Extracellular vesicles for neural regeneration after spinal cord injury
2024-02-13YoungJuLimWookTaeParkGunWooLee
Young-Ju Lim,Wook-Tae Park,Gun Woo Lee
What is spinal cord injury:Spinal cord injury (SCI)is the damage to the structure of the bundles of cells and nerves that communicate signals from the brain to the body and extremities.The pathology of SCI includes both primary and secondary injuries (Morales et al.,2016).Physical forces such as compression,shearing,contusion,and tearing are major causes of primary injury in SCI.There are two main processes in primary injury: acute and subacute.The acute phase includes traumatic disruption of axons and hemorrhage of the blood vessels around the spinal cord.Hemorrhagic injury to the vessels can lead to increased edema within the neural and cord tissues,susceptibility to infiltration by microglia and astrocytes,excitotoxicity,and demyelination.Similarly,disruption of the blood-spinal cord barrier results in the release of inflammatory cytokines from specific cells and vessels.Primary injuries are surgically addressed by surgical decompression and stabilization.Meanwhile,secondary injuries are more complex than primary injuries,and no effective treatment for secondary injuries following SCI has been developed to date.The secondary injury involves several pathomechanisms that seriously affect neural cells,the extracellular matrix within the spinal cord,and the surrounding structures.Among the injury processes,the activity of excitatory toxicity is a main cause for neural cell damage.The activity has been induced by the increased concentration of glutamate and glutamate receptors induce calcium channel permeability,resulting in an abnormal inflow of calcium ions.Guanosine triphosphatebinding protein-coupled metabotropic glutamate receptors induce inositol trisphosphate and release calcium ions from the endoplasmic reticulum.Previous studies have documented the protective role of glutamate receptor or calcium channel antagonists in neural cells.Whilst,the transmission of radicals,production of free radicals,and release of toxic and excitatory amino acids at the injured site are usually involved in the delayed phase of SCI.Specific proinflammatory cytokines,such as interleukin (IL)-1,IL-6,and tumor necrosis factor-α,may initially induce the differentiation of neural stem and progenitor cells to astrocytes.Similarly,cytokines are associated with tissue necrosis,the formation of a cavity of injury,and scarring at the injured area,resulting in the disruption of the healing process after SCI.
Historical perspective on basic research for SCI:Regenerative medicine has become an increasingly important field in recent years,particularly in the context of neural regeneration.Cell-based therapies,such as stem cells,have been investigated as a means of regenerating neural damage by replacing damaged cells and creating cellular environments conducive to healing.Mesenchymal stem cells (MSCs) suppress inflammation,limit secondary injury,secrete paracrine factors that protect the remaining axons,promote axonal regeneration,and differentiate into nerve cells that replace damaged nerve cells.In addition,the synthesis of neurotrophic and angiogenic factors by MSCs promotes their differentiation into neuron-like cells and neuronal survival.Although MSCs can regenerate injured tissues of the nerves and control immunological cascades,they have several limitations to use in clinical trials.First,the survival of MSCs after implantation would be limited at the injured tissue,since the longevity and viability can be prone to the cellular environment and inter-cellular communication of the local tissue.In particular,it has been reported that in the early phase after MSC implantation,proinflammatory activity might temporarily surpass anti-inflammatory activity,which adversely affects MSC function and survival.Second,MSCs are heterogeneous owing to donorassociated differences and variations in cell type and differentiation capacity and are extremely sensitive to environmental factors,thus negatively affecting their ability to attenuate severe inflammation and secondary damage processes.Third,the techniques used to manufacture MSCs,includingex vivoexpansion,isolation techniques,and cultivation methods,have not been standardized,and undetermined or unrecognized factors can result in MSC senescence and loss of function.
New era of regenerative research with extracellular vesicles:As described above,stem cells have shown promising results as a potential therapy for neural regeneration.However,there are still challenges to hinder their use and a development of therapeutic agents,including ethical concerns and difficulties in cell delivery and survival.To date,extracellular vesicles (EVs) have emerged as a promising alternative to MSCs for regeneration after traumatic neural injury,such as SCI,or degenerative pathologies.Stem cellderived EVs have been shown to have many of the same regenerative properties as being MSCs,without the many challenges associated with stem cell therapy.EVs are less likely to be rejected by the immune system and are delivered more easily and efficiently than stem cells.Additionally,EVs can be modified by some methods,such as the addition of specific molecules or engineering them,to enhance their therapeutic potential.Few recent studies have investigated the use of stem cell-derived EVs for neural regeneration.These studies demonstrate that EVs derived from a variety of stem cell sources,including mesenchymal and neural stem cells,can promote neural regeneration in various animal models of neural injury or disease.Our research in an animal model of SCI also demonstrated that MSC-derived EVs would be effective in restoring functional scores and histopathologic conditions by reducing inflammatory responses;more importantly,the effect occurs in a dose-dependent manner (1 × 109particlesvs.5 × 109particles) (Sung et al.,2022b).EVs have been shown to promote the survival and differentiation of neural progenitor cells as well as the regeneration of axons and synapses(Roballo et al.,2019).In addition to promoting neural regeneration,stem cell-derived EVs may have potential applications in neurodegenerative diseases,such as Alzheimer’s and Parkinson’s disease.Studies have shown that EVs promote the clearance of misfolded proteins associated with these diseases and protect neurons from oxidative stress and inflammation (Li et al.,2023).The use of stem cell-derived EVs in neural regeneration is a rapidly developing field of research.Although many challenges remain to be addressed,including the optimization of EV production and delivery as described below,the potential of EVs as a therapeutic agent for neural injury and neurodegenerative disease is promising and warrants further investigation.
