Targeting calcium signaling in Alzheimer’s disease: challenges and promising therapeutic avenues
2024-02-13LinLinSongYongPeiTangBettyYuenKwanLaw
LinLin Song ,YongPei Tang ,Betty Yuen Kwan Law
The critical role of calcium dyshomeostasis in the pathogenesis of Alzheimer’s disease (AD):AD is a progressive neurodegenerative disease characterized by cognitive decline,memory impairment,and behavioral changes.With an estimated 50 million people being affected worldwide,the incidence of AD is constantly increasing globally.The hallmark of AD is the accumulation of amyloid-beta protein (Aβ) in the form of amyloid plaques and hyperphosphorylated tau protein in the form of neurofibrillary tangles.However,increasing evidence suggests that calcium ion (Ca2+) dysregulation also plays a crucial role in the pathogenesis of AD (Calvo-Rodriguez and Bacskai,2021).As a key second messenger,Ca2+regulates a wide range of cellular processes,including the release of neurotransmitters,gene expression,and cell death.Ca2+also regulates the activity of Calcium/calmodulin-dependent protein kinase II,which is critical for synaptic plasticity,learning,and memory (Kaushik et al.,2022).Alternation in the Ca2+signal is an early event in the pathogenesis of AD,which can lead to synaptic dysfunction,neuronal loss,and cognitive impairment.Therefore,dysregulated Ca2+level has a significant impact on the normal function and survival of neurons.The complex interplay among ion channels,pumps,and exchangers maintains intracellular Ca2+levels within a steady range in neurons.Voltage-gated Ca2+channels facilitate Ca2+entry into the cell,which in turn triggers neurotransmitter release at the presynaptic terminal,allowing electrical signals to propagate throughout the brain.In AD,this delicate balance is disrupted partially via the increased level of Aβ oligomers,resulting in excessive Ca2+influx into the cytosol,which may initiate a cascade of events ultimately leading to mitochondrial dysfunction and neuronal cell damage.Recent reports have confirmed that several Ca2+pumps and exchangers responsible for maintaining intracellular Ca2+balance were altered in AD.For example in the brain of AD patients,the expression and function of plasma membrane Ca2+-ATPase and Na+/Ca2+exchanger were reduced,leading to elevated levels of intracellular Ca2+(Calvo-Rodriguez and Bacskai,2021).It has also been found that aberrant Ca2+signaling in AD is caused by the over-activation of ryanodine receptors (RyRs) (Lacampagne et al.,2017) or inositol 1,4,5-trisphosphate receptors(Takada et al.,2017) in the endoplasmic reticulum,leading to excessive Ca2+release into the cytosol.Of note,there are other reported ion channels involved in the progression of AD,including Zn2+,Fe3+,Cu2+and K+channel (Wang and Wang,2017)(Figure 1A).Recent findings support an upstream role for calcium dysregulation in memory decline associated with AD,and clearly implicate that limiting RyR2 opening time prevents and rescues neuronal hyperactivity,memory impairment,and neuronal loss,even in late AD (Yao et al.,2020).Coincidentally,our experiments also confirmed this hypothesis.We found that an increase in Aβ level can cause an elevation of intracellular calcium level by activating RyR2 channels on the endoplasmic reticulum,which further induces mitochondrial dysfunction through the endoplasmic reticulum-mitochondrial signaling cascade leading to neuronal cell damage (Song et al.,2023).
