Iron regulatory protein 1: the deadly switch of ferroptosis
2024-02-13DanielrquezPamelaUrrutia
Daniel A.Bórquez,Pamela J.Urrutia
Ferroptosis,an iron-dependent cell death:Ferroptosis is a type of regulated necrosis,characterized by redox-active iron accumulation and increased free radical production derived by Fenton chemistry,that triggers oxidation of polyunsaturated fatty acids in phospholipids,loss of cellular membranes integrity,and leakage of intracellular contents.
Over the past decade,ferroptosis has garnered enormous interest,since it participates in several diseases including neurodegenerative disorders,acute renal failure,cardiovascular diseases,cancer,and various other age-related disorders.Accordingly,senescent cells are adapted to resist ferroptosis through specific changes in iron homeostasis,contributing to oxidative stress and inflammation (Mazhar et al.,2021).
Most of our knowledge of ferroptosis comes from the use of synthetic small-molecule activators,erastin and RSL3,that target key proteins involved in cellular redox balance.Accordingly,erastin inhibits the xc-system,an amino acid transporter that imports cystine and exports glutamate.Cystine is subsequently reduced to cysteine,the limiting precursor of glutathione (GSH),the main cellular antioxidant.Otherwise,RSL3 inhibits glutathione peroxidase 4 (GPX4),a GSH-dependent enzyme that represses ferroptosis by the reduction of phospholipid hydroperoxides to non-toxic phospholipid alcohols (Tang and Kroemer,2020).Although cellular antioxidant systems hinder the initiation of ferroptosis,redox-active iron availability is essential to their execution.
Iron uptake via endocytosis of the extracellular iron transport protein transferrin (Tf) is the major route of iron entry into most cell types.The transferrin receptor (TfR1) is responsible for Tf targeting the endolysosomal system,where iron is transported to the cytosol through the metal transporters DMT1 and TRPML1.Iron is also stored in a redoxinactive state in ferritin,a 24-subunit spherical nanocage composed of varied ratios of ferritin light chains and ferritin heavy chains (FTH1).The iron contained in ferritin can be released through its degradation by a special type of autophagy,called ferritinophagy.The upregulation of the aforementioned processes has been shown to contribute to ferroptosis.TfR1 accumulation on the cell surface is a cardinal feature of ferroptosis and specific anti-TfR1 antibodies are reliable ferroptosis markers.Otherwise,ferritinophagy is required for ferroptosis,and the FTH1 ferritin subunit plays an important regulatory role,since diminished FTH1 levels promote both processes.
Cytosolic (c)-aconitase (ACO1)-to-iron regulatory protein 1 (IRP1) switch:Considering that the intracellular accumulation of redox-active iron constitutes the main hallmark of ferroptosis,we will discuss below the regulatory pathways that can promote the unrestricted rise of iron levels(reviewed in Urrutia et al.,2021).
Cellular iron levels are regulated by the IRPiron-responsive element (IRE) system,where two RNA-binding proteins (IRP1 and IRP2) posttranscriptionally regulate the expression levels of iron homeostasis-associated proteins by binding to specific stem-loop structures (IRE) in the untranslated regions of its mRNAs.Both proteins are ubiquitously expressed in mammalian cells,although their relative expression levels differ in a tissue-specific manner.IRP2 levels are tightly regulated through iron-and oxygen-dependent proteasomal degradation,dominating the control of iron homeostasis under physiological conditions in most tissues.Otherwise,IRP1 is a bifunctional iron/reactive oxygen species (ROS) sensor that switches between two activities: an iron-sulfur[4Fe-4S] cluster-bearing ACO1 that isomerizes citrate to isocitrate,or an IRE-binding protein(IRP1) after oxidatively disassembly of the [4Fe-4S] cluster.While IRP2 is stabilized only when intracellular iron levels decrease,the sensitivity of the [4Fe-4S] cluster of ACO1 to oxidants (such as hydrogen peroxide or nitric oxide) implies that IRP1 activation is generally decoupled from iron levels.
