1 mice compared with WT mice,which is likely one explanation for the more serious cognitive impairment observed in astrocytic ET-1 mice after stroke.
Our western blot analysis indicated a significant increase in GFAP protein levels in GET-1 mice compared with WT mice at 7 and 28 days after stroke.Double immunofluorescence labeling of the hippocampus revealed a slight increase in GFAP+BrdU+cells in the DG region of GET-1 mice,but no significant difference with respect to WT mice was observed at 28 days after stroke.This may be because GFAP is considered to be a marker of astrocytes,including ischemia-triggered reactivated astrocytes and newborn astrocytes.Our previous study also indicated that the overexpression of ET-1 in astrocytes promotes the proliferation of progenitor stem cells and the differentiation of astrocytes in the SVZ region (Cheng et al.,2019).Monkey SGZ and SVZ progenitor cells were also found to be increased after ischemic insult,and gliogenesis in the hippocampal CA1 regions was susceptible to ischemia(Tonchev,2011),leading to a significant increase in GFAP protein expression in the whole brain.
Prdx6 is widely distributed in different neuronal populations and has glutathione peroxidase,phospholipase A2 (PLA2),and lysophosphatidylcholine acyl transferase activities (Arevalo and Vázquez-Medina,2018).PLA2 can activate NADPH oxidase 2.The PLA2 activity of Prdx6 was shown to be upregulated by superoxidation under oxidative stress conditions (Arevalo and Vázquez-Medina,2018;Patel and Chatterjee,2019).Numerous studies have confirmed the involvement of Prdx6 in neurodegenerative diseases (Krapfenbauer et al.,2003;Drummond et al.,2017).For instance,Yun et al.found that the overexpression of Prdx6 could worsen memory impairment through increased amyloidogenesis in AD patients (Yun et al.,2013).Moreover,Prdx6-overexpressing transgenic mice showed decreased neurogenesis ability compared with WT mice (Yeo et al.,2019).In vitroandin vivostudies have indicated that Prdx6 is primarily expressed in astrocytes(Pankiewicz et al.,2020),although the mechanism of action of Prdx6 in AD remains controversial,and even contradictory (Liao et al.,2021).Thus,further study is warranted.The molecular mechanism of the effect of ET-1 on Prdx6 is not clear.Astrocytic endothelin-1 overexpression exacerbates oxidative stress(Hung et al.,2015),which can increase the production of reactive oxygen species,largely via the activation of NOX (Matsumoto et al.,2008).ET-1 can activate NOX (Duerrschmidt et al.,2000) and PLA2 (Stanimirovic et al.,1993).ET-1 may act on the PLA2 activity of Prdx6,although further research is needed to confirm this hypothesis.
Prdx6 is also critical for lung phospholipid metabolism and lipid peroxidation repair (Arevalo and Vázquez-Medina,2018).Lipid metabolism has been connected with many neuropathological processes,such as changes in AD,and thus plays a vital role in cerebral physiology (Sultana et al.,2013).Unlike neurons that break down glucose for energy,astrocytes store and break down lipid droplets for energy and then support neurons (Barber and Raben,2019).Lipids play a basic role in astrocyte function,including energy production,membrane fluidity,and cell-to-cell signaling.It has been reported that lipids are stored by astrocytes in droplets,and that they are used to perform key physiological and protective functions in the central nervous system (Lee et al.,2021).Interestingly,both our proteomic and metabolite profiling processes showed dysregulation of lipid pathways in the whole brains of GET-1 mice after stroke.Altered lipid metabolism can have complex consequences for learning and memory.Bowers et al.(2020) used transgenic mouse and human tissue genome engineering to show that altered lipid metabolism contributes to intellectual disabilities.However,polyunsaturated free fatty acids (FFAs) are considered beneficial for learning and memory.In a study by Wallis et al.(2021) a targeted lipidomics approach revealed a positive role for saturated FFAs in memory acquisition.In future work,we hope to investigate whether cognitive function after MCAO is affected by regulating targets related to lipid metabolism in GET mice.
Although the results of the proteome profiles indicated that the DEPs were associated with lipid metabolism,the way in which ET-1 expression influences lipid metabolism is not clear.ET-1 has two receptor subtypes: ETAR and ETBR.ET-1 can promote lipolysis through ETAR (Ishida et al.,1992;Juan et al.,2006).Therefore,ET-1 may regulate lipid metabolism-related genes via a precise metabolic pathway,such as the activity of GPC with respect to the ET receptor.This needs to be confirmed via future experiments.