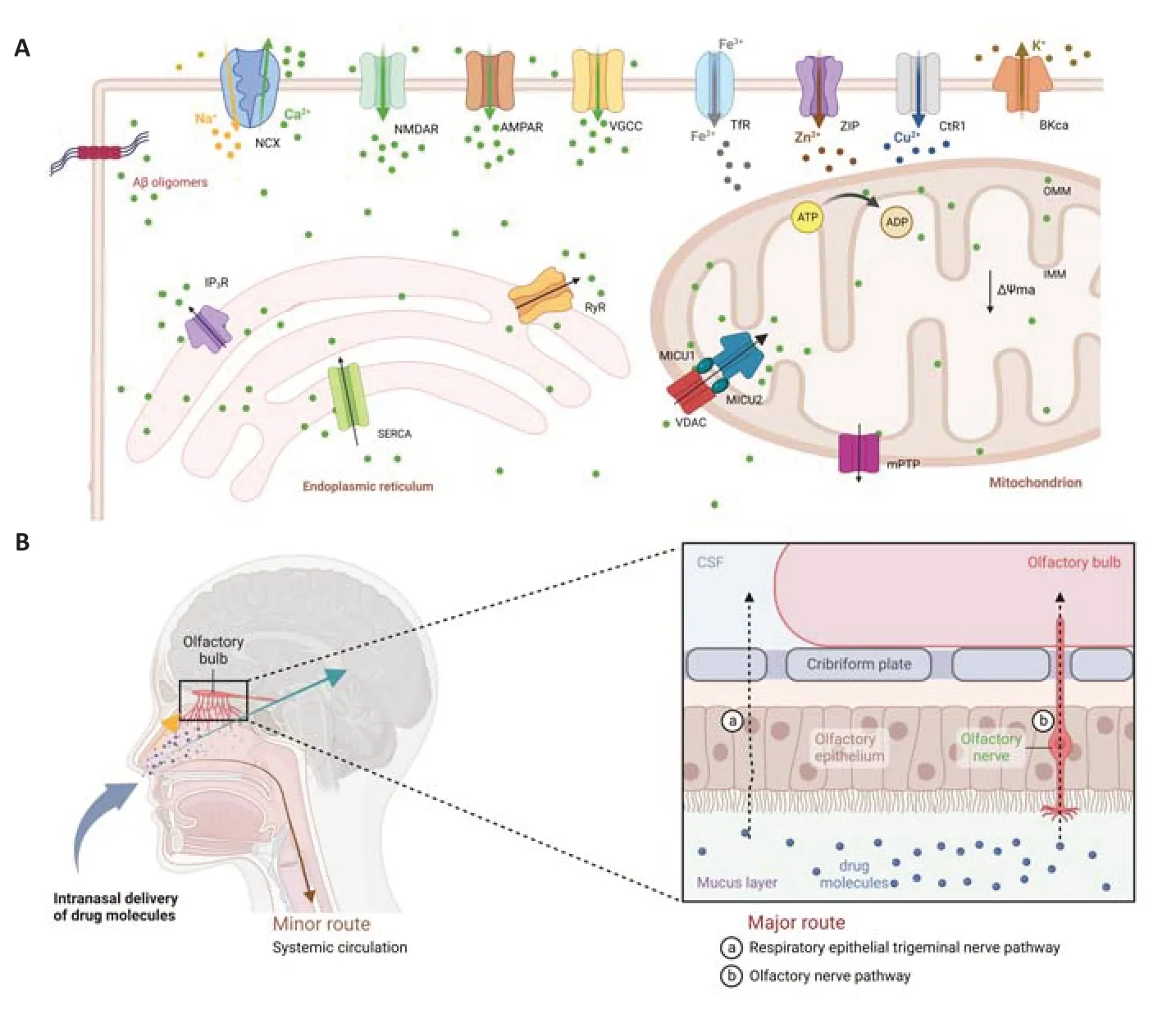
Figure 1|Diagram of the ion channel and the mechanism of intranasal drug delivery.
Furthermore,Ma et al.(2021) found a positive correlation between serum calcium and cerebrospinal fluid-Aβ pathology.This relationship between serum calcium and Aβ pathology was further validated using Aβ-positron emission tomography imaging,demonstrating a positive association with whole-brain amyloid deposition.However,further research is needed to consider calcium dysregulation as a potential biomarker or therapeutic target for AD.Calcium live imaging techniques,such asin vivomultiphoton microscopy and fiber photometry,offer innovative methods to measure brain Ca2+changes in aging or adult mouse models.However,these calcium imaging techniques are currently limited to animal models,and their high cost hinders their widespread use in research.Furthermore,more advancedin vivoimaging techniques are required for practical application in clinical settings.In summary,calcium dysregulation is considered an important factor in the pathogenesis of AD,as evidenced by the positive correlation between serum calcium and cerebrospinal fluid-Aβ pathology,as well as whole-brain amyloid deposition.Targeting calcium signaling holds promise as a potential therapeutic approach for AD.Continued research is necessary to further elucidate the complex role of calcium in AD and to develop new strategies for regulating calcium balance to treat this devastating neurodegenerative disease.
