On-chip physiological mimicry of neurovascular unit: challenges and perspectives
2024-02-13SongIhAhnYongTaeKim
Song Ih Ahn ,YongTae Kim
Neurological disorders including neurodegenerative diseases,brain tumors,and stroke are the second leading cause of death and the greatest cause of disability worldwide.However,it remains challenging to achieve effective drug delivery to the central nervous system for treatments of neurological diseases due to the blood-brain barrier (BBB).The function of the BBB is regulated by the physiological interactions between various types of cells in the neurovascular unit (NVU).In the NVU,the brain vasculature of the BBB is surrounded by brain pericytes,brain astrocytes,neurons,and microglia(Figure 1).Moreover,the NVU at the levels of arteries and veins includes contractile smooth muscle cells (Schaeffer and Iadecola,2021).
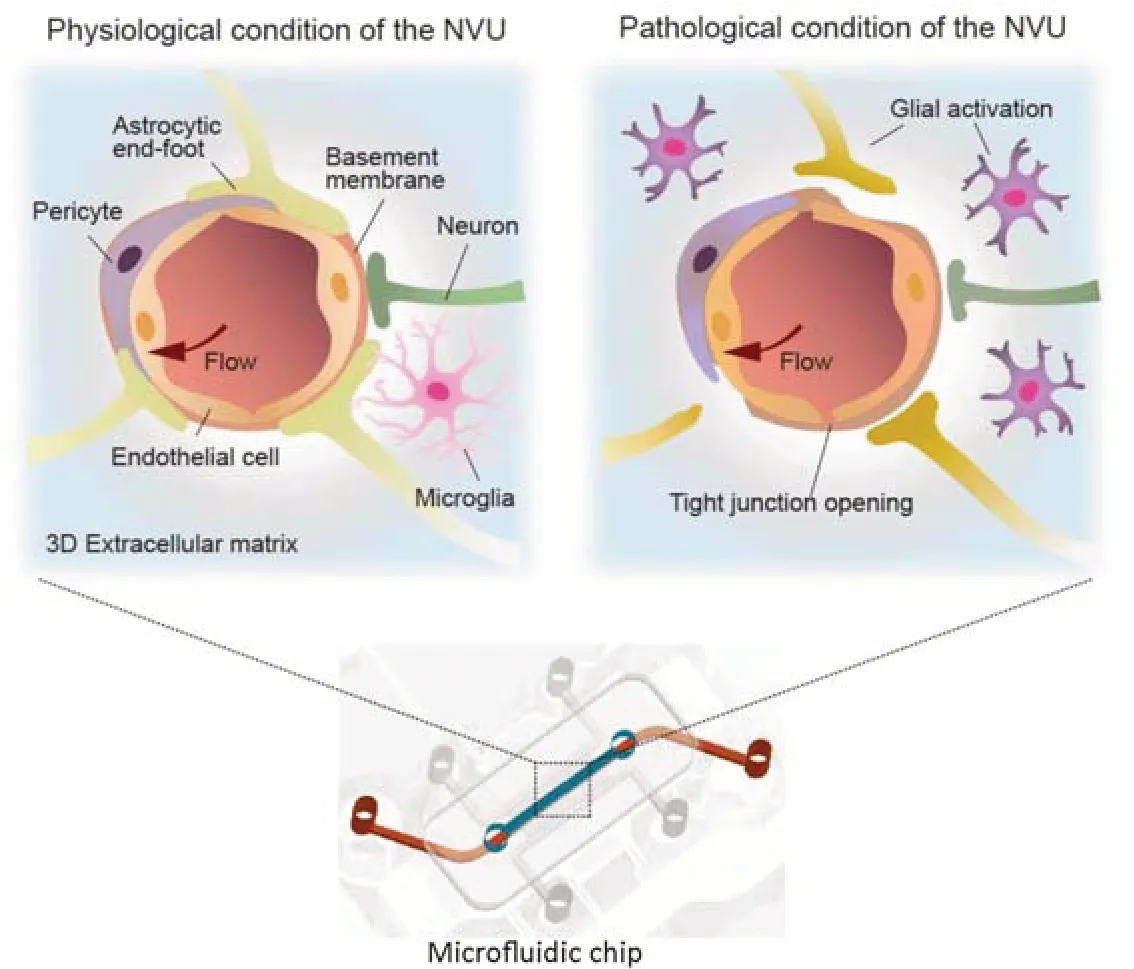
Figure 1|Schematic description of the physiological and pathological conditions of the neurovascular unit (NVU)reconstructed on a chip.
