Migratory mode transition of astrocyte progenitors in the cerebral cortex: an intrinsic or extrinsic cell process?
2024-02-13MichioMiyajimaHidenoriTabataKazunoriNakajima
Michio Miyajima ,Hidenori Tabata,Kazunori Nakajima
The cerebral cortex is comprised of properly localized cell types that exert their specific functions.In the developing brain,cells migrate from the germinal region to their functional locations (Silva et al.,2019;Cossart and Garel,2022).For example,neocortical excitatory neurons are generated in the cerebral ventricular and subventricular zones,move to the developing cortical plate via radial migration,and reside in a radial array of six neuronal layers (Oishi and Nakajima,2018).On the other hand,cortical interneurons are mainly generated in ganglionic eminences,migrate tangentially across the cerebral cortex,and reach their final destinations in the cortex (Lim et al.,2018).The failure of neuronal migration leads to defects in cortical layer formation.While the mechanisms of neuronal distribution have been well examined,how astrocytes are diffusely distributed in the cortex is still unclear.Astrocytes are glial cells in the cerebral cortex with several functions,including metabolic support and synapse formation (Abbottet al.,2006;Bosworth and Allen,2017;Allen and Lyons,2018).For example,astrocytes establish synaptic connectivity in the developing brain while they contact numerous synapses and maintain optimal neuronal activity in the adult brain.In the developing brain,astrocytes are primarily generated from radial glia after the neurogenic period.While a certain type of astrocyte called fibrous astrocytes populates the white matter,protoplasmic astrocytes migrate to the cortical plate during neural network formation.
There are two types of migratory modes used by astrocyte progenitors within the developing cortex: erratic migration and blood vesselguided migration (Tabata et al.,2022).Astrocyte progenitors undergo erratic migration,followed by blood vessel-guided migration.During erratic migration,these cells move rapidly and irregularly within the intermediate zone and cortical plate of the cortex,while blood vessel-guided migration preferentially migrates astrocyte progenitor cells along blood vessels within the cortical plate.Erratically migrating cells move quicker than neurons undergoing radial migration,frequently change direction,and eventually move toward the cortex surface.On the other hand,because blood vessels tend to run radially in the cortical plate,astrocyte progenitors move radially in the cortex toward the brain surface during blood vesselguided migration.C-X-C motif chemokine receptor 4 (CXCR4),C-X-C motif chemokine receptor 7(CXCR7),and integrin beta 1 (ITGB1) were shown to be required molecular mechanisms of blood vessel-guided migration (Tabata et al.,2022).
Although the two migratory modes for astrocyte progenitors have been described and the molecular mechanism of blood vessel-guided migration is partially characterized,how astrocyte progenitors change the migratory mode and distribute in the cortex is still largely unknown.In this article,we discuss how astrocyte progenitors change their migratory mode from erratic to blood vessel-guided migration.In addition,we discuss why astrocyte progenitors have two different migration modes.
Possible molecular mechanisms to switch the migratory mode of astrocyte progenitors:How do astrocyte progenitors change their modes of migration from erratic to blood vessel-guided?A simple answer to this question is that they change the extracellular proteins or matrix to bind so that the anchorage supports migration.Blood vessels are embedded in many specific types of extracellular matrices.The extracellular matrix of blood vessels is different from that of the cortical parenchyma,where erratic migration occurs;therefore,the migratory mode of astrocyte progenitors may become distinct in each place.From this perspective,it is interesting that ITGB1 is required for the blood vessel-guided migration of astrocyte progenitors (Tabata et al.,2022).ITGB1 binds to various integrin alpha subunits (ITGAs) to form a heterodimer.The substrate bound by the integrin heterodimer is determined by the type of ITGA heterodimerized with ITGB1.By changing the binding partner of ITGB1 within the ITGB1-ITGA heterodimer,blood vessel-guided migration may be initiated.Thus,the kind of ITGAs expressed on astrocyte progenitors and those required for blood vessel-guided migration require further research.Moreover,the possibility that expressions of other integrin beta subunits than ITGB1 compete to bind ITGAs on astrocyte progenitors and hence affect their migration mode is also needed to be tested.Another possible mechanism of changes in the migratory mode is that the expression of ITGB1 is a switch for the migratory mode transition.Astrocyte progenitors express ITGB1,which forms a heterodimer with a specific ITGA and binds to blood vessels.Therefore,astrocyte progenitors may initiate blood-vessel-guided migration.It is reported that ITGB1 is activated by CXCR4 and CXCR7 signaling (Tabata et al.,2022),although the activation mechanism is still unclear.Hence,the expression of these molecules may also contribute to migratory mode transition.Further research will be needed to elucidate the detailed expression mechanisms of these molecules and verify whether their expression is the switch of the astrocyte progenitor migratory mode.