Theoretically,EVs have the inherent advantages of being relatively easy to obtain and store and have few ethical restrictions compared to cell-based sources.Because exosomes are remarkably small(50–200 nm),EVs are isolated from the culture medium containing MSCs.The culture medium was collected every 2 days after the cells reached 80% confluence with a viability of > 95%,and cell debris was removed by centrifugation.The supernatant is filtered using a tangential flow filtration system(Pall Corporation,Port Washington,NY,USA),which is one of the methods for obtaining EVs of a specific size.Set the flow rate to 120 r/min and concentrate the EVs in the cell culture medium obtained using tangential flow filtration system.They are barely entrapped in tissues and can penetrate the blood-spinal cord barrier (Wang et al.,2019).The balance between the levels of proinflammatory cytokines (IL-1β,IL-6,and tumor necrosis factor-α) and anti-inflammatory factors are associated with recovery in SCI.Thus,the main focus of treatment for SCI is the inhibition of proinflammatory cytokines.As an example,it has been reported that exosomes isolated from human umbilical cord mesenchymal stem cells inhibit the expression of proinflammatory cytokines through direct interaction with activated microglia during secondary injury.In addition,intravenous injection of human umbilical cord mesenchymal stem cells into an SCI animal model increased motor function recovery by suppressing the expression of IL-1β and IL-6.Neuroinflammation involves resident immune cells;therefore,the activation of resident immune cells is mediated by the nucleotidebinding domain-like receptor protein 3 (NLRP3)inflammasome,which plays an important role in secondary injury.NLRP3 activity has been shown to be increased in models of traumatic brain injury and SCI.NLRP3 is upregulated after SCI,and NLRP3 inhibition promotes functional recovery(Zendedel et al.,2018).Meanwhile,the origin of EVs may be critical for regenerative properties;for example,exosomes isolated from epidural adipose mesenchymal stem cells promoted neuronal function recovery and reduced the volume of tissue damage in histopathologic analysis (Sung et al.,2022a).In a molecular mechanism study,epidural adipose mesenchymal stem cells-derived EVs significantly inhibited the activation of NLRP3 in SCI models,thereby reducing the expression of pro-inflammatory cytokines.Furthermore,epidural adipose mesenchymal stem cells-derived EVs reduced the expression of proapoptotic proteins and increased the expression of antiapoptotic proteins.On the basis of researches reported,regenerative processes regulated by EVs can be associated with specific crosstalk between circulating exosomes and neuronal cells via several independent mechanisms.
To date,much research is being conducted on microRNAs (miRNAs) inside EVs for overcoming SCI (Figure 1A).miRNAs are noncoding RNAs with short nucleotide lengths.miRNAs induce translational repression and mRNA degradation Recently,some miRNAs,including miRNA-21,29,124,126,and 486,have been highlighted as innovative alternatives for SCI treatment (Hu et al.,2015;Yu et al.,2019;Li et al.,2020).These findings suggest that the use of MSC-derived EVs may be a potential strategy for treating SCI,and some pathomechanisms of EVs for neural regeneration of injured spinal cord have been elucidated (Figure 1B).Moreover,EVs with a bilayer membrane structure are valuable for transferring miRNAs safely and effectively to the target area of SCI as a role of the carrier.Exosomes carrying miRNAs have a positive effect on SCI treatment;thus,one of the current hotspot research areas in SCI is about the study of specific miRNAs within EVs.
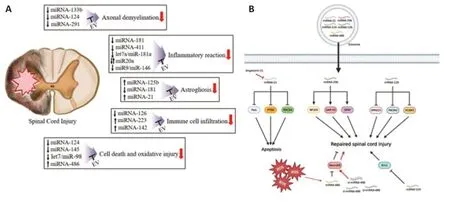
Figure 1|miRNAs in extracellular vesicles and their pathomechanism for neural regeneration.
Future perspectives on EVs:The management of SCI using EVs remains challenging owing to several obstacles.In particular,the development of a reliable potency assay for EVs is challenging because of their complex and diverse characteristics,making it difficult to define potency metrics.Gimona et al.(2021) proposed the use of a matrix array of multiple assays that measure various attributes of EVs that correlate with their intended use,as guidance from regulatory agencies can help with this development.Additionally,developing quality control measures and reproducible manufacturing processes for EVs is challenging because of the wide variability in their preparation caused by the lack of standardized quality assurance and functional assays.To overcome this challenge,Witwer et al.(2019) suggested the development of minimal quantifiable metrics to harmonize the definition of EVs and provide a denominator for comparative manufacturing and functional testing.They also recommended the use of identifying metrics (the ratio of MSC to non-MSC surface antigens,the ratio of membrane lipids to proteins,the ratio of specific lipids,the concentration of membrane lipid vesicles,vesicle integrity,and biological activity) and validating each metric in comparison with that of a well-characterized EV preparation.This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.2022R1C1C1005410) (to GWL).
Young-Ju Lim,Wook-Tae Park,Gun Woo Lee*
Department of Orthopedic Surgery,Yeungnam University Medical Center,Yeungnam University College of Medicine,Daegu,Korea
*Correspondence to:Gun Woo Lee,MD,PhD,gwlee1871@gmail.com.
https://orcid.org/0000-0002-8441-0802(Gun Woo Lee)
Date of submission:March 12,2023
Date of decision:May 11,2023
Date of acceptance:May 24,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380894
How to cite this article:Lim YJ,Park WT,Lee GW (2024) Extracellular vesicles for neural regeneration after spinal cord injury.Neural Regen Res 19(3):491-492.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
