Presynaptic endoplasmic reticulum architecture and hereditary spastic paraplegia
2024-02-13JuanJosrezMoreno
Juan José Pérez-Moreno
Hereditary spastic paraplegia (HSP) is a clinically and genetically heterogeneous neurodegenerative disorder,characterized primarily by progressive spasticity and weakness in the lower limbs.Patients can also experience peripheral neuropathy,cognitive impairment,and other neurological symptoms.To date,more than 80 genes have been implicated in HSP,encompassing various cellular components,although mutations in genes encoding endoplasmic reticulum (ER)-shaping proteins are the most prevalent (Parodi et al.,2017).ER-shaping proteins are generally known for regulating the tubulation and curvation of the ER,but most of them show additional functions,including fusion of ER tubules,microtubulesevering,ER autophagy,lipid droplet synthesis,contact sites with other organelles (Öztürk et al.,2020).This highlights the complexity of studying the role of these proteins and the link between ER function and HSP.
In neurons,the ER extends along dendrites,the cell body,and the axon,forming a continuous network that physically communicates the whole cell and needs a lot of engineering (Öztürk et al.,2020).ER-shaping proteins have a main role in shaping axonal ER,which shows a predominant presence of ER tubules interconnected with occasional cisternae (Wu et al.,2017),and remarkably,the main hallmark feature of HSP is progressive distal axon degeneration,preferentially affecting long neurons (reaching up to 1 m in humans) (Parodi et al.,2017).This suggests an important role for the ER architecture (Figure 1) in maintaining axonal integrity and highlights the importance of its study to understand the pathogenic mechanisms underlying HSP.This article specifically focuses on the role of the ER architecture (the organization of the ER network) at the terminal part of the axon,beyond additional pathways by which the ER might control neuronal function (Öztürk et al.,2020).
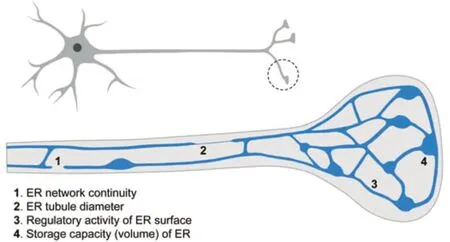
Figure 1|Architectural features of the presynaptic endoplasmic reticulum (ER) with potential influence on its function.
Presynaptic ER architecture influence on Ca2+dynamics:ER morphology and distribution are tightly regulated,varying not only between cell types but also between regions within the same cell,and adapting to physiological changes.This is important when considering axonal ER distribution,since the axon terminal is a highly specialized region,at both functional and structural levels.A potential scenario is that axonal ER may be mostly involved in ensuring the continuity of the network from the soma,while presynaptic ER might show features that differ from the rest of the axonal network,providing the ability to properly regulate specific processes related to the synaptic function,such as vesicle trafficking,Ca2+dynamics,mitochondrial function,or the lipid composition of the plasma membrane,among others.In fact,the ultrastructural analysis performed to date suggests that the presynaptic ER shows a more extensive network than the rest of the axonal ER,showing more branching (Wu et al.,2017).
Recent research supports a specific and critical role for the presynaptic ER in managing Ca2+fluxes,where the architecture of the ER network seems to have two main features,area,and volume,with distinguishable physiological influence.Presynaptic ER volume,arguably more dependent on cisternae than on tubules,determines the capacity to store Ca2+.This capacity has a role in buffering presynaptic Ca2+to control neurotransmitter release,depending on Kv2.1-mediated tethering between ER and the plasma membrane (Panzera et al.,2022).Neurotransmission is triggered by a local cytosolic increase of Ca2+levels,but the role of the Ca2+released from presynaptic ER seems to be limited to facilitating extended Ca2+signaling(i.e.,long-term neuronal firing) (de Juan-Sanz et al,2017).Importantly,the presynaptic ER capacity to store Ca2+also controls Ca2+exchange between different compartments.ER Ca2+depletion activates SOCE (store-operated Ca2+entry)response through the ER proteins STIM,which mediate contact sites with the plasma membrane to allow Ca2+exchange.STIM2 activation drives spontaneous neurotransmission (Chanaday et al.,2021),and STIM1 activation reduces release probability (de Juan-Sanz et al.,2017).
Apart from cisternae,the presynaptic ER network predominantly shows tubules,which given their high surface/volume ratio are critical to determine the amount of ER surface.Altered integrity and dynamics of presynaptic ER tubules lead to increased basal cytosolic Ca2+levels,impairing synaptic function (Lindhout et al.,2019).This might suggest a reduced ER Ca2+uptake capacity,although not necessarily a reduced volume to store Ca2+.In fact,recent work suggests that a specific decrease of the presynaptic ER surface,but not its volume or basal ER Ca2+levels,might impact the number of contacts with other compartments (i.e.,STIM-mediated contacts),and in turn the ability to transfer Ca2+between them during neuronal activity (Pérez-Moreno et al.,2023).In conclusion,the current data suggest that in the presynaptic ER network,cisternae would provide volume to buffer Ca2+,while tubules would regulate Ca2+dynamics by physical interaction with other membranes.
