Harnessing the power of pericytes and hypoxia-inducible factor-1 to modulate stroke outcome
2024-02-13OmolaraOgunsholaChihChiehTsao
Omolara O.Ogunshola,Chih-Chieh Tsao
The human brain has exceedingly high metabolic demands.The cerebral vasculature has the critical task of providing sufficient blood supply to meet this demand.The sudden interruption of blood flow to the brain,as observed during ischemic stroke,results in acute neurological injury with devastating consequences– a high rate of adult disability and death.Ischemic stroke is thus a vascular disorder with a dramatic neurological impact.There is an urgent need for more effective disease management to combat stroke as current treatment paradigms,focusing on vascular recanalization and neuroprotection,have only limited clinical success.Since accumulating data suggests successful long-term neuroprotection is unlikely to be achievable without a functional microvascular network,strategies that reduce endothelial dysfunction could be indispensable.Encouragingly,very recent evidence highlighted herein suggests protecting the humble pericyte may be a new approach to reaching this goal.
The blood-brain barrier (BBB):The brain vascular compartment at the microcapillary level,namely the BBB,plays a critical role in maintaining brain homeostasis and neuronal function (Engelhardt et al.,2014a).This is achieved by transporting essential nutrients and metabolites from blood to the brain while preventing noxious substances circulating in the bloodstream from gaining access to the parenchyma,as well as removing toxins.Cerebral endothelial cells lining the microvessels perform the “gate-keeper” function per se due to the high expression of restrictive tight and adherens junction proteins and various transporters (Engelhardt et al.,2014a).Perivascular pericytes,astrocytes,and the vascular basement membrane that surround the vessels are known to play critical roles in supporting endothelial function and vascular integrity (Figure 1A),however,the complex communication that takes place between these components is still being unraveled.
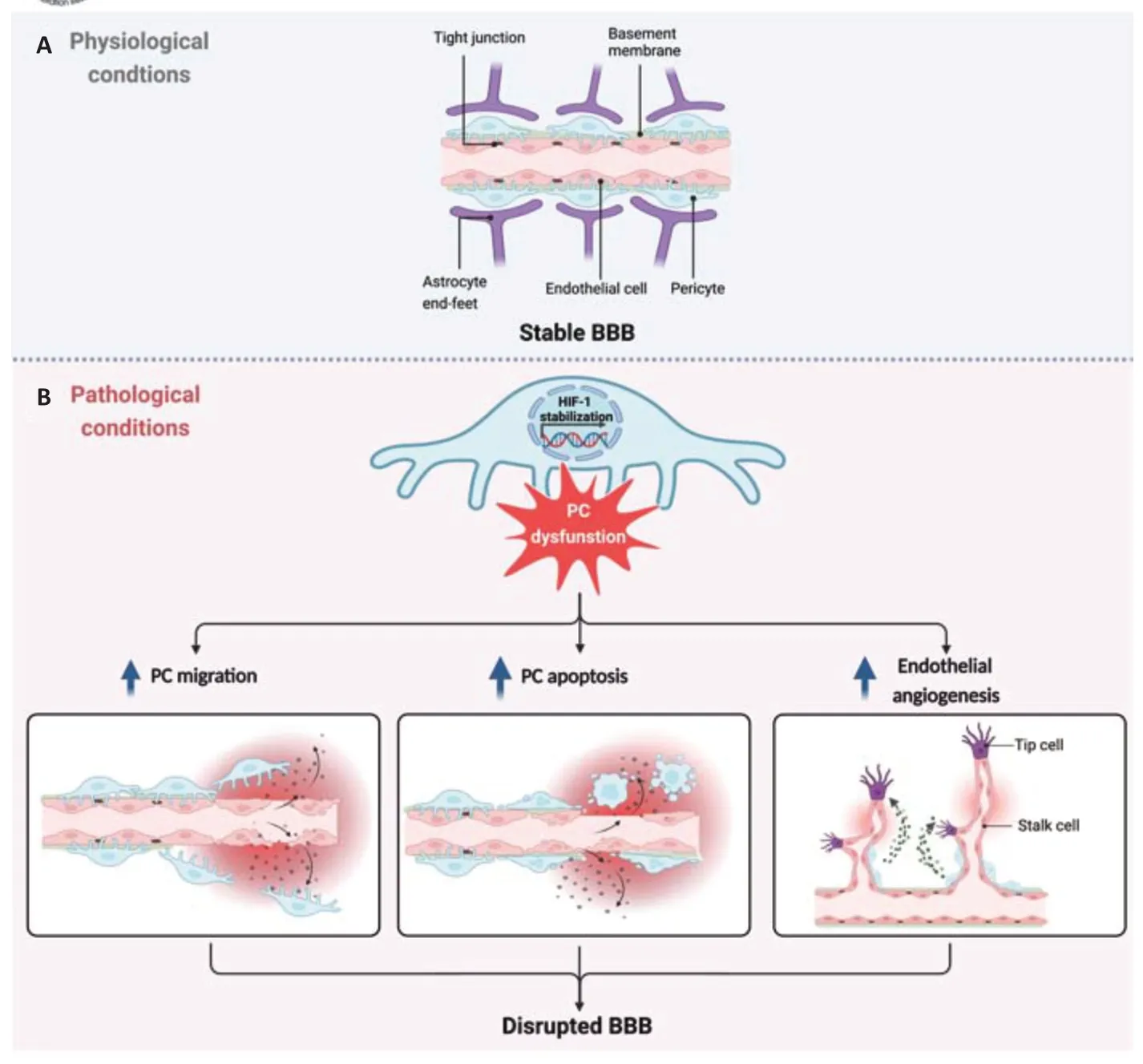
Figure 1|Impact of the pericyte-HIF-1 axis on blood-brain barrier (BBB) stability.