The premise of examining cognitive function after MCAO is to determine whether the MCAO model was successfully established.Indeed,it would be helpful to use magnetic resonance imaging to observe the infarct volume at different time points after MCAO in small animals,which could help in judging more precisely whether the model was successful.In our previous study (Hung et al.,2015),we investigated cognitive deficits after 30 minutes of MCAO with long-term reperfusion.Because no infarction could be seen in brain slices after TTC staining,we also used magnetic resonance imaging of small animals to observe the degree of cerebral edema and signs of neurodegeneration.In this study,we observed an obvious infarct area after TTC staining in brain slices when mice were subjected to 1 hour of MCAO with long-term reperfusion.We used the following two methods to judge whether the MCAO model was successful.First,when the blood flow of the middle cerebral artery decreased to 30% or less than the baseline via laser Doppler flowmetry during MCAO surgery,middle cerebral artery embolization was considered successful (Zhang et al.,2013;Hung et al.,2015).Second,we investigated brain shrinkage in brain slices after the behavior test,and mice subjected to MCAO exhibited substantial hemispheric shrinkage on the ipsilateral side of the brain (data not shown).
We found no MWM differences between the WT-sham group and the WTMCAO group.These results are consistent with those of other studies showing that mice undergoing transient (60 minutes) intraluminal occlusion and 1 month of reperfusion exhibited normal spatial learning capacities in the MWM test;however,learning deficits were observed in the passive avoidance test (Bouët et al.,2007).Kathner-Schaffert et al.(2019) found that WT mice subjected to 45 minutes of MCAO showed impaired performance in the MWM test,and we consider this to be mainly related to the degree of brain ischemia and reperfusion time.A longer ischemia period is associated with a greater degree of brain damage,whereas a longer reperfusion time(3 months,6 months,or even longer) is associated with greater MWM test performance.Of course,the number of animals in each group could also affect the results.In this study,we did not conduct the MWM test with longer reperfusion times.There were significant differences between the GET-1 MCAO and WT MCAO groups,which explain why astrocytic ET-1 accelerated the occurrence of PSCI at 1 month of reperfusion.
This study has some limitations that should be noted.First,we validated the Prdx6 data by western blot analysis,indicating that the protein sequencing results were credible.However,more research is needed to reveal the relationship between astrocytic ET-1 overexpression and Prdx6.Second,although we showed that some lipid metabolites were altered in GET-1 mice compared with WT mice,the exact mechanisms require further study.
In conclusion,this study demonstrates that astrocytic ET-1 overexpression had a negative effect on poststroke cognitive function.Specifically,astrocytic ET-1 overexpression impaired hippocampal neurogenesis and lipid metabolism.Our results suggest that astrocytic ET-1 may be identified as a potential target for the development of drugs to treat cognitive impairment following cerebrovascular disease.
Acknowledgments:We would like to thank Dr.Sookja Kim Chung (The University of Hong Kong,China) for providing GET-1 mice.
Author contributions:JL,WJ,YC and XC conceived and designed the experiments.WJ,YC,ZN and YZ performed the experiments.CW and MD analyzed the data.JL,WJ,YC wrote the manuscript.SC,YH,JS,LZ and XC provided revision of the paper.All authors read and approved the final manuscript.
Conflicts of interest:The authors declare no competing interests.
Data availability statement:All relevant data are within the paper and its Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Editor’s evaluation:Post-stroke cognitive impairment (PSCI) is one of the most common stroke complications.At present,there is no specific drug for PSCI,and the underlying mechanism is still unclear.In this manuscript,the authors studied how astrocytic ET-1 leads to PSCI following transient MCAO.The authors found that astrocytic ET-1 overexpression played a negative role in the cognitive function of MCAO animals,and the mechanism is associated with hippocampal neurogenesis’s impairment and lipid metabolism change in the brain.The results of this study will provide help for understanding the molecular mechanisms of PSCI.
Additional files:
Additional Figure 1:Identification of ET-1 transgenic mice and intraoperative cerebral blood flow images.
Additional Figure 2:Astrocytic ET-1 overexpression impaired neurogenesis in the DG region at 3 months after MCAO.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Treatment with β-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload,endoplasmic reticulum stress,and apoptosis