Potentially by limiting Ca2+transfer through the number of contacts with other membranes,it seems that the amount of presynaptic ER directly influences the levels of neurotransmission:reduced presynaptic ER leads to reduced neurotransmission (Pérez-Moreno et al.,2023),and in agreement,increased presynaptic ER leads to increased neurotransmission (Kuijpers et al.,2021).An intriguing question to address in future research is how the amount of presynaptic ER is physiologically adjusted.The main finding to date is the identification of autophagy as one of the mechanisms controlling the excess of presynaptic ER (Kuijpers et al.,2021).
Assessing axonal and presynaptic ER roles on HSP:In HSP patients,the motor-evoked potentials are reduced,suggesting a useful biomarker for monitoring disease progression and therapeutic response in HSP patients (Brighente et al.,2021).Among other neuronal defects,animal models defective for different ER-shaping proteins show altered presynaptic ER network and decreased synaptic function (Öztürk et al.,2020;Pérez-Moreno et al.,2023),supporting a role for presynaptic ER in modulating this process.However,given the continuity and pervasiveness of the ER network,a main challenge when studying presynaptic ER is to discriminate between its specific functions and those involving the whole axonal ER network.Besides the specialization of the presynaptic region,it is not clear whether presynaptic ER might be considered a different structure of the same network,as it is for example established for rough and smooth ER.Therefore,a question that arises is whether mutations affecting presynaptic ER organization inevitably affect axonal ER in a general way.Loss of Rtnl1(Drosophila ortholog of the HSP protein RTN2)causes decreased axonal ER levels and occasional gaps in the network only in long motor neurons(Yalçın et al.,2017),while showing reduced presynaptic ER in all motor neurons (Pérez-Moreno et al.,2023).This model opens the possibility to study the local disruption of presynaptic ER in shorter neurons without having additional defects in the network,but it would be expected to have unnoticed defects in the axonal ER,given the general dependency of the network on the role of ER-shaping proteins.
Another important aspect to consider when studying the local roles of axonal ER is the relevance of keeping a continuous network.Arguably,continuity might provide communication(i.e.,long-range Ca2+waves) and/or allow to respond to local changes through the local distribution of ER-related molecules.For example,physical continuity might allow for quick expansion of the network by adding new elements from adjacent areas.This idea is supported byin vivoimaging from different organisms,revealing a quick and dynamic exchange of tubules between different areas of the network.Besides its physical membrane continuity,axonal ER can be very narrow,with no noticeable lumen (Terasaki,2018).This suggests a potential mechanism of dynamic compartmentalization to isolate local regions from unwanted diffusion of molecules along the network while keeping a continuous network.Whether this type of mechanism is physiologically relevant and influences the presynaptic ER network needs to be addressed.
In the context of HSP,the role of ER-shaping proteins is known to be key for keeping a continuous and interconnected ER network.For example,depletion of atlastin leads to ER fragmentation and reduces the branching of ER tubules (Öztürk et al.,2020),and loss of Rtnl1 and REEP proteins causes sporadic gaps in the axonal ER network (Yalçın et al.,2017),increasing the chances of a discontinuity of the network with distance from the cell body.The tubular ER network spreads ubiquitously throughout the cytoplasm,and its local amount is approximately proportional to the local cytoplasmic volume.Therefore,axon length but also axon width should determine the susceptibility of the network to lose its continuity,a hypothesis that might fit with the type of neurons preferentially affected in HSP patients (Parodi et al.,2017).We ignore whether disconnected parts of the network can perform some of its functions (equivalent to what is observed with Golgi outposts in dendrites),but assuming an essential role for the network continuity,a disconnected presynaptic ER from the soma could show dysfunction even having a normal local architecture.Therefore,finding suitable approaches to specifically affect presynaptic ER will be essential to understand its local roles and links with HSP.
Concluding remarks:Mutations in genes associated with axonal ER are tightly associated with HSP,but the underlying mechanisms remain elusive.Current research supports an important role for presynaptic ER in regulating synaptic function through Ca2+dynamics,opening a promising hypothesis of pathogenesis.Model organisms are essential to identify general and conserved mechanisms involving presynaptic ER,and mammalian models are particularly necessary to study specific molecular mechanisms,given the different functions observed in some members of the same protein families.Further research should shed light on fundamental questions,such as why ER is continuous along the axon until the presynaptic terminals,or which are the pathological consequences of a discontinuous network,which will help to determine the relevance of the presynaptic ER network in HSP.Uncovering these processes will likely inspire rational approaches to future therapies.
The present work was supported by Juan de la Cierva Incorporación grant (IJC2019-038819-I)from the Spanish State Research Agency (MCIN/AEI/10.13039/501100011033) (to JJPM).
I thank Cahir O’Kane for helpful discussions and comments on the article.
Juan José Pérez-Moreno*
Instituto de Biomedicina de Sevilla (IBiS),Hospital Universitario Virgen Del Rocío/CSIC/Universidad de Sevilla,and Departamento de Biología Celular,Facultad de Biología,Universidad de Sevilla,Seville,Spain
*Correspondence to:Juan José Pérez-Moreno,PhD,jpmoreno@us.es.
https://orcid.org/0000-0002-8497-3066(Juan José Pérez-Moreno)
Date of submission:April 21,2023
Date of decision:May 25,2023
Date of acceptance:June 7,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380885
How to cite this article:Pérez-Moreno JJ (2024)Presynaptic endoplasmic reticulum architecture and hereditary spastic paraplegia.Neural Regen Res 19(3):485-486.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and buildupon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
