Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
2024-02-18LongqingZhangXiTanFanheSongDanyangLiJiayiWuShaojieGaoJiaSunDaiqiangLiuYaqunZhouWeiMei
Longqing Zhang,Xi Tan,Fanhe Song,Danyang Li,Jiayi Wu,Shaojie Gao,Jia Sun,Daiqiang Liu,Yaqun Zhou,Wei Mei
Abstract Activated G-protein-coupled receptor 39 (GPR39) has been shown to attenuate inflammation by interacting with sirtuin 1 (SIRT1) and peroxisome proliferatoractivated receptor-γ coactivator 1α (PGC-1α).However,whether GPR39 attenuates neuropathic pain remains unclear.In this study,we established a Sprague-Dawley rat model of spared nerve injury-induced neuropathic pain and found that GPR39 expression was significantly decreased in neurons and microglia in the spinal dorsal horn compared with sham-operated rats.Intrathecal injection of TC-G 1008,a specific agonist of GPR39,significantly alleviated mechanical allodynia in the rats with spared nerve injury,improved spinal cord mitochondrial biogenesis,and alleviated neuroinflammation.These changes were abolished by GPR39 small interfering RNA (siRNA),Ex-527 (SIRT1 inhibitor),and PGC-1α siRNA.Taken together,these findings show that GPR39 activation ameliorates mechanical allodynia by activating the SIRT1/PGC-1α pathway in rats with spared nerve injury.
Key Words: G-protein-coupled receptor 39 (GPR39);neuroinflammation;neuropathic pain;nuclear respiratory factor 1 (NRF1);peroxisome proliferatoractivated receptor-γ coactivator 1α (PGC-1α);sirtuin 1 (SIRT1);spinal cord;mitochondrial transcription factor A (TFAM)
From the Contents
Introduction 687
Methods 687
Results 689
Discussion 690
Introduction
Disruption of the somatosensory system is known to cause neuropathic pain(Jensen et al.,2011;Colloca et al.,2017).Approximately 7–10% of the world’s population suffers from neuropathic pain (Bouhassira et al.,2008;van Hecke et al.,2014).However,the specific mechanism underlying neuropathic pain remains unclear (Chen et al.,2018a;Inoue and Tsuda,2018).Previous studies have shown that G-protein-coupled receptor 39 (GPR39) is expressed in the hippocampus and auditory cortex (Jackson et al.,2006;Egerod et al.,2007).Moreover,studies have reported that activation of GPR39 can promote wound healing (Zhao et al.,2015),inhibit inflammatory bowel disease(Pongkorpsakol et al.,2019),and attenuate depression (Starowicz et al.,2019).Recent studies have shown that GPR39 activation inhibits inflammation and ameliorates oxidative stress and mitochondrial dysfunction (Muneoka et al.,2018;Jing et al.,2019;Xu et al.,2019).Furthermore,evidence indicates that GPR39 protects against neuronal lesions by decreasing endoplasmic reticulum stress (Dittmer et al.,2008;Mo et al.,2020a;Sánchez-Temprano et al.,2022).However,the mechanisms underlying GPR39-mediated attenuation of neuropathic pain remain unclear.
Sirtuin 1 (SIRT1),a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase,plays a critical role in regulating metabolic disease pathogenesis,aging,neurodevelopment,and brain senescence (Bordone and Guarente,2005;Chang and Guarente,2014;Herskovits and Guarente,2014;Cui et al.,2023;Gu et al.,2023;Wang et al.,2023).Moreover,spinal SIRT1 expression is down-regulated by neuropathic pain,whereas SIRT1 activation ameliorates neuropathic pain (Zhou et al.,2017a;Chen et al.,2018b;Mo et al.,2020b).Recent evidence has demonstrated that GPR39 activation with TC-G 1008 dampens neurological deficits and reduces neuroinflammation caused by ischemic injury,and that this effect is mediated by the SIRT1/peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α) pathway (Xie et al.,2021).Previous studies have demonstrated that activation of the SIRT1/PGC-1α pathway can alleviate mechanical allodynia (Li et al.,2019;Hao et al.,2022).However,whether GPR39 exerts its analgesic effects by activating SIRT1 is unclear.
Mitochondrial biogenesis is a dynamic process that generates new mitochondria,which contribute to mitochondrial homeostasis.PGC-1α significantly induces mitochondrial biogenesis,and promotes the transcription and replication of mitochondrial DNA (mtDNA) by interacting with nuclear respiratory factor 1 (NRF1) and mitochondrial transcription factor A (TFAM)(Santos et al.,2011;Fan et al.,2021).Furthermore,our previous studies showed that impaired mitochondrial biogenesis may lead to chronic pain,and that PGC-1α activation ameliorates mechanical allodynia (Chen et al.,2021;Sun et al.,2021,2022;Zhang et al.,2022b).In addition,another study indicated that PGC-1α activation dampens neuroinflammation (Han et al.,2021).However,whether GPR39 exerts analgesic effects by activating PGC-1α is unclear.The aim of the present study was to identify whether GPR39 activation improves mechanical allodynia by promoting mitochondrial biogenesis and attenuating neuroinflammation through activation of the SIRT1/PGC-1α pathway in a rat model of neuropathic pain.
Methods
Animals
A total of 415 male Sprague-Dawley rats (8 weeks old,220–240 g,specific pathogen-free (SPF) level) were purchased from the Laboratory Animal Center of Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology (Wuhan,China,license No.SYXK (E) 2019-0106).The rats were housed under controlled conditions (temperature: 22–25°C,relative humidity: 45–65%,and 12-hour light to dark cycle and,water and food pellets availablead libitum).This study was approved by the Experimental Animal Care and Use Committee of Tongji Hospital,Tongji Medical College,Huazhong University of Science and Technology (approval No.TJH-202107011) on July 11,2021.All experiments were conducted in accordance with the Animal Research Reporting ofin VivoExperiments (ARRIVE) guidelines (Percie du Sert et al.,2020).The study flow chart,including the animal groups,is shown in the figure describing the study design (Additional Figure 1).According to previous studies (Xie et al.,2017;You et al.,2018;Hu et al.,2020;Li et al.,2021),spared nerve injury (SNI) quickly induces long-lasting mechanical allodynia in male rats;for this reason,only male rats were used in this study.
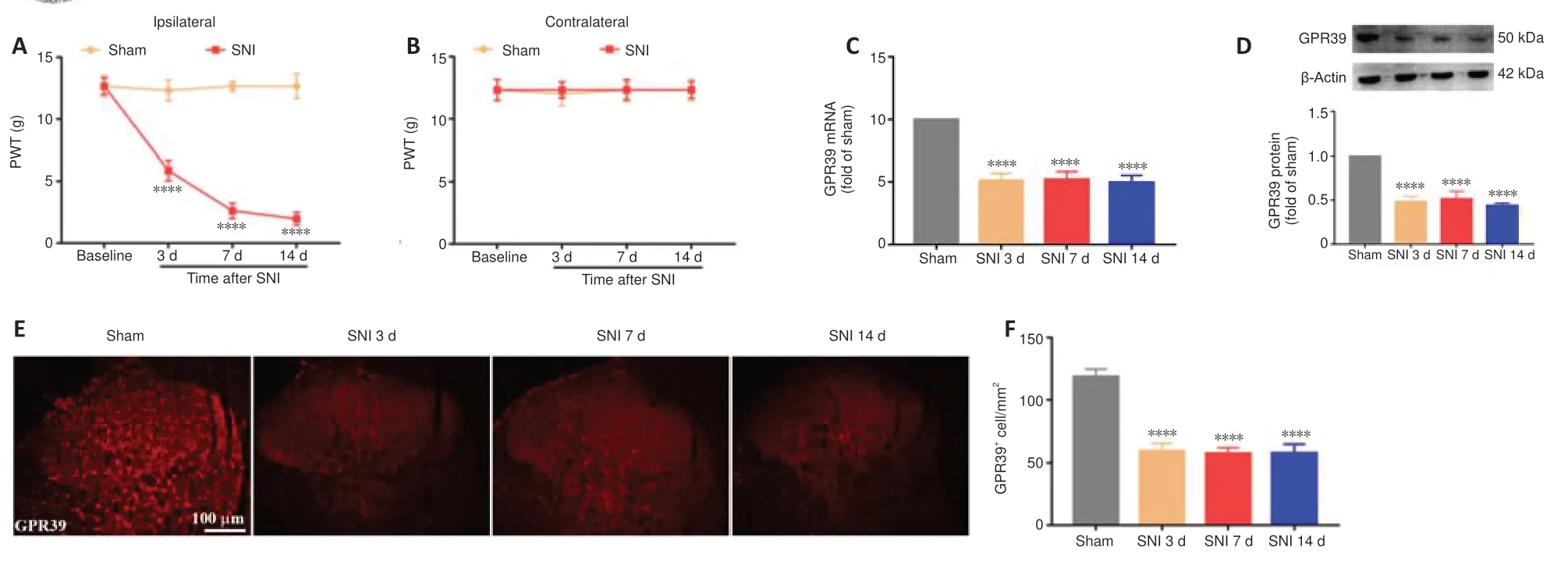
Figure 1|SNI decreases PWT and lowers GPR39 expression in the spine.