IRE binding by IRPs enhances iron uptake through the upregulation of TfR1 and DMT1.IRPs also repress the expression of both subunits of ferritin(ferritin light chains and FTH1),decreasing iron storage and promoting ferritinophagy,and also diminishes ferroportin-1 expression,the unique transporter that allows iron export from cells.Therefore,IRPs activation can fuel ferroptosis by increasing redox-active iron levels.
IRP1: a self-sustained deathly switch for ferroptosis:Currently,the regulatory mechanisms of ferroptosis remain obscure.Although ferroptosis can be initiated through an extrinsic pathway,e.g.,by inhibition of the cystine/glutamate antiporter(also known as amino acid transport system xc-),or through an intrinsic pathway,involving the blocking of expression or activity of GPX4 (Tang and Kroemer,2020),is unlikely that these mechanisms can be used endogenously in a cell fate decision.
IRP1 has several characteristics that make it an excellent candidate to act as the molecular switch to initiate and sustain ferroptosis processes: (i) Is present in two interconvertible forms,ACO1 and IRP1,with the first acting as a large reservoir of potential activity,mostly insensitive to cellular iron status under physiological conditions (Meyron-Holtz et al.,2004);(ii) ACO1-to-IRP1 switching is triggered under oxidative stress conditions,which coincides with the effector mechanism of the main ferroptosis inducers;(iii) Iron-induced ROS activates IRP1,which in turn increases redox-active iron levels,in a positive feedback loop leading to cell death and (iv) diminished activity of ACO1 can reduce glutamate levels,impairing cystine uptake through cystine/glutamate antiporter and finally reducing GSH synthesis.In a nutshell,ACO1-to-IRP1 conversion can act as a self-sustaining molecular switch coordinating the two main effector pathways of ferroptosis (Figure 1).
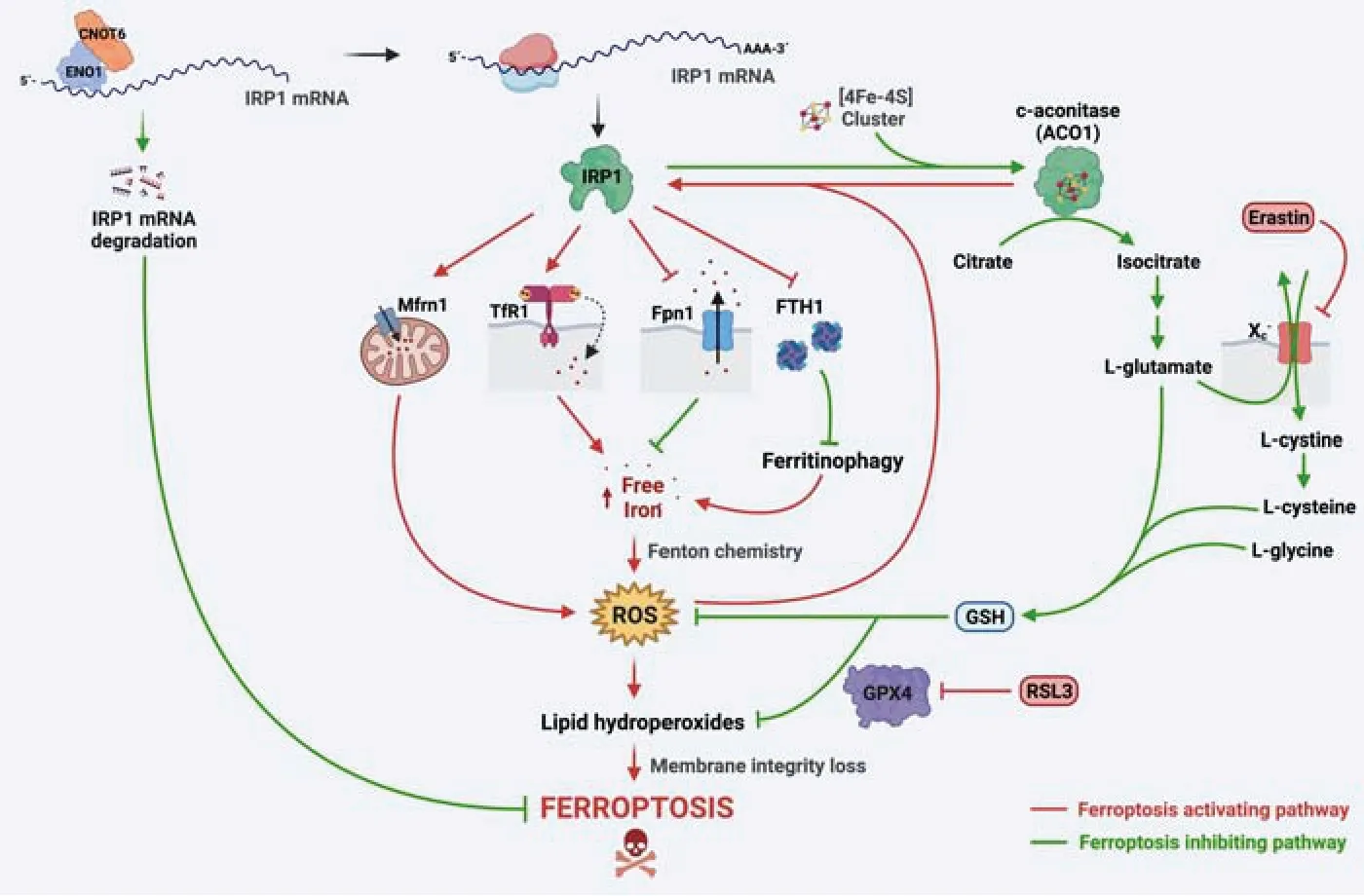
Figure 1|ACO1-to-IRP1 switch controls ferroptosis.