Prospects for the use of calcium signalingtargeted pharmaceuticals in the treatment of AD:Over the past two decades,the drug development process for AD has been found to be challenging and debatable.Despite the decreasing acceptance of the Aβ hypothesis,amyloidtargeted therapy remains the primary strategy in the AD pharmaceutical development pipeline,which is becoming increasingly diversified in terms of targets,therapeutic approaches,and efficacy.Currently,the majority of candidate compounds for AD are developed via targeting Aβ,tau,or inflammation,but with relatively limited medicines targeting calcium signaling.Small molecule compounds and monoclonal antibodies remain the significant primary candidates for the development of AD medicine (van Bokhoven et al.,2021).For example,Aducanumab is a monoclonal antibody drug that has been highly controversial.It selectively targets pathological oligomeric and fibrillar forms of Aβ.However,there is insufficient evidence to suggest that it can slow down or halt the progression of AD,and it is associated with the side effect of cerebral edema (Vaz et al.,2022).Due to the attention garnered by the approval of Aducanumab,the Food and Drug Administration(FDA) has recently granted breakthrough status to two future AD treatment approaches: Donanemab by Eli Lilly and Lecanemab by Biogen.Lecanemab has already received FDA approval,with its effect in selectively binding to large soluble Aβ fibrils,reducing Aβ fibrils in the brain and cerebrospinal fluid (Decourt et al.,2021).Donanemab,on the other hand,reduces amyloid plaques in AD by targeting Aβ (p3–42),and its clinical studies are expected to be completed by 2024 (Decourt et al.,2021).Recent studies have also explored several new therapeutic targets by attenuating calcium dysregulation-mediated downstream deleterious effects such as oxidative stress and mitochondrial dysfunction.Some antioxidant and mitochondrialtargeted drugs have shown promising efficacy in preclinical studies and are undergoing clinical trial evaluations (Park et al.,2021).In terms of AD drugs that regulate calcium signaling,memantine is worth mentioning.Memantine is one of the two FDA-approved drugs to treat AD patients and the only N-methyl-D-aspartate receptor antagonist,which preferentially blocks excessive(pathological/extrasynaptic) N-methyl-D-aspartate receptor activity while relatively sparing normal(physiological/synaptic) activity.Memantine restricts excessive Ca2+influx,thus reducing neuronal excitotoxicity.Unfortunately,memantine can only be approved for the treatment of patients with moderate-to-severe AD and offers only palliative benefits to patients (Figueiredo et al.,2013).
Our study discovered that hyperoside (HYP),a naturally occurring compound derived from medicinal plants,can directly bind to Aβ and reduce Aβ aggregation.By regulating RyR2,HYP maintains the Ca2+balance in the endoplasmic reticulum-mitochondrial signaling cascade,thereby restoring neuronal vitality.Moreover,HYP demonstrated the ability to attenuate shortterm memory impairment and hippocampusdependent learning deficits in APP/PS1 doubletransgenic mice.Furthermore,we conducted preliminary toxicity assessments of HYP and found no adverse effects.However,further in-depth research is warranted to provide a comprehensive evaluation of its safety profile.These findings offer potential avenues for the clinical application of HYP,but additional investigations are necessary for a more thorough understanding.Although the development of calcium signaling targeting natural compounds for AD represents a promising therapeutic approach,the prerequisite for compounds that can selectively target specific calcium channels or pumps without disrupting normal neuronal cell function is required.As the calcium channel is ubiquitously expressed in the body,for example,RyR2 is expressed in the brain and heart;sarco/endoplasmic reticulum Ca2+-ATPase mainly exists in the sarcoplasmic reticulum and heart;gated voltage channels are distributed in skeletal muscle,cardiac muscle,and neurons,etc.,the potential off-target systemic effects of the candidate compounds still worth further investigation.Additionally,due to the heterogeneity of AD pathology and the multifactorial nature of the disease,a combinational therapeutic approach targeting multiple targets of the disease may be necessary.Despite these challenges,the development of calcium signaling-targeted drugs is still an important area for the pharmacological research of AD.In summary,the development of calcium signaling-targeted medicine represents a promising avenue for AD.However,further research is still required to characterize the protective mechanisms involved in targeting calcium signaling without compromising normal neuronal and systemic cellular function.