Experimental design
The experimental design is shown inAdditional Figure 1.
Experiment 1: The aim of this experiment was to identify changes in the GPR39 and SIRT1/PGC-1α signaling pathways in the spinal cord of rats after nerve injury.The paw withdrawal threshold (PWT) was assessed on day 2 before surgery and on days 3,7,and 14 after nerve injury.The target molecules were evaluated using western blotting (WB),immunofluorescence (IF) staining,and quantitative polymerase chain reaction (qPCR).The experimental groups used were as follows: sham (n=15 rats) and SNI (n=45 rats).
Experiment 2: The aim of this experiment was to explore the effective analgesic dose of TC-G 1008 in rats with SNI.A single dose of TC-G 1008 (5,20,or 50 μg) was injected 7 days after SNI,and the PWT was assessed 2,4,and 6 hours after injection.The experimental groups used were as follows:SNI+vehicle (n=5 rats),SNI+TC-G 1008 5 μg (n=5 rats),SNI+TC-G 1008 20 μg (n=5 rats),and SNI+TC-G 1008 50 μg (n=5 rats).
Experiment 3: The aim of this experiment was to determine whether repeated injection of TC-G 1008 (20 μg) reduced mechanical allodynia in rats with SNI.TC-G 1008 was intrathecally administered on days 7–11 after the operation.The PWT was tested on days 7–11 after SNI,and locomotion was assessed by an open field test (OFT).The target molecules were evaluated by WB,IF staining,qPCR,and enzyme-linked immunosorbent assay (ELISA).The experimental groups used were as follows: sham+vehicle (n=20 rats),sham+TC-G 1008 (n=20 rats),SNI+vehicle (n=20 rats),and SNI+TC-G 1008 (n=20 rats).
Experiment 4: This experiment was conducted to verify whether pretreatment with TC-G 1008 could suppress the development of neuropathic pain.TC-G 1008 (20 μg,intrathecal) was administered on days 1–5 after the operation.PWT was assessed on days 3,5,and 6 after SNI.The experimental groups used were as follows: sham+vehicle (n=5 rats),sham+TC-G 1008 (n=5 rats),SNI+vehicle (n=5 rats),and SNI+TC-G 1008 (n=5 rats).
Experiment 5: The aim of this experiment was to verify whether GPR39 small interfering RNA (siRNA) could reverse the analgesic effect of TC-G 1008 in rats with SNI.GPR39 siRNA or negative siRNA was injected on days 1,3,5,and 7 after nerve injury,and TC-G 1008 (20 μg) was intrathecally administered on days 7–11 after the operation.The PWT was assessed on days 7–11 after SNI,and locomotion was evaluated by OFT.The target molecules were evaluated using WB,IF staining,qPCR,and ELISA.The experimental groups used were as follows: sham (n=20 rats),SNI+vehicle (n=20 rats),SNI+TC-G 1008 (n=20 rats),SNI+TC-G 1008+negative siRNA (n=20 rats),and SNI+TC-G 1008 +GPR39 siRNA (n=20 rats).
Experiment 6: The aim of this experiment was to identify whether a SIRT1 antagonist (Ex-527) could dampen the improved mechanical allodynia induced by TC-G 1008 in rats with SNI.TC-G 1008 (20 μg) or Ex-527 (1.5 μg)was administered on days 7–11 after the operation.The PWT was assessed on days 7–11 after SNI,and locomotion was evaluated by OFT.The target molecules were evaluated using WB,qPCR,and ELISA.The experimental groups used were as follows: sham (n=15 rats),SNI+vehicle (n=15 rats),SNI+TC-G 1008 (n=15 rats),and SNI+TC-G 1008+Ex-527 (n=15 rats).
Experiment 7: The aim of this experiment was to verify whether PGC-1α siRNA could block the analgesic effect of TC-G 1008 in rats with SNI.PGC-1α siRNA or negative siRNA was injected on days 1,3,5,and 7 after nerve injury,and TC-G 1008 (20 μg) was intrathecally administered on days 7–11 after the operation.The PWT was assessed on days 7–11 after SNI,and locomotion was evaluated by OFT.The target molecules were evaluated using WB,qPCR,and ELISA.The experimental groups used were as follows: sham (n=15 rats),SNI+vehicle (n=15 rats),SNI+TC-G 1008 (n=15 rats),SNI+TC-G 1008+negative siRNA (n=15 rats),and SNI+TC-G 1008+PGC-1α siRNA (n=15 rats).
Spared nerve injury
On the basis of previous studies,a model of mechanical allodynia was established by inducing SNI (Decosterd and Woolf,2000;Hu et al.,2020;Li et al.,2021).The rats were anesthetized by inhalation anesthesia (induction with 2.5% isoflurane (RWD,Shenzhen,China),maintenance with 1.5% isoflurane),and the right sciatic nerve was exposed.The right common peroneal andtibial nerves were ligated and excised.Finally,the muscle and skin layers were sutured.For sham rats,the nerves were exposed but not ligated.
Mechanical allodynia
We used a 50% PWT to examine mechanical allodynia caused by SNI (Zhou et al.,2017a).Von Frey filaments (1.0–15.0 g;RWD) were used to assess PWT according to the “up-and-down” method (Wang et al.,2020).The investigator who performed the mechanical allodynia assessments was blinded to the group assignments.
Open field test
As previously described (Zhang et al.,2022a),the OFT was performed to evaluate locomotor function in rats.Each rat was allowed to explore an open field (60 cm × 60 cm × 40 cm) for 300 seconds.Total distance and average speed during the exploration period were assessed.The researcher who performed the OFT assessments was blinded to the group assignments.
Intrathecal catheterization
Intrathecal catheterization was performed as previously described (Zhang et al.,2022a).Briefly,under anesthesia with 2.5% isoflurane,PE10 polyethylene catheters (RWD) were inserted into the L5–L6 spinous processes.Intrathecal injection of 2.0% lidocaine (RWD) was used to confirm the correct position of the catheter.The rats were allowed to rest for 7 days after intrathecal catheterization.