There has been significant research to investigate the multicellular network of the NVU and to understand the drug transport mechanisms at the BBB.However,it is difficult to investigate the accurate BBB physiology in such simple models as Transwell in which brain endothelial cells are cultured in a static two-dimensional microenvironment.Moreover,unsuccessful clinical trials have demonstrated the limitations of animal models in modeling the pathophysiology of human neurological diseases.
In the last decade,in vitroorgan-on-a-chip models have shown a great potential to explore the pathophysiology and therapeutics of brain diseases by mimicking the pathological conditions of the multicellular microenvironment of the NVU(Caffrey et al.,2021).
On-chip physiological mimicry of neurovascular unit:The NVU is a functional unit of the brain that underscores the structural and functional interconnection between the BBB,neuronal,and non-neuronal cells in the brain.Significant attempts have been made to mimic the structure and function of the NVU on chips.Especially,several types of NVU component cells were cocultured in controlled microchannels that can closely recapitulate the brain microenvironment.In general,brain endothelial cells are cultured to form a tight brain endothelium which expresses a high level of tight junctions (Zlokovic,2008),where brain pericytes and astrocytes are co-cultured to support the vascular integrity and function.Recent studies have recapitulated the three-dimensional(3D) cellular networks in the perivascular spaces of the NVU on chips (Ahn et al.,2020;Jang et al.,2022).Astrocytes cultured in a 3D hydrogel have shown their end feet extended to the brain endothelium (Ahn et al.,2020).Neurons or microglia can be also co-cultured on chips to recreate their interface with the brain vessels(Pediaditakis et al.,2021).
Physico-chemical microenvironments of the brain have also been established to reconstitute the NVU on chips.Taking advantages of a microfluidicbased system,the perfusion of culture medium into a vascular channel has enabled to mimic the blood flow in the brain vessels (Ahn et al.,2020;Pediaditakis et al.,2021).Perivascular spaces were filled with hydrogels that mimic the brain extracellular matrix.In a recent study,the decellularized extracellular matrix from brain tissue has been used to provide a native microenvironment with brain-specific properties(Yi et al.,2019).
In vitro neurovascular unit models for disease modeling:Under the pathological process in the brain,the NVU undergoes profound changes,including glial activation,tight junction opening and changes in transport systems,resulting in dysregulation of the BBB function (Figure 1).To understand the interactions between the dysregulated BBB and potential therapeutics,pathological microenvironments and dysfunction of NVU components need to be precisely recapitulated (Caffrey et al.,2021).Recent studies have constructed neurological disease models on chips by introducing pathological factors into NVU chips.Neurodegenerative diseases,such as Alzheimer’s disease (AD) and Parkinson’s disease(PD),have been reconstructed by introducing neurotoxic proteins into the NVU chip models.These NVU chips have been used to understand the pathomechanisms of AD and PD which remain largely unknown.A recent study has investigated a molecular mechanism of hyperglycemic-induced AD by mimicking the diabetic microenvironment in the brain on an NVU chip (Jang et al.,2022).An NVU chip under hyperglycemia conditions showed BBB disruption,resulting in dysregulated transport of amyloid beta.The hyperglycemia conditions induced the most common features of AD like the accumulation of amyloid beta and phosphorylated Tau in neurons in the chip,indicating that hyperglycemia induces AD pathogenesis at the NVU.PD is characterized by a loss of the dopaminergic neurons in the substantia nigra,a part of the midbrain.A substantia nigra brain chip was developed to replicate the NVU in human PD brains by culturing human dopaminergic neurons from the substantia nigra (Pediaditakis et al.,2021).This model recapitulated synucleinopathy by incorporating alpha-synuclein fibril.Especially,the key pathological characteristics of PD,including phosphorylated alpha-synuclein accumulation,mitochondrial dysfunction,and neuronal death,have been observed.More importantly,the critical roles of microglia in the pathogenesis of neurodegenerative diseases have been appreciated by studies that reproduced the microglia-mediated neuroinflammation on chips.An NVU chip that incorporates the brain endothelium,pericytes,astrocytes,microglia,and cortical neurons has been developed to investigate the association between neuroinflammation,neurodegeneration,and neuronal death(Pediaditakis et al.,2022).In this model,tumor necrosis factor-alpha,a key inflammatory cytokine,was added either through the brain channel or via the vascular channel to characterize tissueor systemic-induced inflammation.In both cases,exposure to tumor necrosis factor-alpha induced activation of microglia and astrocytes,followed by major hallmarks of neuroinflammation,such as the BBB disruption,increased secretion of proinflammatory cytokine,and neuronal damage.