Recent findings support the previously stated hypothesis.In lung cancer cells,a ferroptosis inducer (trabectedin) increases IRP1 mRNA expression,and IRP1 knockdown prevents TfR1 upregulation,iron accumulation,and cell death induced by trabectedin (Cai et al.,2023).
Additionally,IRP1 knockdown significantly inhibits ferroptosis triggered by erastin and RSL3 in melanoma cells.Conversely,IRP1 overexpression sensitizes melanoma cells to erastin and RSL3-induced cell death.Furthermore,erastin and RSL3 induce ACO1-to-IRP1 switching,increasing its IRE binding activity,resulting in the upregulation of TfR1 and the downregulation of ferroportin-1 and FTH1 levels,supporting iron accumulation and lipid oxidation (Yao et al.,2021).IRP1 overexpression also promotes ferroptosis through enhanced expression of mitoferrin-1,a mitochondrial iron transporter,which carries iron from the cytoplasm into the mitochondria.In this way,the IRP1-mitoferrin-1 axis enhances mitochondrial iron accumulation,which increases ROS levels,resulting in lipid peroxidation and membrane damage (Zhang et al.,2022).
Highlighting the key role of IRP1 in regulating ferroptosis,cancer cells have developed a mechanism to decrease its expression and confer resistance to this type of cell death.In hepatocellular carcinoma cells,ɑ-enolase 1 recruits the CCR4-NOT deadenylase complex to promote the removal of poly (A) tail of IRP1 mRNA,inducing its degradation and inhibiting IRP1 expression.Accordingly,diminished IRP1 and mitoferrin-1 expression in hepatocellular carcinoma patients correlates with poor clinical prognosis (Zhang et al.,2022).Recently,it has been shown that enolase-3 upregulation in colonic epithelial cells suppresses ferroptosis by targeting IRP1 mRNA (Arenbaoligao et al.,2023),suggesting that this mechanism also operates in non-cancer cells.Moreover,markedly reduced IRP1 expression is associated with ferroptosis resistance in senescent mouse embryonic fibroblasts,although its contribution to this phenotype should be the subject of future studies (Masaldan et al.,2018).