Drug administration
TC-G 1008 (a specific agonist of GPR39;Cat# HY-103007,MedChemExpress,Monmouth Junction,NJ,USA) was dissolved in dimethyl sulfoxide (Sigma,St.Louis,MO,USA).The TC-G 1008 dose was selected based on our preliminary experiments.Different doses of TC-G 1008 (5,20,or 50 μg) were assessed to determine whether a single intrathecal injection could attenuate mechanical allodynia.Furthermore,TC-G 1008 (20 μg,intrathecal) was administered to identify whether repeated or early injection of TC-G 1008 can reverse mechanical allodynia or suppress the development of neuropathic pain.To identify whether the analgesic effect of TC-G 1008 depended on SIRT1,a SIRT1 antagonist (Ex-527,1.5 μg/rat,intrathecal administration,Cat# HY-15452,MedChemExpress,dissolved in dimethyl sulfoxide (Sigma)) was administered 1 hour before TC-G 1008 injection.The Ex-527 dose was selected based on a previous study (Mo et al.,2020b).The time points for treatment with TC-G 1008 and/or Ex-527 are shown in the figure depicting the experimental design (Additional Figure 1).
siRNA transfection
The GPR39 siRNA (sense 5′-GCU AUG CUC UGU CCU GCA ATT-3′;anti-sense 5′-UUG CAG GAC AGA GCA UAG CTT-3′),PGC-1α siRNA (sense 5′-CGG AUA UAC UCA UUA ACA UTT-3′;anti-sense 5′-AUG UUA AUG AGU AUA UCC GTT-3′),and negative siRNA (sense 5′-UUC UCC GAA CGU GUC ACG UTT-3′ and anti-sense 5′-ACG UGA CAC GUU CGG AGA ATT-3′) sequences were designed by Tsingke Biotechnology (Beijing,China).Each siRNA (1 μg/μL,dissolved in RNase-free water (Tsingke Biotechnology)) was mixed with transfection reagent (Tsingke Biotechnology) and injected intrathecally at 1,3,5,and 7 days after SNI.qPCR was used to evaluate the effect of the siRNAs.
Quantitative polymerase chain reaction
The rats were euthanized with CO2(the CO2concentration was continuously increased at a replacement rate of 60% until respiratory and cardiac arrest,which occurred within 30 minutes) as previously described (Zhang et al.,2022a).Total RNA was isolated from L4–L6 spinal cord sections,and complementary DNA was synthesized.The qPCR reactions were as follows:5 μL Premix Ex TaqII (Vazyme,Nanjing,China),0.5 μL primer,2 μL diethyl pyrocarbonate water (Vazyme),and 2 μL complementary DNA.Reactions were performed using a LightCycler 480 qPCR instrument (Roche,Basel,Switzerland) and the following standard conditions: 95°C for 5 minutes,40 cycles (95°C for 15 seconds,56°C for 30 seconds,and 72°C for 30 seconds).The relative expression levels were quantified using the 2–∆∆CTmethod (Wang et al.,2021).The following primers were used: β-actin forward: 5′-CCC ATC TAT GAG GGT TAC GC-3′,reverse: 5′-TTT AAT GTC ACG CAC GAT TTC-3′;GPR39 forward: 5′-GGT CTT CCA GTC CAG CAT CTT TG-3′,reverse: 5′-CAC CAC GAT CAG TCT CAG GAA TA-3′;PGC-1α forward: 5′-GTG CAG CCA AGA CTC TGT ATG G-3′,reverse: 5′-GTC CAG GTC ATT CAC ATC AAG TTC-3′.
Western blot assay
The rats were euthanized with CO2as described above,and the L4–L6 spinal cord segments were quickly excised.The protein concentration was quantified using a bicinchoninic acid protein assay kit (Boster Biological Technology,Wuhan,China,Cat# AR0146).Equal amounts of protein (25 μg) were separated using 10% sodium dodecyl-sulfate-polyacrylamide gel electrophoresis and transferred onto polyvinylidene fluoride membranes.The membranes were blocked with 5% bovine serum albumin (Boster Biological Technology) for 1.5 hours at 25°C and incubated with the corresponding primary antibodies overnight at 4°C,including rabbit anti-β-actin (1:10,000;ABclonal,Wuhan,China,Cat# AC026;RRID: AB_2768234),rabbit anti-GPR39(1:500;Bioss,Beijing,China;Cat# bs-5789R;RRID: AB_11095358),rabbit anti-SIRT1 (1:1000;ABclonal;Cat# A0230;RRID: AB_2757043),rabbit anti-PGC-1α (1:1000;ABclonal;Cat# A12348;RRID: AB_2759191),rabbit anti-NRF1(1:1000;Abcam,Cambridge,UK;Cat# ab 175932;RRID: AB_2629496),and rabbit anti-TFAM (1:500;Abcam;Cat# ab 131607;RRID: AB_11154693).The membranes were then incubated with horseradish peroxidase-conjugated goat anti-rabbit (1:5000;ABclonal;Cat# AS014;RRID: AB_2769854) for 1.5 hours at 25°C.The bands were detected and quantified using Image Lab software (Bio-Rad,Hercules,CA,USA).
Immunofluorescence staining
Under anesthesia with isoflurane (2.5%),the rats were perfused intracardially with 0.1 M phosphate-buffered saline,followed by 4% ice-cold paraformaldehyde.Then,the L4–L6 spinal cord segments were collected and dehydrated in a 30% sucrose solution overnight at 4°C.The L4–L6 spinal cord segments were sectioned to a thickness of 20 μm using a cryostat (Leica,Wetzlar,Germany).For immunofluorescence staining,the sections were blocked with 5% donkey serum (Boster Biological Technology) and 0.3% Triton X-100 (Boster Biological Technology) for 1 hour at 25°C,then incubated with the following primary antibodies overnight at 4°C: rabbit anti-GPR39 (1:50;Bioss;Cat# bs-5789R;RRID: AB_11095358),rabbit anti SIRT1 (1:50;ABclonal;Cat# A0230;RRID: AB_2757043),mouse anti-glial fibrillary acidic protein(GFAP,1:100;Cell Signaling Technology,Danvers,MA,USA;Cat# 3670;RRID:AB_561049),mouse anti-neuronal nuclei (NeuN,1:50;Abcam;Cat# ab 104224;RRID: AB_10711040),and goat anti-ionized calcium-binding adapter molecule 1 (Iba-1,1:50;Abcam;Cat# ab5076;RRID: AB_2224402).The sections were then incubated with corresponding secondary antibodies for 1 hour at 25°C:CoraLite594-conjugated donkey anti-rabbit (1:100;Proteintech,Chicago,IL,USA;Cat# SA00013-8;RRID: AB_2857367),CoraLite488-conjugated donkey anti-mouse (1:50;Proteintech;Cat# SA00013-5;RRID: AB_2890971),and Fluorescein IsoThioCyanate-donkey anti-goat (1:100;ABclonal;Cat# AS032;RRID: AB_2769473).A fluorescence microscope (BX53,Olympus,Tokyo,Japan) was used to capture the images.As described previously (Ji et al.,2008;Zhang et al.,2013,2022a),ImageJ 1.8 software (National Institutes of Health,Bethesda,MD,USA) was used to analyze the images.The number of immunopositive cells in a 500 μm × 500 μm measuring frame in the spinal dorsal horn was determined.The results are expressed as the total number of double-positive cells per square millimeter.
Enzyme-linked immunosorbent assay
The rats were euthanized with CO2as described above,and the L4–L6 spinal cord segments were quickly excised.The spinal cord segments were homogenized in phosphate-buffered saline.The supernatant was collected through centrifugation at 3000 ×gat 4°C for 25 minutes.The supernatant was analyzed using rat interleukin-1β (IL-1β) (Boster Biological Technology;Cat#EK0393),interleukin-6 (IL-6) (Boster Biological Technology;Cat# EK0412),and tumor necrosis factor-α (TNF-α) (Cat# EK0526;Boster Biological Technology)ELISA kits,according to the manufacturer’s instructions.
mtDNA copy number estimation
The rats were euthanized with CO2as described above.DNA was extracted from the L4–L6 spinal cord segments of rats using a Tissue Genomic DNA Extraction Kit (EP007,ELK Biotechnology,Wuhan,China).qPCR was performed to detect NADH dehydrogenase 1 (mitochondrial gene) and β-actin (nuclear gene) levels (Scholpa et al.,2018).The qPCR reactions were as follows: 5 μL Premix Ex TaqII (Vazyme),0.5 μL primer,2 μL diethyl pyrocarbonate water(Vazyme),and 2 μL complementary DNA.Reactions were performed using a LightCycler 480 qPCR instrument (Roche,Basel) and the following standard conditions: 95°C for 5 minutes,40 cycles (95°C for 15 seconds,56°C for 30 seconds,and 72°C for 30 seconds).The ratio of NADH dehydrogenase 1 to β-actin represents the relative mtDNA copy number.The following primers were used: β-actin forward: 5′-CCC ATC TAT GAG GGT TAC GC-3’,reverse: 5′-TTT AAT GTC ACG CAC GAT TTC-3′;NADH dehydrogenase 1 forward: 5′-TTA TCC TCT TAT CCG TCC TC-3′,reverse: 5′-TGT TAA GTC GAA GGG AGC-3′.The data were normalized by β-actin.