Possible external factors that promote the migratory mode transition of astrocyte progenitors:In addition to the molecular mechanism of migratory mode transition,the extrinsic factors that regulate this transition are also important.Do environmental factors regulate this transition,and if so,what are these factors?As mentioned above,astrocyte progenitors in both migration modes move toward the surface of the cortex,with erratic migration followed by blood vessel-guided migration.The cortex is covered by meninges,which might secrete and form a concentration gradient of some external factor that promotes the migratory mode transition of astrocyte progenitors.It would be interesting to examine whether any of the molecules that are known to be secreted from meninges has such an activity.A higher concentration of such factors on the surface of the cortex may lead to a higher percentage of astrocyte progenitors with blood-vessel-guided migration.Another possible extrinsic factor that regulates the migratory mode transition of astrocyte progenitors is the neuronal layer.The cerebral neocortex is organized into six stacked layers.During cortical development,newly born neurons migrate into the cortical plate and organize themselves in an inside-out fashion so that they populate deeper layers,while lateborn neurons migrate past them to populate more superficial layers (Oishi and Nakajima,2018).Toward the completion of neurogenesis,neural progenitors transition to a gliogenic phase and start to generate astrocytes.Therefore,a major part of the neuronal layer already exists in the cortex when astrocyte progenitors migrate into the cortical plate.Thus,neurons in the cortical plate may send some signals to astrocyte progenitors to cause a migratory mode transition.Indeed,it was reported that layer-specific molecular and morphological differences in astrocytes depend on neuronal layers (Lanjakornsiripan et al.,2018).Hence,migratory mode transition may also be dependent on neuronal layers.For example,the differential expression of cell surface molecules or extracellular matrix proteins between deep and superficial layer neurons may promote the changes in the migration mode of astrocyte progenitors.
As a third extrinsic mechanism for migratory mode transition of astrocyte progenitors,a lack of oxygen and nutrients may promote changes in migratory mode.Erratically migrating cells were reported to be derived from the cortical ventricular zone during the late stages of cortical plate development.By roaming the substantial space away from the blood vessels,erratically migrating cells may lack oxygen and nutrients.Interestingly,CXCR4 expression is regulated by hypoxia-inducible factor-1,which is induced in response to reduced oxygen availability (Staller et al.,2003).Hypoxic conditions may change the migratory mode of astrocyte progenitors from erratic to blood vesselguided migration in search of oxygen-rich blood.Another environmental factor that potentially regulates the migratory mode transition of astrocyte progenitors is microbiota.It is known that neonates are exposed to microbes from just after birth when changes in the migratory mode transition are often observed,which can produce microbe-derived factors,including metabolites and microbe-associated molecular patterns,that enter the blood circulation.As the bloodbrain barrier is immature during the perinatal period,these factors may penetrate the cortex.It is worth noting that among such microbederived factors,lipopolysaccharide can promote the expression of the Cxcr4 gene through tolllike receptor 4 in macrophages (Tian et al.,2019).Similarly,in astrocyte progenitors,microbiotaderived lipopolysaccharide may stimulate toll-like receptor 4 and induce the expression of CXCR4 protein,leading to astrocyte progenitors’ CXCR4-dependent migratory mode transition.
Possible internal factors that regulate the migratory mode transition of astrocyte progenitors:It is also possible that the migratory mode transition of astrocyte progenitors occurs autonomously through cell-intrinsic mechanisms.Astrocyte progenitors actively divide and proliferate and the migratory mode transition may be intrinsically programmed to occur after certain rounds of cell division.Another possibility is that migratory mode transition is regulated by the lineage commitment program.Erratically migrating cells express oligodendrocyte lineage transcription factor 2,which is known to be expressed in astrocyte progenitors in the cortex and in oligodendrocyte progenitor cells.Interestingly,oligodendrocyte progenitor cells also migrate along blood vessels (Tsai et al.,2016).As astrocyte progenitors and oligodendrocyte progenitor cells have common features to express oligodendrocyte lineage transcription factor 2 and are closely related cell types,they may have a common mechanism to start blood vessel-guided migration.ALDH1L1 (aldehyde dehydrogenase 1 family member L1) is an astrocyte lineage-specific marker molecule that is expressed during the period of migratory mode transition (as early as P0) and may be another candidate lineage marker molecule required in a migratory mode transition.One interesting cell-intrinsic mechanism driving the attraction of oligodendrocyte progenitor cells to blood vessels is CXCR4,whose expression is promoted by autocrine Wnt signaling (Tsai et al.,2016).Astrocyte progenitors may also use similar,if not identical,mechanisms to initiate the migration mode transition (Figure 1).