Robust human cell sources of functional NVUspecific cells have enabled the establishment of reliable NVU models which are predictive of human clinical results.Co-culture of human neural stem cells with brain endothelial cells in a chipinduced spatially regulated NSC differentiation that mimics cellular morphogenesis in the human brain (Kim et al.,2021).Moreover,the model has been utilized to study the mechanism of fungal brain infection by adding the human fungal pathogenC.neoformans.This fungal brain infection model has enabled the real-time observation and quantification of BBB-microbial interactions and neurotropism.A patient-specific glioblastoma model has been developed by culturing glioblastoma cells obtained from patients in an NVU chip (Yi et al.,2019).With the tumor cells isolated from patients,the glioblastoma chip could reproduce the patient-specific resistance to therapeutics observed in clinical trials.Moreover,the glioblastoma-on-a-chip showed its potential for use in the high-throughput point-of-care testing.
In vitro neurovascular unit models for drug screening:Functional NVU chip models can then be used for predicting therapeutic efficacy,toxicity,and interactions at the BBB.A BBB chip with astrocytes cultured in a 3D microenvironment has shown its potential to be used for quantification of drug distribution across the BBB (Ahn et al.,2020).This platform has enabled the 3D mapping of nanoparticles in both tissue and cellular levels.Moreover,the nanoparticle transport mechanism over the BBB could be identified at a molecular level.Another NVU-on-a-chip model was developed to study the interactions between the NVU cells and therapeutic stem cells,which have emerged as a powerful therapeutic strategy for neurological diseases (Lyu et al.,2021).Especially,the peri-infarct zone of ischemic stroke was recapitulated on the chip by establishing the ischemic condition with depletion of serum and glucose for 24 hours.The ischemic stroke NVU model has then enabled to track the therapeutic stem cell infiltration and to evaluate the neurorestorative effects of the stem cell therapy.In another study,a hypoxia-induced BBB chip model showed increased barrier function with significant increases in the mRNA levels for genes encoding the transport proteins highly expressed at the BBBin vivo(Park et al.,2019).This BBB chip exhibitedin vivo-like shuttling of targeting peptides,nanoparticles,and antibodies across the BBB.
The NVU controls brain metabolic homeostasis by regulating the dynamic influx and efflux of molecules and drugs between the brain vasculature and the parenchyma.The metabolic interactions between the BBB and neurons in the brain parenchyma have been investigated using a linked organ-on-a-chip system that links an influx BBB chip,a brain chip,and an efflux BBB chip (Maoz et al.,2018).This model was used to study the effect of the psychoactive drug methamphetamine on the metabolic pathways in the NVU.
Challenges and perspectives:Various types of human NVU-on-a-chip models have been developed to establish the physiological function and structure of the human NVU.As one of their major applications,these models are used to recapitulate the pathological features of neurological diseases for studying pathophysiology and therapeutic strategies of neurological diseases.The on-chip NVU disease models allow for realtime monitoring of the NVU pathophysiology in a spatiotemporally controlled manner.Moreover,the models can be used for screening of potential therapeutic candidates.
Despite remarkable progress being made in the NVU-on-a-chip technology,several challenges remain to be accomplished for successful clinical translation in neurological diseases.For its application in a wide range of fields,robust fabrication and experimental protocols need to be established.Recently,advanced technologies of device fabrication,such as 3D printing and injection molding,have enabled high-throughput fabrication of the devices (Kim et al.,2023).The manufactured chips with standardized protocols may reduce the chip-to-chip variations and provide user-friendly platforms for non-expert end-users.