Statistical analysis
All data are presented as the mean ± standard error of the mean (SEM) and were analyzed using GraphPad Prism version 6.0.0 for Windows (GraphPad Software,San Diego,CA,USA,www.graphpad.com).Unpairedt-test and oneor two-way analysis of variance followed by Bonferroni’spost hoctest was used to analyze IF staining,OFT,ELISA,qPCR,mtDNA copy number,WB,and PWT data.Statistical significance was set atP< 0.05.
Results
Effects of neuropathic pain on spinal GPR39 expression
A neuropathic pain model was conducted by SNI in rats.The behavioral results showed that SNI decreased the ipsilateral PWT (Figure 1A),but not the contralateral PWT (Figure 1B).We then assessed GPR39 expression levels in rats with neuropathic pain.The results indicated that GPR39 mRNA(Figure 1C) and protein (Figure 1D) levels were down-regulated following SNI.Furthermore,single immunostaining confirmed that SNI decreased GPR39 immunopositivity in the spinal dorsal horn (Figure 1EandF).Moreover,as shown inFigure 2,double immunostaining indicated that GPR39 mainly colocalized with NeuN and Iba-1,and that SNI reduced GPR39 immunopositivity in neurons and microglia.These results substantiated SNI diminished the expression of GPR39 in the spinal cord of rats.
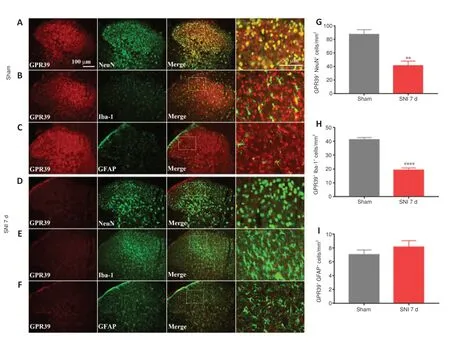
Figure 2|GPR39 localization in the spinal cord.
SNI inactivates the SIRT1/PGC-1α pathway and disrupts mitochondrial biogenesis
It was demonstrated that activation SIRT1/PGC-1α promoted mitochondrial biogenesis.We then examined the expression levels of the mitochondrial biogenesis pathway-related proteins SIRT1,PGC-1α,and NRF1/TFAM after SNI.The immunostaining results indicated that SIRT1 expression was downregulated in the spinal dorsal horn of the neuropathic pain group compared with the sham group (Figure 3AandB).Moreover,double immunostaining demonstrated that SNI decreased the SIRT1 expression in neurons (Figure 3C–E).Furthermore,the WB results revealed that SIRT1 (Figure 3F),PGC-1α (Figure 3G),NRF1 (Figure 3H),and TFAM (Figure 3I) expression were significantly decreased in rats with SNI,compared with sham rats.In addition,neuropathic pain significantly decreased the mtDNA copy number (Figure 3J).The results indicated that SNI suppressed SIRT1/PGC-1α and NRF1/TFAMmediated mitochondrial biogenesis in the spinal cord of rats.
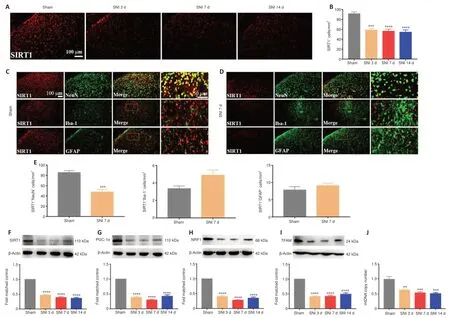
Figure 3|Spared nerve injury inactivates the SIRT1/PGC-1α pathway and impairs mitochondrial biogenesis.
Analgesic effects of TC-G 1008 in rats with SNI
To determine whether GPR39 activation ameliorated mechanical allodynia in rats with SNI,TC-G 1008 was administrated in rats with intrathecal injection.As shown inFigure 4AandB,a single intrathecal injection with 20 and 50 μg TC-G 1008 elevated the ipsilateral PWT,but not the contralateral PWT,4 hours after treatment,compared with the SNI+vehicle group.Next,we evaluated whether repeated administration of TC-G 1008 ameliorated neuropathic pain.The PWT results showed that multiple intrathecal administrations of 20 μg TC-G 1008 distinctly up-regulated the ipsilateral PWT,but not the contralateral PWT in rats with SNI (Figure 4CandD).We also found that early treatment with TC-G 1008 did not delay the development of SNI-induced mechanical allodynia (Figure 4EandF).Furthermore,locomotor activity was not affected by TC-G 1008,as determined by OFT (Figure 4G–I).These results confirmed that activation of GPR39 with TC-G 1008 mitigated SNI-induced mechanical allodynia.
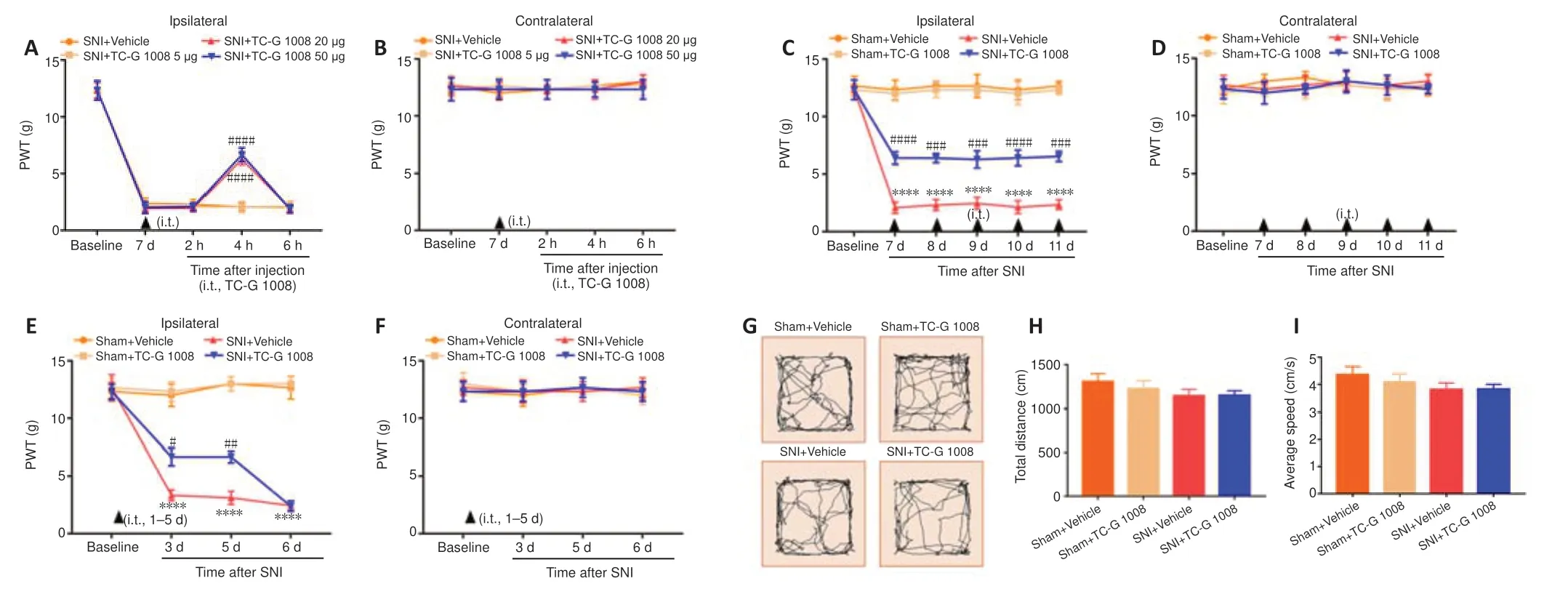
Figure 4|Analgesic effect of TC-G 1008 on neuropathic pain in rats with SNI.