The potential application of nasal drug delivery for combinational therapy in Alzheimer’s disease:The high failure rate in the development of drugs for AD can be attributed to several factors.One reason is the complexity and heterogeneity of the disease,which makes it difficult to identify specific drug development targets.Another reason is the incomplete understanding of the underlying causes of AD,which poses a challenge to the development of effective drugs that can target the disease.Currently,the treatment for AD is limited only to a few orally administered drugs approved by the FDA,which mainly act on the symptoms (from mild to moderate) without ameliorating the pathogenesis of the disease.In addition,the blood-brain barrier presents an extra challenge to the treatment of AD as it can impede the penetration of drugs into their intended targets inside the brain.This challenge has led to the exploration of various novel drug delivery strategies,such as using nanoparticles,liposomes,and other nano-carrier systems to help drugs penetrate the blood-brain barrier and enter the brain.Nasal drug delivery is a non-invasive drug administration method that exploits the highly vascularized mucosa lining of the nasal cavity to absorb drugs,either directly into the brain via the olfactory nerve or into the systemic circulation(Figure 1B).Compared to other delivery routes,nasal drug delivery offers several advantages,especially for drugs that need to reach the brain.For example,it provides a direct and rapid way to the brain by bypassing the blood-brain barrier and avoiding the first-pass effect in the liver,resulting in a higher concentration of available drugs that can reach the brain with reduced systemic exposure,thus increasing safety by reducing offtarget side effects with higher targeted efficacy(Lee and Minko,2021).Onzetra Xsail®,a marketed formulation approved for intranasal administration for the treatment of brain disorders,offers better migraine relief and statistically significantly fewer adverse effects than its conventional oral counterpart (Cady,2015).Together with the success of intranasal delivery of Onzetra Xsail®,our research has confirmed that nasal drug delivery can prolong the half-life of HYP,slow down the elimination rate,and significantly increase its bioavailability.Therefore,nasal drug delivery for treating AD and other neurodegenerative diseases should be further evaluated and advocated.However,nasal drug delivery also has limitations,for example,since the nasal mucosa is sensitive,repeated exposure to certain drugs or delivery tools can cause irritation or damage.In addition,drug absorption via the nasal cavity is unstable and affected by several factors such as nasal congestion,drug solubility,and mucosal clearance(Agrawal et al.,2018).Now,most of the research works related to nasal-to-brain delivery of braintargeting drugs are limited to preclinical studies,and only a few of them such as the Esketamine hydrochloride anti-depression nasal spray have received market approval.In summary,nasal drug delivery holds promise as a viable method for delivering drugs to the brain and treating neurological disorders such as AD,but further research is needed to fully evaluate its efficacy and safety with optimization on drug formulations and pharmacokinetic parameters.
This work was supported by grants from the Macao Science and Technology Development Fund(Project code: 002/2023/ALC) and Foshan Medicine Dengfeng Project of China 2019–2021 (to BYKL).
LinLin Song#,YongPei Tang#,Betty Yuen Kwan Law*
Neher’s Biophysics Laboratory for Innovative Drug Discovery,State Key Laboratory of Quality Research in Chinese Medicine,Macau University of Science and Technology,Macao Special Administration Region,China
*Correspondence to:Betty Yuen Kwan Law,PhD,yklaw@must.edu.mo.
https://orcid.org/0000-0002-8926-3960(Betty Yuen Kwan Law)
#Both authors contributed equally to this article.
Date of submission:April 26,2023
Date of decision:May 30,2023
Date of acceptance:June 13,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380898
How to cite this article:Song L,Tang Y,Law Y(2024) Targeting calcium signaling in Alzheimer’s disease: challenges and promising therapeutic avenues.Neural Regen Res 19(3):501-502.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