Pericytes at the BBB:In recent years interest in the role of the humble pericyte has dramatically increased.Widely distributed throughout the microvascular network,pericytes locate at the abluminal capillary surface where they share a basement membrane with the endothelial cells(Figure 1A).Pericyte density in the CNS and retina is the highest of all capillary networks reflecting their vital contribution to modulation of these vascularized tissues (Engelhardt et al.,2014a;Zhou et al.,2022).Pericytes influence a wide variety of vascular characteristics and participate in diverse physiological processes including formation and maintenance of the BBB,regulation of blood flow and inflammatory responses,angiogenesis,as well as being a potential source of pluripotent stem cells (Engelhardt et al.,2014a;Zhou et al.,2022).Additional contact with astrocytes means they may also act as a middleman relaying signals from the circulatory system to the neuronal compartment and back.Over the last decade,studies have consistently shown that pericytedriven signaling processes and/or secretion of pericyte-derived factors stabilize the microvascular endothelium (Engelhardt et al.,2014a;Zhou et al.,2022).In contrast,injury-induced pericyte dysfunction correlates with vascular disturbance and injury progression in stroke and other diseases(Engelhardt et al.,2014a;Zhou et al.,2022).For example,mild events such as hypoxic exposure induce the secretion of vascular endothelial growth factor by pericytes thereby stimulating angiogenesis (Engelhardt et al.,2014a),a process that inherently increases vascular permeability.In more severe scenarios such as post-ischemic events pericytes rapidly migrate away from vessel walls and/or constrict and die (Tsao et al.,2021;Zhou et al.,2022),again compromising vascular integrity with hugely detrimental outcomes.Microvascular dysfunction,pathological angiogenesis,and hemorrhage due to loss of vessel pericyte coverage is also seen in diabetic patients and associated with hemorrhage in prematurity,amyotrophic lateral sclerosis,and Alzheimer’s disease (Bennett and Kim,2021).As pericyte loss is increasingly reported in conditions that are risk factors for brain disease,e.g.,aging and hypertension,stroke,and neurodegenerative disease (Bennett and Kim,2021;Tsao et al.,2021;Zhou et al.,2022),limiting pericyte dropout might improve vascular function in these patients.However,a much better understanding of the key events that disturb pericyte functionality is needed before such strategies can be developed.