TC-G 1008 promotes mitochondrial biogenesis and attenuates neuroinflammation through the SIRT1/PGC-1α pathway
SIRT1/PGC-1α played a crucial role in regulating mitochondrial biogenesis and inflammatory activities,and GPR39 could regulate the down-stream of SIRT1.But it was unclear whether activation of GPR39 could enhance mitochondrial biogenesis and alleviate neuroinflammation through the SIRT1/PGC-1α pathway in the spinal cord of rats with SNI.Immunofluorescence staining revealed that TC-G 1008 reversed SNI-induced down-regulation of SIRT1 in neurons (Figure 5AandB).In agreement with this,WB showed that administration of TC-G 1008 significantly augmented SIRT1 levels (Figure 5C)and rescued the decrease in PGC-1α (Figure 5D),NRF1 (Figure 5E),and TFAM(Figure 5F) expression caused by SNI.Moreover,TC-G 1008 counteracted the impairment of mitochondrial biogenesis observed in rats with nerve injury(Figure 5G).In addition,TC-G 1008 inhibited the IL-1β (Figure 5H),IL-6 (Figure 5I),and TNF-α (Figure 5J) secretion induced by neuropathic pain.The data demonstrated that activation of GPR39 promoted NRF1/TFAM-mediated mitochondrial biogenesis and attenuated neuroinflammation through the SIRT1/PGC-1α pathway in the spinal cord of rats with SNI.
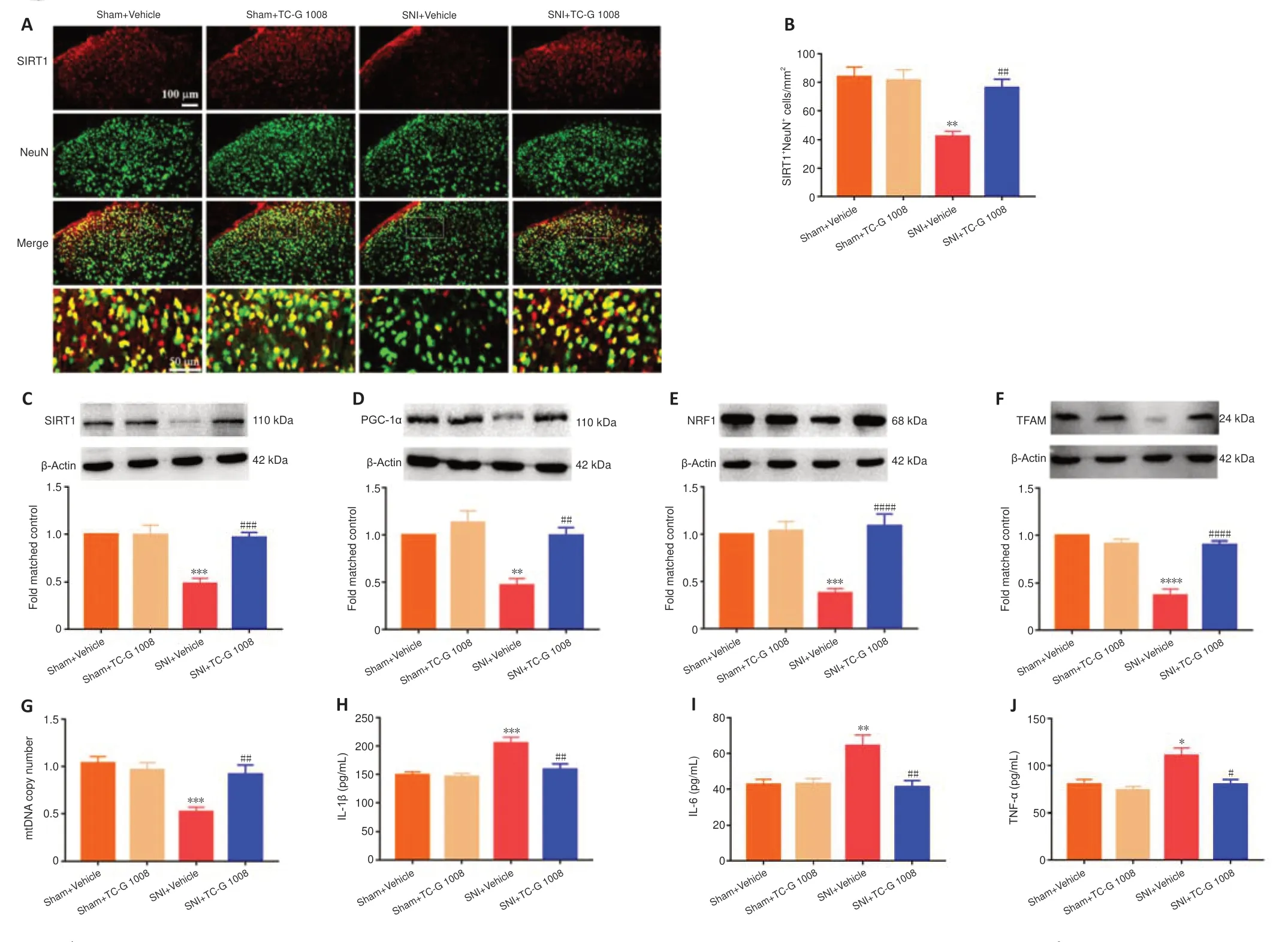
Figure 5|TC-G 1008 reverses neuropathic pain-induced impairment of mitochondrial biogenesis and neuroinflammation by activating the SIRT1/PGC-1α pathway.
The analgesic effects of TC-G 1008 in a rat model of neuropathic pain were abolished by GPR39 siRNA
Next,we used GPR39 siRNA to determine whether the analgesic effects of TC-G 1008 are mediated by GPR39.The qPCR results showed that administration of GPR39 siRNA suppressed GPR39 expression compared with the not treated (NT) rats (Figure 6A).Furthermore,the ipsilateral PWT results indicated that GPR39 siRNA reversed the mechanical allodynia induced by TC-G 1008 in rats with nerve injury (Figure 6BandC).In addition,GPR39 siRNA treatment did not affect locomotor activity,as assessed by OFT (Figure 6D–F).The data further confirmed that spinal GPR39 played a prominent role in regulating the development of neuropathic pain and activation of GPR39 dampened SNI-induced mechanical allodynia.
The beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation are blocked by GPR39 siRNA in rats with SNI
To further determine the role of GPR39 in neuropathic pain,GPR39 siRNA was used in this study.Double immunofluorescence staining showed that the increase in SIRT1 expression in neurons induced by TC-G 1008 was abolished by GPR39 siRNA (Figure 7AandB).Additionally,WB showed the increase in SIRT1 (Figure 7C),PGC-1α (Figure 7D),NRF1 (Figure 7E),and TFAM (Figure 7F) expression levels induced by TC-G 1008 was reversed by injection with GPR39 siRNA in rats with SNI.TC-G 1008 mitigated the impairment of mitochondrial biogenesis,whereas GPR39 siRNA abolished this effect (Figure 7G).However,GPR39 silencing reversed the improved neuroinflammation induced by TC-G 1008 in rats with SNI (Figure 7H–J).The data further demonstrated that activation of GPR39 promoted NRF1/TFAM-mediated mitochondrial biogenesis and attenuated neuroinflammation through the SIRT1/PGC-1α pathway in the spinal cord of rats with SNI.