IRP1 and ferroptosis at the crossroads of neurodegenerative diseases:Beyond the current evidence as a death switch in cancer cells,IRP1 also constitutes an excellent candidate as a ferroptosis regulator in central nervous system cell types and therefore a promising therapeutic target for acute and chronic neurodegenerative diseases.Ferroptosis has recently been proposed as a key neuronal death mechanism in several central nervous system diseases,including stroke,Parkinson’s disease,Alzheimer’s disease,and amyotrophic lateral sclerosis (reviewed in Masaldan et al.,2019).Coincidentally IRP1 deregulation has been observed in these neurodegenerative conditions (reviewed in Zhou and Tan,2017).
The hallmarks of many neurodegenerative diseases including iron accumulation,oxidative stress,and neuroinflammation,and current evidence supports the hypothesis that they are mechanically connected by IRP1 (Urrutia et al.,2021).During neuroinflammation,hydrogen peroxide,nitric oxide,and peroxynitrite are produced downstream of NADPH oxidase and inducible nitric oxide synthase,two key enzymes activated in proinflammatory microglia/macrophages.These reactive species promote[4Fe-4S] disruption and ACO1 conversion into IRP1,and therefore can paracrinely affect neuronal sensitivity to ferroptosis.Accordingly,the removal of microglia in tri-cultures with neurons and astrocytes significantly delays iron-induced neurotoxicity.Interestingly,pro-inflammatory phagocytes are resistant to ferroptosis,conceivably by the transcriptional upregulation of FTH1,which inhibits ferritinophagy and therefore ferroptosis.
The main risk factor for all chronic neurodegenerative diseases is aging,and aged microglia is poised to trigger the activation of neuronal IRP1.Aged microglia show a rise in the redox-active iron levels,increased NADPH oxidase 2 expression,high ROS production,and exacerbated neuroinflammatory response by noxious stimuli,potentially contributing to IRP1 deregulation through the previously described mechanisms.
名师的成长与成名主要靠教师自身的努力进取,但也离不开学校的发展平台和有意识、有计划的培育,因此,学校要把名师培育作为师资队伍建设的一项重要工作来抓,强化顶层设计和统筹谋划,形成有利于名师干事创业、成长发展的良好机制。
Recent findings also suggest that reactive astrocytes could modulate neuronal ferroptosis in a phenotype-dependent manner.In this way,astrocytes can either exert an anti-ferroptotic effect in neurons,for example,through the downregulation of neuronal DMT1 by GDNF released by astrocytes (Zhang et al.,2014),or promote neuronal ferroptosis through the secretion of chemokines such as CXCL10,that impairs antioxidant synthesis in neurons (Liang et al.,2023).
IRP1 also emerges as a promising therapeutic target to prevent neurodegeneration associated with increased neuronal iron and ferroptosis.However,to date,no small molecule inhibitors of IRP1 activity have been identified.Both the protection of [4Fe-4S] of IRP1 from oxidative damage by antioxidant molecules or the downregulation of IRP1 expression by short harpin RNA has been effective interventions to attenuate iron accumulation,oxidative stress,and neuronal death induced by rotenone,a mitochondrial toxin used to model Parkinson’s disease.In the coming years,it is expected that this evidence will consolidate and expand to other neurodegenerative conditions.
This perspective is dedicated to my PhD mentor Dr.Marco Tulio Núñez from the Universidad de Chile.We thank him for all training,support,and guidance.
This work was supported by FONDECYT Initiation in Research,grant number 11201141,awarded to PJU.
Daniel A.Bórquez,Pamela J.Urrutia*
Center for Biomedical Research,Faculty of Medicine,Universidad Diego Portales,Santiago,Chile (Bórquez DA)Institute of Nutrition &Food Technology (INTA),Universidad de Chile,Santiago,Chile (Urrutia PJ)Geroscience Center for Brain Health and Metabolism,Santiago,Chile (Urrutia PJ)
*Correspondence to:Pamela J.Urrutia,PhD,pamela.urrutia@uchile.cl.
https://orcid.org/0000-0002-2381-9073(Pamela J.Urrutia)
Date of submission:April 10,2023
Date of decision:May 27,2023
Date of acceptance:June 15,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380889
How to cite this article:Bórquez DA,Urrutia PJ(2024) Iron regulatory protein 1: the deadly switch of ferroptosis.Neural Regen Res 19(3):477-478.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
猜你喜欢
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