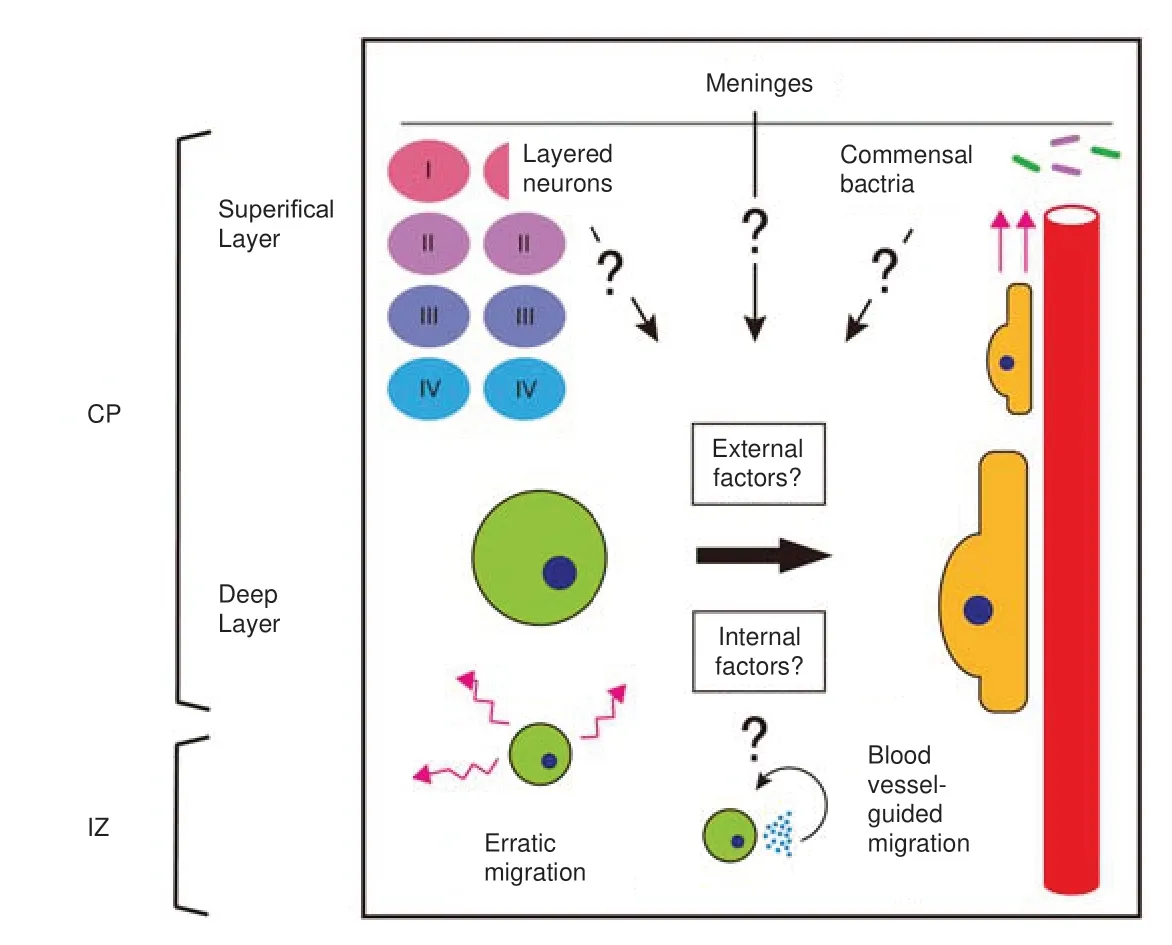
Figure 1|Possible mechanisms of migratory mode transition in astrocyte progenitors.