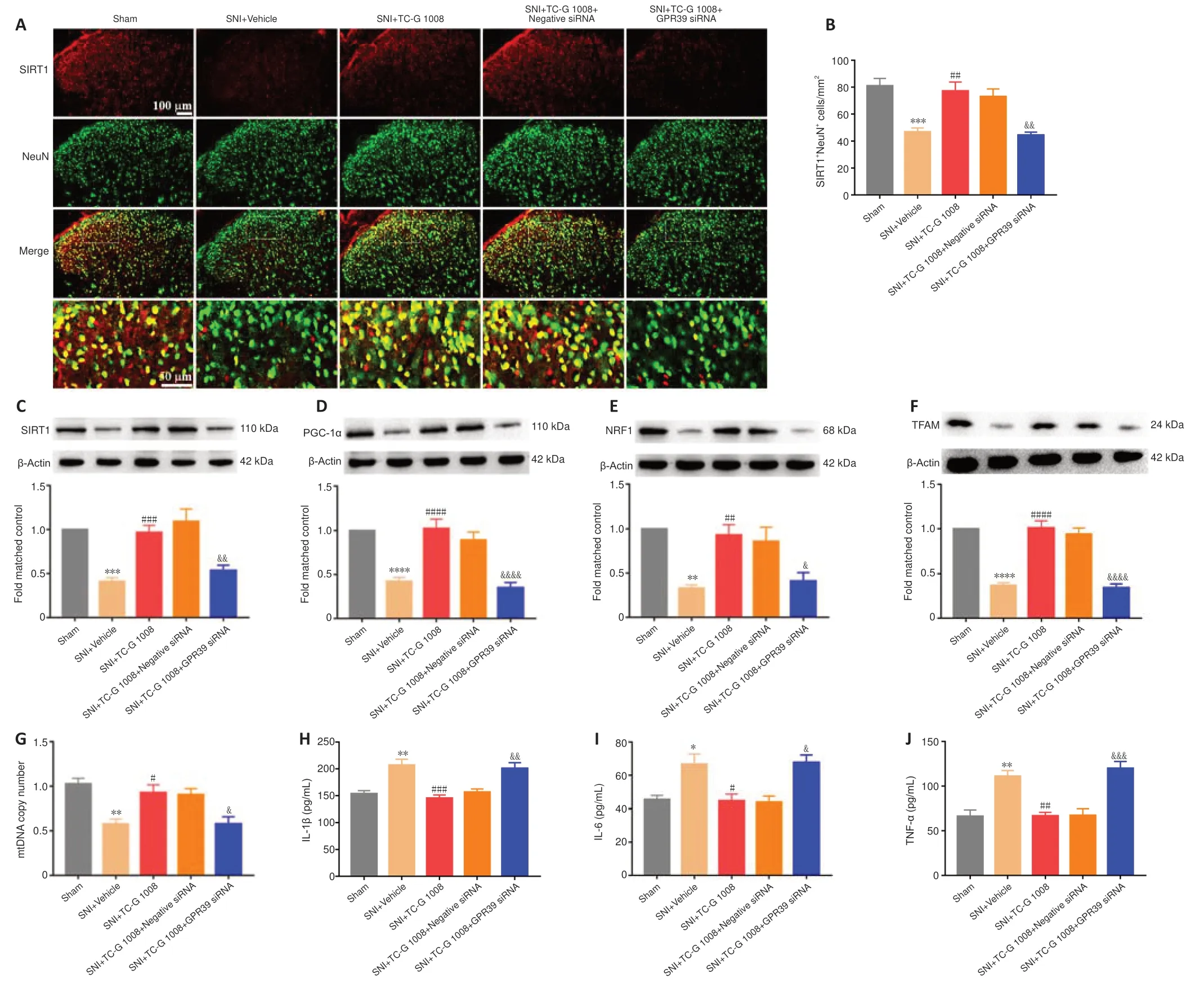
Figure 7|Beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation are abrogated by intrathecal injection with GPR39 siRNA in rats with SNI.
A SIRT1 antagonist reverses the improved neuropathic pain induced by TC-G 1008
SIRT1 restrained the progress of neuropathic pain.A SIRT1 inhibitor (Ex-527)was used to explore whether the analgesic effects of TC-G 1008 are mediated by SIRT1.The result demonstrated that Ex-527 reversed the increase in ipsilateral PWT induced by TC-G 1008 in rats with SNI (Figure 8AandB).Moreover,the OFT results demonstrated that locomotion was not affected by Ex-527 (Figure 8C–E).These results substantiated that GPR39 activation with TC-G 1008 alleviated mechanical allodynia caused by SNI by activating SIRT1.
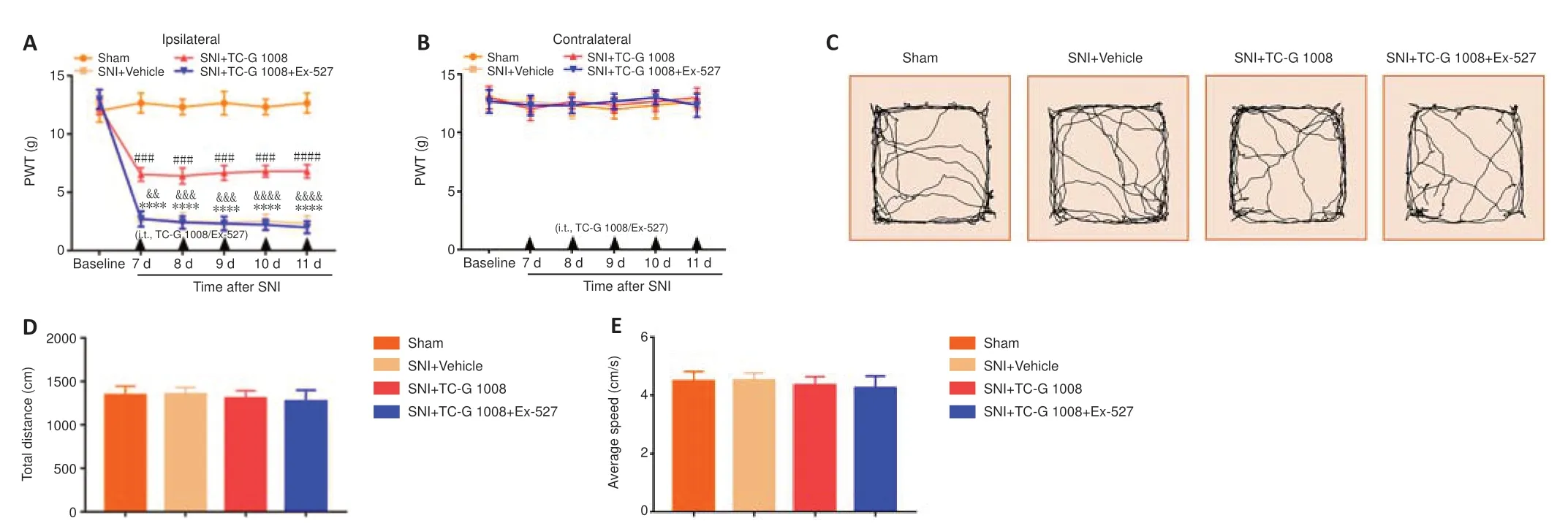
Figure 8|A SIRT1 antagonist reverses the TC-G 1008-induced improvement in neuropathic pain in rats after nerve injury.
The beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation are reversed by treatment with a SIRT1 antagonist
It was found that SIRT1 facilitated mitochondrial biogenesis and suppressed neuroinflammation.We then tested whether the effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation are mediated by SIRT1.First,we found that,compared with SNI+Vehicle,TC-G 1008 treatment upregulated GPR39 protein expression;however,Ex-527 treatment had no effect on the increase in GPR39 protein expression induced by TC-G 1008 in rats with SNI (Additional Figure 2A).Moreover,TC-G 1008 restored the decrease in PGC-1α (Figure 9A),NRF1 (Figure 9B),and TFAM (Figure 9C) expression;however,Ex-527 markedly reversed these changes.Moreover,TC-G 1008 mitigated impaired mitochondrial biogenesis;however,Ex-527 abolished this effect (Figure 9D).Furthermore,TC-G 1008 decreased IL-1β,IL-6,and TNF-α expression in the spinal cords of rats with SNI,but Ex-527 abrogated this change (Figure 9E–G).The data demonstrated that GPR39 activation with TC-G 1008 promoted NRF1/TFAM-mediated mitochondrial biogenesis and attenuated neuroinflammation through activating SIRT1 in the spinal cord of rats with SNI.
