Heterogeneous patterning of blood-brain barrier and adaptive myelination as renewing key in gray and white matter
2024-02-13BarbaraPavan
Barbara Pavan
Background:Development and homeostasis of the brain are enabled through the precise control of the cell microenvironment by the blood-brain barrier (BBB),which interfaces between the brain parenchyma and the lumen of blood microvessels,and by the blood-cerebrospinal fluid barrier,which separates the cerebrospinal fluid from the blood vessels of the choroid plexus (Villabona-Rueda et al.,2019).Here,the focus will be on the BBB,the impairment of which is considered the earliest common denominator in neurovascular diseases.
BBB includes specialized brain microvascular endothelial cells (BMECs),which act as a “biological filter” by selectively restricting the transport of substances from blood to neural tissue (Langen et al.,2019),through tight junction (TJ) protein complexes.In fact,the transport of compounds across BBB is determined by the expression in BMECs of TJs,which very dynamically modulate the paracellular diffusion route between neighboring cells,in concert with the expression and activity of different types of active efflux transporters,that extrude from the brain both xenobiotics,and endogenous lipophilic compounds and neural waste products,such as amyloid beta(Villabona-Rueda et al.,2019).The BBB,moreover,controls the intrathecal passage of macrophages,T and B lymphocytes as well as immunoglobulins or antibodies,acting as an “immunological filter”(Langen et al.,2019).All functions of BMECs are preserved by their close and steady interactions with surrounding astrocytes,pericytes,neurons,and perivascular macrophages,which collectively form the so-called neurovascular unit (NVU).NVU is physiologically regulated by neuronal signaling,whereas dysregulation of one of the NVU element can lead to disease (Langen et al.,2019).Impairment of NVU and BBB are also known to be correlated to the demyelinating processes in the brain (Call and Bergles,2021).
The myelin-forming oligodendrocyte precursor cells (OPCs) are generated during embryonic development,while myelination mostly occurs postnatally (Werkman et al.,2021),when oligodendrocytes wrap neuronal axons with myelin sheaths,i.e.,a tight stack of several phospholipid bilayers formed by their plasma membranes,which provides metabolic support to axons and facilitates fast saltatory impulse propagation along axons by electrical isolation (Werkman et al.,2021).OPCs continuously generate new myelinating oligodendrocytes in the somatosensory cortex from birth up to middle age;then,in old age,the oligodendrocyte population declines,accompanied by a reduction in myelin layers.Remyelination is the process of spontaneous regeneration of myelin following demyelinating events taking place in the adult brain.Unlike axonal myelination,whose patterns scale between cortical regions in a close association between axon diameter and myelin thickness (Call and Bergles,2021),remyelination implies that myelin sheath thickness is independent of axon diameter.Finally,myelination is mainly driven by axonal signals,while neuroinflammation is the trigger for remyelination,where the myelin sheaths are thinner and shorter than the original ones generated during development (Call and Bergles,2021).
Blood-brain barrier features in gray and white matter:There is remarkable variation in the cell composition of the NVU among different brain regions,and along the vascular tree (Villabona-Rueda et al.,2019),where specialized vascular heterogeneity is essential to support the high metabolic requirements of neurons and the dynamic pattern of neural activity (Villabona-Rueda et al.,2019).In this view,specific features of the BBB phenotype,such as changes in barrier function based on TJs tightness of TJs,expression of efflux and influx transporters,receptors,and adhesion molecules can be influenced by different signals from the surrounding vascular environment(Villabona-Rueda et al.,2019).Furthermore,NVU heterogeneity is particularly evident between white matter (WM) and gray matter(GM),consisting of significant dissimilarities in morphology,cellular content such as astrocytes,pericytes and oligodendrocytes,microvascular density (Noumbissi et al.,2018),and a differenttightness of BBB (Nyúl-Tóth et al.,2016;Noumbissi et al.,2018).Variability in BBB tightness has been investigated measuring transendothelial electrical resistance of cultured porcine BMEC monolayers derived from different brain regions (Nyúl-Tóth et al.,2016).Interestingly,transendothelial electrical resistance of WM-derived BMECs increases more rapidly than transendothelial electrical resistance of GM-derived BMECs,reaching a significantly higher plateau and indicating a higher BBB tightness in WM (Nyúl-Tóth et al.,2016).The different types of neurons involved in the NVU are known both to regulate brain blood flow,and directly influence BBB tightness and/or permeability through direct innervations of BMECs in WM and GM (Nyúl-Tóth et al.,2016).Consequently,the need to distinguish between WM-derived from GM-derivedin vitroBBB models should be recommended (Nyúl-Tóth et al.,2016;Guzzo et al.,2023).