Biological significance of two different migratory modes of astrocyte progenitors:Why astrocytic progenitors have two different migratory modes is an important biological question,but not yet clearly answered in the current literature.One conceivable answer is that blood vesselguided migration may be required for the efficient migration of astrocyte progenitors to the superficial region of the cortex.As already mentioned,many neurons settle in the cortical plate when astrocyte progenitors invade this zone.Therefore,it is possible that it is difficult for the astrocyte progenitors to reach the superficial part of the cortical plate by passing through the celldense neuronal region using the erratic migration mode.Using a radially oriented blood vessel as a scaffold for migration would likely make it easier for astrocyte progenitors to move through the cortical plate toward the brain surface,similar to neuronal migration along the radial glial fiber.Defects in blood vessel-guided migration can result in a decrease in astrocytes in the surface region of the cortex (Tabata et al.,2022),which also supports our hypothesis.In contrast to neuronal migration,blood vessel-guided migration of astrocyte progenitors is more frequently observed in the superficial part than in the deep part of the cortical plate,while radial glia-guided migration of neurons is observed throughout the cortical plate.This suggests that neuronal migration is more strictly controlled regarding radial positioning than astrocyte progenitor migration.It should be noted that astrocytes are aligned in a roughly birthdate-dependent outside-in pattern (Tabata et al.,2022),as opposed to the relatively strict inside-out pattern of neuronal positioning,which is achieved by each neuron reaching the top of the cortical plate.This difference in the birthdatedependent pattern of cell positioning between neurons and astrocytes may partly be attributed to the difference in their dependency on the radial scaffold of migration.Another potential reason why astrocyte progenitors use blood vesselguided migration in addition to erratic migration may be the attachment of astrocyte progenitors to the blood vessels,which is required for the organized formation of the blood vessel network.As astrocyte progenitors have been observed to bridge neighboring blood vessels (Tabata et al.,2022),they seem to control angiogenesis.For normal angiogenesis,astrocyte progenitors may need to bind to and move around blood vessels.
Conclusions:The research on the mechanism cortical astrocyte progenitors use to switch between erratic migration and blood vessel-guided migration has only recently been conducted,and many questions remain.Answering these questions would lead to the elucidation of how astrocytes are diffusely distributed in the cerebral cortex and how brains are formed in the development stage.Astrocytes are pivotal for normal brain function and are expected to contribute to many psychiatric disorders caused by neuronal dysfunction (Kruyer et al.,2023).Since defects in the blood vesselguided migration of astrocyte progenitors can lead to defective localization of astrocytes in the cortex,the elucidation of the mechanisms of how astrocyte progenitors transition between migration modes may lead to a novel remedy for such psychiatric disorders.
This work was supported by the Japan Science and Technology Agency–Precursory Research for Embryonic Science and Technology (JPMJPR22SA to MM),Japan Society for the Promotion of Science KAKENHI Grant-in-Aid for Scientific Research(JP21K07309 to HT,JP20H05688 and JP22K19365 to KN),Takeda Science Foundation (to KN),Keio Gijuku Academic Development Funds (to KN),and Keio Gijuku Fukuzawa Memorial Fund (to KN).
Michio Miyajima*,Hidenori Tabata,Kazunori Nakajima*
Department of Anatomy,Keio University School of Medicine,Shinjuku-ku,Tokyo,Japan (Miyajima M,Tabata H,Nakajima K)
Department of Molecular Neurobiology,Institute for Developmental Research,Aichi Developmental Disability Center,Kasugai,Aichi,Japan (Tabata H)
*Correspondence to:Kazunori Nakajima,MD,PhD,kazunori@keio.jp;Michio Miyajima,PhD,michio.miyajima@keio.jp.
https://orcid.org/0000-0003-1864-9425(Kazunori Nakajima)
https://orcid.org/0000-0003-6223-2915(Michio Miyajima)
Date of submission:March 21,2023
Date of decision:May 23,2023
Date of acceptance:June 9,2023
Date of web publication:July 20,2023
https://doi.org/10.4103/1673-5374.380886
How to cite this article:Miyajima M,Tabata H,Nakajima K (2024) Migratory mode transition of astrocyte progenitors in the cerebral cortex: an intrinsic or extrinsic cell process? Neural Regen Res 19(3):471-472.
Open access statement:This is an open access journal,and articles are distributed under the terms of the Creative Commons AttributionNonCommercial-ShareAlike 4.0 License,which allows others to remix,tweak,and build upon the work non-commercially,as long as appropriate credit is given and the new creations are licensed under the identical terms.
Open peer reviewers:Kim M A De Kleijn,Radboud University,The Netherlands;Ruben Deogracias,University of Salamanca,Spain.
Additional file:Open peer review reports 1 and 2.
杂志排行
中国神经再生研究(英文版)的其它文章
- Activation of G-protein-coupled receptor 39 reducesneuropathic pain in a rat model
- Chitosan-based thermosensitive hydrogel with longterm release of murine nerve growth factor for neurotrophic keratopathy
- Fasudil-modified macrophages reduce inflammation and regulate the immune response in experimental autoimmune encephalomyelitis
- Artificial intelligence-assisted repair of peripheral nerve injury: a new research hotspot and associated challenges
- Treadmill exercise improves hippocampal neural plasticity and relieves cognitive deficits in a mouse model of epilepsy
- Astrocytic endothelin-1 overexpression impairs learning and memory ability in ischemic stroke via altered hippocampal neurogenesis and lipid metabolism