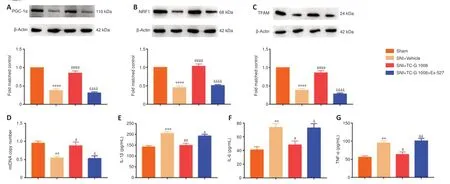
Figure 9|Ex-527 abrogates the beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation in rats with SNI.
PGC-1α siRNA dampens the analgesic effects of TC-G 1008 on neuropathic pain rats
It was confirmed that PGC-1α activation was conducive to attenuate neuropathic pain.PGC-1α siRNA was used to examine whether PGC-1α mediates the analgesic effect of TC-G 1008.The qPCR results demonstrated that administration of PGC-1α siRNA suppressed PGC-1α expression compared with NT rats (Figure 10A).Furthermore,the ipsilateral PWT results indicated that PGC-1α siRNA abolished the analgesic effects of TC-G 1008 (Figure 10B).Moreover,the contralateral PWT was not affected by administration of PGC-1α siRNA or negative siRNA (Figure 10C).In addition,on the basis of the OFT results,PGC-1α siRNA had no effect on rat motor function (Figure 10D–F).These results substantiated that GPR39 activation with TC-G 1008 alleviated SNI-induced mechanical allodynia through activating spinal PGC-1α.
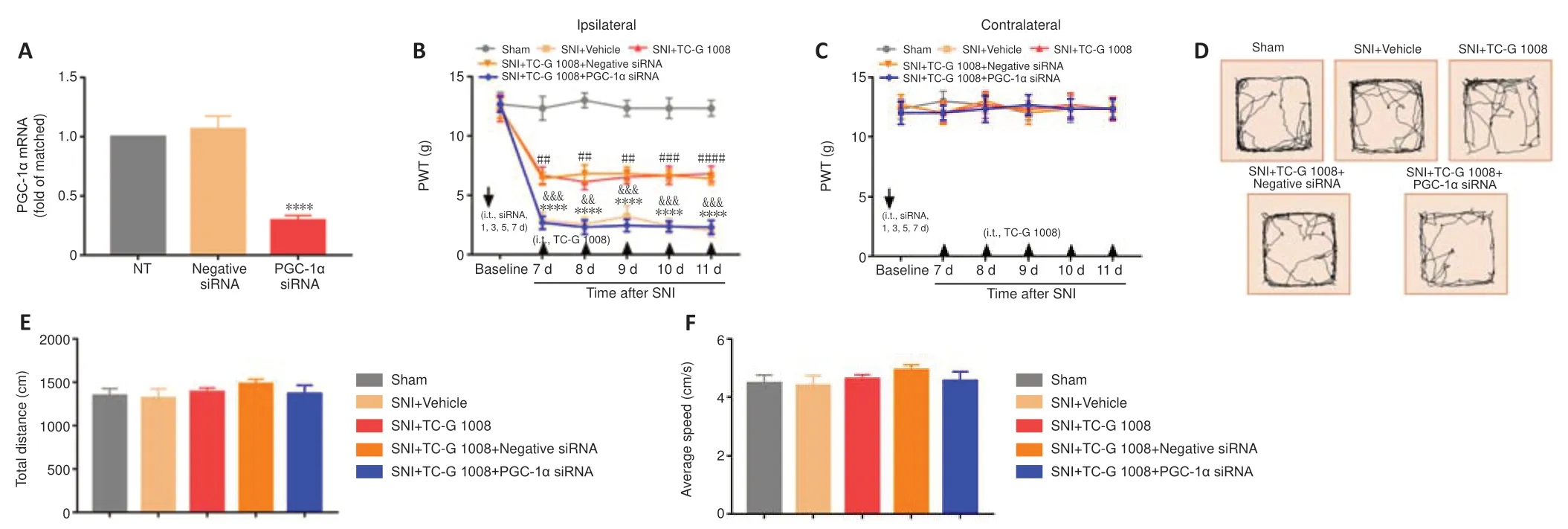
Figure 10|Beneficial effects of TC-G 1008 on mechanical allodynia are reversed by PGC-1α siRNA in rats with SNI.
PGC-1α siRNA reverses the beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation in rats with SNI
Activation of PGC-1α substantially redounded mitochondrial biogenesis and dampened neuroinflammation.PGC-1α siRNA was used to explore whether the effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation were dependent on PGC-1α.First,we found that PGC-1α siRNA or negative siRNA treatment did not affect the increase in GPR39 protein expression induced by TC-G 1008 in rats with SNI (Additional Figure 2B).The WB results showed that TC-G 1008 reversed the decrease in PGC-1α,NRF1,and TFAM expression induced by neuropathic pain,but that PGC-1α siRNA markedly abolished this effect (Figure 11A–C).PGC-1α siRNA abolished the enhanced mitochondrial biogenesis induced by TC-G 1008 in rats with nerve injury (Figure 11D).Furthermore,PGC-1α siRNA reversed the attenuation in neuroinflammation induced by TC-G 1008 in rats with SNI (Figure 11E–G).The data demonstrated that GPR39 activation with TC-G 1008 promoted NRF1/TFAM-mediated mitochondrial biogenesis and diminished neuroinflammation through activating PGC-1α in the spinal cord of rats with SNI.
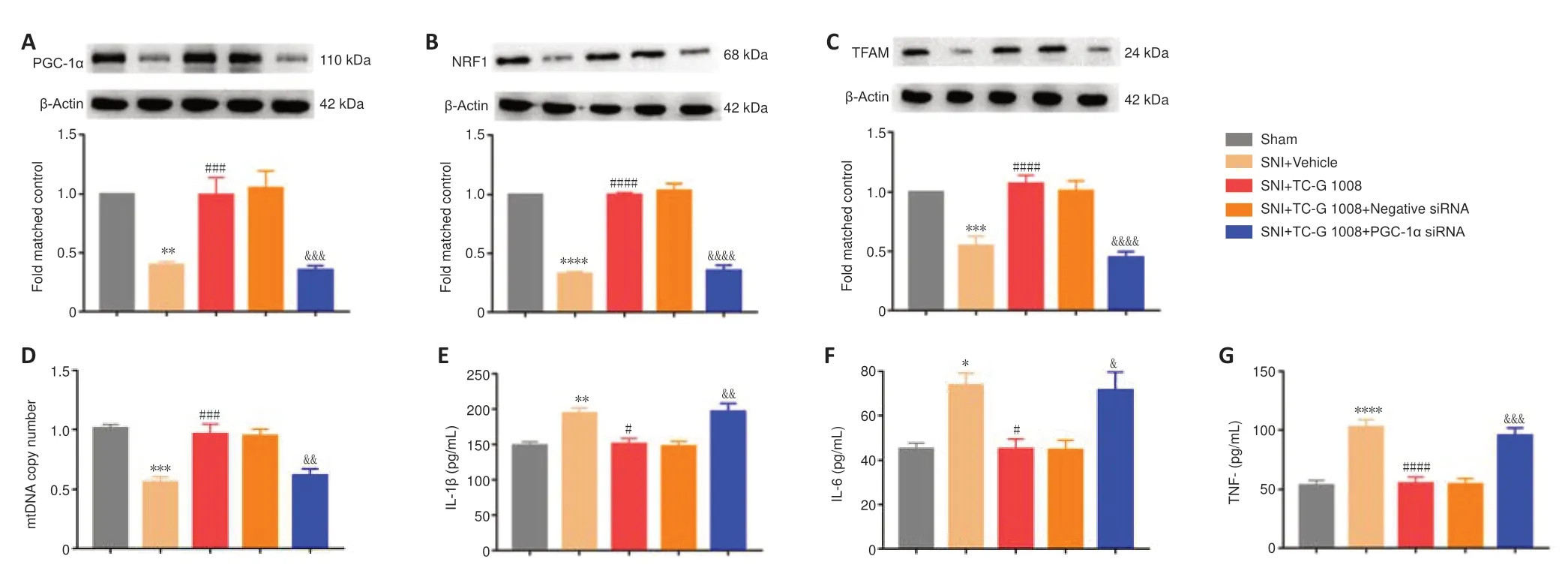
Figure 11|PGC-1α siRNA inhibits the beneficial effects of TC-G 1008 on mitochondrial biogenesis and neuroinflammation in rats with SNI.