Considering the mutual influence between neighboring cells within the NVU together with the metabolic needs and differences in blood flow/shear stress related to the differential vulnerability of WMvs.GM,this perspective suggests a potential functional coupling between BBB heterogeneity and variable patterning of myelination and remyelination in WM and GM(Figure 1).
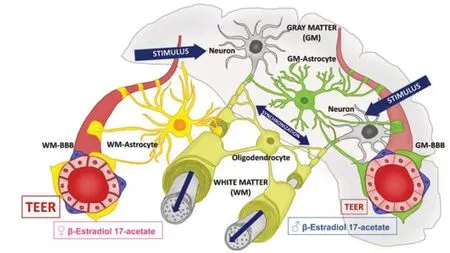
Figure 1| Heterogeneity of neurovascular unit contributes to heterogeneous blood-brain barrier (BBB) and myelination patterns in white matter (WM) and gray matter (GM).
Myelination patterns in gray and white matter:Heterogeneity in oligodendrocyte morphology and myelination/remyelination patterns in WM and GM has been widely reviewed (Werkman et al.,2021).Besides,the post-injury remyelination pattern,the possibility of modifying neural circuits also persists in adulthood,due to the large neuronal territory available for the addition of new myelin on axons suitable to be myelinated and the persistence of oligodendrocyte progenitors.This phenomenon is called adaptive myelination or myelin plasticity (Gibson et al.,2018),and occurs by modulating the thickness of myelin in WM in response to the neuronal activity strengthening the synapses in GM,known as synaptic plasticity.Indeed,motor learning,exposure to new environments and other life experiences are related to changes in oligodendrogenesis and dynamic shaping of myelin (Gibson et al.,2018).This new form of brain plasticity is controlled by the addition and subtraction of myelin sheaths to increase or decrease,respectively,the speed of communication required to sustain oscillations that synchronize different brain regions at the same frequency (Gibson et al.,2018).Accordingly,non-pathogenic myelin plasticity acts together with synaptic plasticity in learning and memory consolidation (Gibson et al.,2018).
This perspective,therefore,focuses on the potential relationship between BBB heterogeneity and the neuronal activity-dependent adaptive myelination pattern in WM and GM.BMECs secreting both soluble factors and extracellular vesicles are found to enhancein vitrosurvival,proliferation,and motility of OPCs,whose different morphology within the brain reflects inherent differences in the programs that instruct sheath formation (Noumbissi et al.,2018).Therefore,appropriate systemic endogenous or exogenous stimuli,acting on the different properties of the BBB,could influence the adaptive myelination process in various brain areas (Figure 1).
Experimental evidence indicates that microvascular heterogeneity and adaptive myelination in brain regions are associated with a synchronized state of neuronal activity for clearance and repair of the brain.Indeed,neuronal activity has been reported inversely related to the expression levels and function of the efflux transporters in BBB through the regulation of the clock genes in BMECs (Pulido et al.,2020).Consistently,increased clearance of waste products from the brain has been observed during synchronized neuronal activity related to the phases of the non-rapid eye movement sleep (Pulido et al.,2020).Furthermore,the manipulation of sensory inputs has been shown to induce structural changes in vascular trees along the different regions of the brain cortex (Lacoste et al.,2014),such as an increase in vascular density and branching following the enhancement of desynchronized neural activity (Lacoste et al.,2014),which usually occurs during wakefulness and rapid eye movement sleep (Pavan et al.,2022).Conversely,the increase in myelin thickness arising after ischemic cortical lesions has been found to be associated with synchronization of neuronal activity (Ganguly et al.,2022).It has also been reported that the vasculature responds to changes in neuronal activity associated with experience by regulating endothelin-1 release at the BBB level,which in turn affects the myelinating capacity of oligodendrocytes (Swire et al.,2019).Besides,endothelin-1 released during neuroinflammation is known to increase P-gp transport activity(Harati et al.,2012).It is,therefore,conceivable that neuronal activity,along with the BBB,influences OPC function and selectively instructs myelination of active neural circuits to promote oligodendrogenesis and adaptive myelination of the brain (Gibson et al.,2018).A functional link between increased BBB clearance activity during synchronized neuronal activity and myelin adaptive plasticity could also be hypothesized.Astrocytes,the crosstalk between neuronal synapses and BBB,as well as between oligodendrocytes and axons(Lacoste et al.,2014;Figure 1),may be the best candidates to mediate this functional link (Pavan et al.,2022).