Establishing a human brain cell source that can provide human brain-specific cells with robust and scalable manners is another challenge to be addressed.Recent advances in induced pluripotent stem cell (iPSC) technology have provided an access to stable cell resources to generate human brain-specific cells.The iPSC technology has also enabled the development of patient-specific NVUon-a-chip models.Establishing a personalized NVU chip model that reconstructs the patientspecific pathological characteristics could open the route for discovering the most proper treatment for individual patients.Genetic features of the patients can be also recapitulated by establishing a model with the entire component cells obtained from the patient-derived iPSC.Recently,organoids,self-organized 3D tissue constructs derived from stem cells,have been integrated with organ-on-achip technology to achievein vivolike pathological phenotypes with organ-and patient-specific characteristics.This organoid-on-a-chip model enhances the similarity of thein vitromodel to native NVU while providing highly reproducible and controllable platforms for therapeutic screening.
Not only the cellular components,but the extracellular microenvironment also needs to be considered to precisely recapitulate the pathophysiological conditions.For example,the composition and stiffness of the extracellular matrix depend on the brain region,age,and pathological conditions.The brain interstitial fluid and the cerebrospinal fluid flow are altered in pathological conditions due to the accumulation of proteins,cells,and fluid.Thus,recreating the disease-specific extracellular microenvironment including mechanical,chemical,and physical microenvironment is critical to mimic the dynamic nature of human NVU.
Customizable design of multichannel devices allows for their application in various fields for preclinical testing.The central nervous system is an immunologically privileged site where the BBB controls the entry of immune cells into the central nervous system parenchyma.NVU chip models can be used for real-time monitoring of immune cell trafficking across the BBB.Moreover,the coculture of microglia,the resident immune cells of the brain,with peripheral immune cells in NVU chips could provide a promising tool for studying their dynamic interactions in neuroinflammation.Neural implants for neuromodulation are emerging as a promising approach to treating and diagnosing neurological diseases,including AD,PD,stroke,amyotrophic lateral sclerosis,and multiple sclerosis.The biocompatibility,stability,and efficacy of the implants as well as the neural response to the implants should be tested for successful implantation.The NVU chip models can be used for preclinical testing of surgical neural implants by monitoring both the implants and thetissues.
The NVU chip technology has offered novelin vitropreclinical platforms to overcome the current limitations of conventional preclinical models.The on-chip mimicry of the pathophysiological conditions combined with innovations in fabrication methods,stem cell technology,and microfluidic technology may provide an effective and reliable tool for disease modeling and drug screening as an alternative to the conventional preclinical models.
This work was supported by a 2-Year Research Grant of Pusan National University (to SIA).
Song Ih Ahn*,YongTae Kim*
School of Mechanical Engineering,Pusan National University,Busan,South Korea (Ahn SI)George W.Woodruff School of Mechanical Engineering,Georgia Institute of Technology,Atlanta,GA,USA (Kim Y)Parker H.Petit Institute for Bioengineering and Bioscience,Georgia Institute of Technology,Atlanta,GA,USA (Kim Y)Wallace H.Coulter Department of Biomedical Engineering,Georgia Institute of Technology,Atlanta,GA,USA (Kim Y)Institute for Electronics and Nanotechnology,Georgia Institute of Technology,Atlanta,GA,USA(Kim Y)
*Correspondence to:Song Ih Ahn,PhD,
songihahn@pusan.ac.kr;YongTae Kim,PhD,ytkim@gatech.edu.
https://orcid.org/0000-0001-8923-7079(Song Ih Ahn)
https://orcid.org/0000-0002-8835-8247(YongTae Kim)
Date of submission:April 11,2023
Date of decision:May 25,2023
Date of acceptance:June 5,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380892
How to cite this article:Ahn SI,Kim Y (2024)On-chip physiological mimicry of neurovascularunit: challenges and perspectives.Neural Regen Res 19(3):499-500.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Katrine Dahl Bjornholm,Karolinska Institutet,Sweden.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