Discussion
This study demonstrated that (1) activation of GPR39 by intrathecal administration of TC-G 1008 improved mechanical allodynia in rats with SNI;(2) TC-G 1008 promoted mitochondrial biogenesis and decreased neuroinflammation in rats with SNI by activating the SIRT1/PGC-1α pathway;and (3) GPR39 siRNA,SIRT1 antagonist,or PGC-1α siRNA reversed the improved mechanical allodynia,mitochondrial biogenesis,and neuroinflammation induced by TC-G 1008 in rats with SNI.In summary,our study shows that the activation of GPR39 with TC-G 108 improves mechanical allodynia by promoting mitochondrial biogenesis and ameliorating neuroinflammation through activation of the SIRT1/PGC-1α pathway in rats with SNI.
An increasing number of studies have indicated that GPR39 is expressed in the amygdala,hippocampus,and auditory cortex (Jackson et al.,2006;Egerod et al.,2007).Moreover,our qPCR and WB results demonstrated that GPR39 is expressed in the spinal cord of rats.Zn2+is a physiological agonist of GPR39 (Depoortere,2012).Activation of GPR39 (ZnR) by extracellular Zn2+triggers the expression of G protein α-subunit and G protein q-subunit/phospholipase C/inositol triphosphate (Rychlik and Mlyniec,2020).A previous study indicated that Zn2+attenuates thermal hyperalgesia in diabetic rats(Bădescu et al.,2014),promotes mitochondrial biogenesis in human dermal melanocytes (Rudolf and Rudolf,2017),and protects against cadmiuminduced oxidative stress (Pan et al.,2017).Moreover,GPR39 activation promotes wound healing (Zhao et al.,2015) and alleviates depression(Starowicz et al.,2019),inflammatory bowel disease (Pongkorpsakol et al.,2019),alcohol addiction (Cuzon Carlson et al.,2019),and hypoxic ischemic injury (Xie et al.,2021).Previous studies have indicated that activation of GPR39 with TC-G 1008 promotes osteoblast differentiation and mineralization(Chai et al.,2019),enhances tight junction assembly in intestinal epithelial cells through the phospholipase C/calmodulin-dependent protein kinase kinase β/AMP-activated protein kinase pathway (Pongkorpsakol et al.,2019),and attenuates neuroinflammation through the SIRT1/PGC-1α/Nrf2 pathway(Xie et al.,2021).Our results suggest that SNI decreased GPR39 expression in spinal neurons and microglia.Moreover,we found that TC-G 1008 attenuated mechanical allodynia in rats with nerve injury.GPR39 siRNA abrogated these effects.Consistent with previous studies,this study indicated that GPR39 activation strengthened mitochondrial biogenesis and ameliorated neuroinflammation.Furthermore,in this study,we demonstrated that spinal GPR39 played a critical role in regulating the process of neuropathic pain and activation of GPR39 alleviated mechanical allodynia in rats with SNI.
SIRT1 (an NAD+-dependent histone deacetylase) regulates a variety of cellular processes,including inflammation,aging (Herskovits and Guarente,2014).SIRT1 activation contributes to the treatment of neuropathic pain (Zhou et al.,2017a).GPR39 activation has been reported to attenuate neuroinflammation by interacting with SIRT1 (Xie et al.,2021).Furthermore,some studies have indicated that SIRT1 dampens inflammation (Xie et al.,2021;Zhang and Wu,2021),as well as promoting mitochondrial biogenesis by interacting with PGC-1α (Guo et al.,2014;Zhou et al.,2017b;Wang et al.,2019).Consistent with previous studies,our results show that SNI induced a decrease in SIRT1 expression in neurons.Additionally,we found that GPR39 activation reversed the decrease in SIRT1 levels induced by SNI and ameliorated mechanical allodynia by promoting mitochondrial biogenesis and attenuating neuroinflammation,while these changes were reversed by administration of a SIRT1 antagonist.This study confirmed that spinal SIRT1 mediated the effect of GPR39 on mechanical allodynia,mitochondrial biogenesis,and neuroinflammation in rats with SNI.
Impaired mitochondrial biogenesis leads to ATP depletion,oxidative stress,and cell apoptosis (Lin and Beal,2006).Mitochondrial biogenesis,fusion,and fission have been shown to regulate mitochondrial homeostasis (Vyas et al.,2016).PGC-1α (an important transcription co-activator) is the primary factor inducing mitochondrial biogenesis (Zhu et al.,2013).Previous studies have suggested that disruption of mitochondrial biogenesis exacerbates neuropathic pain,and that PGC-1α activation ameliorates mechanical allodynia (Chen et al.,2021;Sun et al.,2021,2022;Zhang et al.,2022b).NRF1 has been shown to promote nucleo-mitochondrial interactions and mitochondrial biogenesis (Hickson-Bick et al.,2008).TFAM,a regulator of mitochondrial genes,plays a critical role in mtDNA maintenance,mitochondrial dynamics,reactive oxygen species (ROS) scavenging,and cell survival (Ekstrand et al.,2004).More importantly,substantial evidence indicates that PGC-1α is essential for promoting mtDNA transcription and replication through its interaction with NRF1 and TFAM (Santos et al.,2011;Fan et al.,2021).Recent evidence shows that PGC-1α exerts neuroprotective effects by inhibiting neuroinflammation (Yang et al.,2017;Wang et al.,2018;Liu et al.,2019;Han et al.,2021).In this study,we found that SNI decreased PGC-1α,NRF1,and TFAM expression and decreased mtDNA copy number.Furthermore,GPR39 activation with TC-G 1008 ameliorated mechanical allodynia and reversed the decrease in PGC-1α,NRF1,and TFAM expression and mtDNA copy number,attenuating neuroinflammation,whereas PGC-1α siRNA abrogated these changes.Indeed,PGC-1α activation contributed to spark NRF1/TFAM-dependent mitochondrial biogenesis,which played a crucial role in regulating the process of neuropathic pain.Moreover,this study substantiated that PGC-1α mediated the effect of GPR39 on mechanical allodynia,mitochondrial biogenesis,and neuroinflammation in rats with SNI.
Despite these positive results,our study had some limitations.The use of transgenic mice may be helpful in assessing the role of GPR39 in the development of neuropathic pain.Moreover,our study focused on the role of GPR39 in mitochondrial biogenesis and neuroinflammation in rats with SNI.However,the relationship between GPR39,autophagy,and redox imbalance in the context of neuropathic pain is worth investigating.
Collectively,our findings show that GPR39 activation with TC-G 1008 ameliorated mechanical allodynia by triggering NRF1/TFAM mediatedmitochondrial biogenesis and dampening neuroinflammation through activation of the SIRT1/PGC-1α pathway.These results suggest that GPR39 is a potential therapeutic target for relieving mechanical allodynia after SNI.
Acknowledgments:The authors thank the Experimental Animal Center of Tongji Hospital for raising rats.
Author contributions:WM conceived the study;LZ designed the study;XTand FS provided experimental support;DL,JW,SG,JS,DL,and YZ analyzed and interpreted the data.LZ wrote the paper.All authors have read and approved the final manuscript.
Conflicts of interest:The authors have no conflicts of interest to declare.
Data availability statement:All data relevant to the study are included in the article or uploaded as Additional files.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Valeria Bortolotto,University of Piemonte OrientaleNovara,Italy.
Additional files:
Additional Figure 1:The schedule of this study.
Additional Figure 2:GPR39 protein levels in rats treated with TC-G 1008 after SIRT1 antagonist and PGC-1α siRNA.
Additional file 1:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
- Treatment with β-sitosterol ameliorates the effects of cerebral ischemia/reperfusion injury by suppressing cholesterol overload,endoplasmic reticulum stress,and apoptosis