The pericyte-hypoxia-inducible factor (HIF-1) axis:Recent work demonstrates that a key driver of pericyte susceptibility to injury is the stabilization of HIF-1.A ubiquitously expressed transcription factor,HIF-1 is a master regulator of injury adaptation that enables cells to switch from aerobic to anaerobic metabolism to improve their survival chances during adverse conditions(Engelhardt et al.,2014a).Its well-described neuroprotective properties have stimulated much debate about utilizing HIF-1 stabilizers to treat neurological damage.But HIF-1 has a dark side -it can also activate cell death pathways(apoptotic and autophagic) that remove cells unable to adapt to environmental challenges(Engelhardt et al.,2014a).Studies have shown the negative effects of HIF-1 signaling on barrier functionality with intrinsic stabilization by brain endothelial cells compromising BBB stability primarily through driving angiogenic remodeling(Engelhardt et al.,2014a,b).However,we must remain mindful that capillary endothelial cellsin vivoalso receive extensive input from the everpresent perivascular cells that also induce HIF-1 in response to injury.Indeed,the consequences of perivascular HIF-1 stabilization deserve much more study as both astrocytes and pericytes cells can store,and in response to environmental cues secrete,a plethora of growth factors and cytokines-many of which are downstream targets of HIF-1.This might suggest that perivascular cells elicit similar responses to environmental stimuli that ultimately result in vascular disturbance.However,this is not the case.Astrocytes constantly support endothelial barrier functionality during injury conditions despite HIF-1 stabilization as astrocyte-specific HIF-1 blockadein vivodid not alter hypoxia-induced permeability (Baumann et al.,2022).In complete contrast,pericyte HIF-1 stabilization acutely compromised BBB stability.Other data showed HIF-1 stabilization with cobalt chloride stimulated pericytes to a pro-angiogenic state,promoting wound healing and increased vessel remodeling– an outcome dependent on pericyte exosome secretion and blocked by HIF-1 inhibitors (Mayo and Bearden,2015).In vivo,using Cre-driven pericyte-specific PHD2 and PHD3 deficiency to induce HIF-1 stabilization significantly increased normoxic BBB permeability (Urrutia et al.,2016).Another insightful comparativein vivostudy from our group showed that pericytetargeted HIF-1 loss of function prevented hypoxiainduced barrier dysfunction whereas astrocytetargeted HIF-1 loss of function had no effect(Baumann et al.,2022).Notably,pericyte survival is largely unaffected by hypoxia but more severe injury causes significant cell death as observed in different stroke models (Tsao et al.,2021;Zhou et al.,2022).The data advocates that HIF-1 is closely entwined with pericyte death as pericyte-targeted HIF-1 loss of function ameliorated pericyte loss and maintained their coverage at vessel walls during acute reperfusion stages (Tsao et al.,2021).As this observation correlated with diminished vascular permeability,substantially decreased infarction and better functional recovery (Tsao et al.,2021),it seems that pericyte loss also undermines neuronal repair.More investigation is clearly needed but a causal relationship seems to be emerging that HIF-1-induced pericyte dysfunction increases vascular leak (Figure 1B)and ultimately endangers neuronal survival.The mechanisms underlying this dramatic outcome are largely unclear although as stated above activation of cell death pathways likely plays a role.Another possibility worth considering is that hypoxia-triggered pro-angiogenic factors might push pericytes into an activated and proangiogenic state (Engelhardt et al.,2014a;Mayo and Bearden,2015) whereas HIF-1 blockade would inactivate the signaling and favor quiescence.Extrapolating from published data and due to their links with HIF-1,we speculate that Notch and/or Angiopoietin signaling pathways could be major players in pericyte activation (Figure 1B).
Is HIF-1 a feasible target?The HIF system is an exciting target for therapeutic development but its diverse roles mean global activation of all its downstream components is unlikely to be universally beneficial.During stroke,the clear conundrum is that stabilizing HIF-1 at acute time points might protect neurons but simultaneously induce widespread pericyte death driving vascular compromise and sub-optimal long-term neuronal recovery.In the first instance,employment of pericyte-specific strategies that solely aim to retain pericyte coverage of vessels,particularly at early disease stages may be a way to preserve vascular function.Preclinical studies suggest how to broadly achieve this goal as highlighted in the excellent review by Zhou et al.(2022).Potential therapeutic strategies include promoting pericyte proliferation and maturation,increasing pericyte survival,and regulating pericyte recruitment.Edaravone and cilostazol for example were shown to protect and boost pericyte proliferation and thereby help repair the BBB in a rat stroke model(Zhou et al.,2022).In ischemic brain injury,Sigma-1 receptor agonists ameliorated pericyte death and miantained their coverage of blood vessels.Modulation of the PDGFR axis to promote pericyte proliferation and protect them from apoptosis and thereby restore vascular integrity has also shown potential in Alzheimer’s disease models (Smyth et al.,2022).However,it cannot be disputed that even better specificity and outcome would likely be achieved by directly targeting brain pericytes,and pericyte HIF-1 specifically as the data above indicates.The hindrance here is that a pericyte-specific marker is still to be discovered although transcriptomic analyses have identified potential cerebral pericyte markers (He et al.,2016) that can now be tested.