Responsiveness of BBB and myelination development to sex steroids:Among the various endogenous systemic stimuli,sex steroids are here suggested as essential coupling factors between the dynamic heterogeneity of the BBB and myelin plasticity.Indeed,heterogeneity of the BBB depends not only on the specific brain region but also on gender (Guzzo et al.,2023).In this regard,it has been reported that the estrogen hormone 17β-estradiol strongly enhances the yield of adhesion and proliferation of human BMECs in culture,depending on whether they derive from WM or GM of male or female patients(Guzzo et al.,2023).Based on its physiological concentrations,this potent estrogenic hormone might be a key molecule stimulating the phenotypical and functional patterning of vascularization across the different regions of the human brain,particularly regarding female WM microvessels,mainly after menopausal transition,and male GM microvessels (Guzzo et al.,2023;Figure 1).In vitroexperiments show estrogens are directly implicated in promoting neural stem cell and OPC proliferation,differentiation,and myelination,as well as ameliorating outcomes of the remyelination process to neuroinflammatory conditions in humans (Seeker et al.,2022),probably together with their trophic effects on BMECs (Guzzo et al.,2023;Figure 1).
Future directions:Understanding how the brain area-dependent heterogeneity of NVU may be expressed in parallel with the corresponding patterns of adaptive myelination and remyelination can provide important therapeutic indications for neuro-pathologies that sprout differently in various brain regions.Depending on therapeutic strategies,characterization of WM-and GMdependent BBB barrier function and expression of transporters and receptors can lead to innovative drug delivery devices,allowing selective drug targeting to the specific areas of the brain.Moreover,the identification of specific proteins for adhesion and tight junctions expressed by WMand GM-derived BMECs (Figure 1) could be applied to improve or achieve chronic biocompatibility of intracortical implants in male and female patients(Guzzo et al.,2023).In this context,sex steroid mimetic compounds,like the long-acting estrogen β-estradiol 17-acetate,could be a gold standard in gender-specific estrogen hormone-related effects on vascular health (Figure 1).The latter aspect may not be negligible in predicting the efficacy and duration of a potential estrogen-eluting device at the brain microvascular level (Guzzo et al.,2023),as well as providing personalized neuro-medicine for individual patients (Pavan et al.,2022).
It is also advisable to consider brain regional differences in BBB permeability and NVU composition,when developing and/or assessing remyelination based treatments (Werkman et al.,2021).To this end,therapeutic strategies against the most lethal brain tumors such as glioblastomas may be improved by an in-depth investigation into neuronal activity-dependent adaptive myelination coupled with the dynamic heterogeneity of the BBB.Indeed,there is emerging evidence for neuronal activity-dependent regulation of glioma progression,due to the robust mitogenic effect of neuronal activity on normal OPCs,a putative cellular origin for many forms of glioma (Gibson et al.,2018).In particular,OPCs and macrophages/microglia constitute a special microenvironment for glioblastoma cells at the tumor border or peritumoral zone of cell migration and invasion of healthy tissue (Gibson et al.,2018).As a result,neuronal activity regulates both normal and neoplastic glial cell behavior.Therefore,dysregulated or maladaptive mechanisms of myelination have a drastic impact on neural network functioning,as myelin alterations can promote or disrupt the synchrony of signals and thus modify neural coherence,leading to disease states of the brain like malignant cell proliferation(Gibson et al.,2018).Finally,modulation of sensory inputs by drug delivery and/or transcranial magnetic stimulation could be a useful tool to induce structural changes of the vascular tree and concomitant adaptive myelin remodeling along brain regions,in the counteracting glioma progression when combined with surgery,or as a therapeutic benefit against neurodegenerative diseases.
The present work was supported by Fondo per la Ricerca di Ateneo (FAR) 2022,University of Ferrara,Ferrara,Italy (to BP).
Barbara Pavan*
Department of Neuroscience and Rehabilitation,University of Ferrara,via L Borsari,Ferrara,Italy;Center for Translational Neurophysiology of Speech and Communication (CTNSC),Italian Institute of Technology (IIT),via Fossato di Mortara,Ferrara,Italy
*Correspondence to:Barbara Pavan,PhD,pvnbbr@unife.it.
https://orcid.org/0000-0001-8942-9310(Barbara Pavan)
Date of submission:March 29,2023
Date of decision:May 11,2023
Date of acceptance:May 22,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380884
How to cite this article:Pavan B (2024)Heterogeneous patterning of blood-brain barrier and adaptive myelination as renewing key in gray and white matter.Neural Regen Res 19(3):481-482.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewer:Myriam Cayre,Laboratoire de Neurosciences Cognitives,Aix-Marseille Université,France.
Additional file:Open peer review report 1.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