Another matter of contention is the intricate etiology of many neurological diseases.Stroke pathogenesis is extremely complex and the fact that HIF-1 levels fluctuate during different disease stages presumably underlies conflicting data from different studies (Tsao et al.,2021).We advocate that future therapeutic strategies must factor in the opposing actions of HIF-1 modulators on multiple brain cellular compartments (e.g.,neuronal,non-neuronal,and vascular) during disease progression.Achieving such a delicate balancing act within effective time windows will be challenging but hugely rewarding.To this aim more in-depth studies of cell-specific [HIF-1] responses during sub-acute and late stages must be performed and,as sustained recovery is the ultimate therapeutic goal,particular focus on long-term vascular remodeling and plasticity is critical.A glaringly open question that needs to be answered from the study of Tsao et al.(2021)is will loss of pericyte HIF-1 benefit chronic stroke stages? Will the positive early outcome persist?At later stroke stages pericytes have been shown to foster functional recovery in lesioned sites by inducing fibrotic processes via enhancement of oligodendrogenesis and astrogliosis,and aid tissue remodeling by promoting macrophage clearance and extracellular matrix deposition (Zhou et al.,2022).Through the expression of neurogenic factors pericytes may also participate in poststroke neurogenesis,neuroblast recruitment and act as a neural stem cell source (Engelhardt et al.,2014a).Thus,this multi-functional cell can acquire stemness-like features post-injury and produce various trophic and immunomodulatory factors to activate other cell compartments (Engelhardt et al.,2014a;Zhou et al.,2022).Whether HIF-1 impacts these processes,de-differentiation and/or proliferation of pericyte populations is an intriguing black box that we are currently exploring.
Outside the realms of pericyte-specific HIF-1 modulation,the intrinsic potential of pericytes to repair vascular damage opens the door to cell therapy-directed microvascular targeting as a therapeutic option.Very recently,excellent work by Sun et al.(2020) showed transplantation of human pluripotent stem cell-derived pericytes preserved BBB integrity and promoted functional stroke recovery in mice highlighting the huge potential of pericyte-based cell therapies.The use of patient iPSC-derived pericytes would be of considerable value in this regard.In addition,extrinsically providing/supplementing the pericyte-specific signals that maintain endothelial functionality and barrier stability under physiological conditions may also be a successful tactic within defined time windows.In this respect,metabolomics might give an insight into effective molecules as cell-specific adaptation to injury correlates well with metabolic reprogramming at the BBB (Huang et al.,2020).This approach enabled Huang et al.(2020) to show astrocyte-endothelial glutathione shuttling supports barrier stability and that exogenous glutathione supplementation can reverse injuryinduced BBB disturbance.The metabolomic profile of brain pericytes is still to be reported but similar research will undoubtedly increase the chances of identifying new therapeutic opportunities.
Conclusion:Targeting pericytes to safeguard vascular functionality is certainly a promising therapeutic strategy for stroke and other neurological diseases.To take advantage of the pericyte-HIF-1 axis,however,future studies will need to identify optimal windows of therapeutic opportunity and overcome the challenge of harnessing the neuroprotective effects of HIF-1 while avoiding its detrimental vascular effects.Considerably more exploration is needed.Although it is undeniable that these goals will be more easily achieved after the identification of the elusive pericyte-specific marker,attempts to harness the power of pericytes are already well underway.
This work was supported by Swiss National Science Foundation grant 31003A_150062 (to OOO).
Omolara O.Ogunshola*,Chih-Chieh Tsao
Institute of Veterinary Physiology,Vetsuisse Faculty,University of Zurich,Zurich,Switzerland
*Correspondence to:Omolara O.Ogunshola,PhD,larao@access.uzh.ch.
https://orcid.org/0000-0002-1197-4914(Omolara O.Ogunshola)
Date of submission:September 12,2022
Date of decision:June 1,2023
Date of acceptance:June 15,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380902
How to cite this article:Ogunshola OO,Tsao CC(2024) Harnessing the power of pericytes and hypoxia-inducible factor-1 to modulate stroke outcome.Neural Regen Res 19(3):473-474.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons Attribution Non Commercial-Share Alike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
