Stress relaxation properties of calcium silicate hydrate: a molecular dynamics study
2024-02-05ZhichengGENGShengwenTANGYangWANGHubaoZhenHEKaiWULeiWANG
Zhicheng GENG, Shengwen TANG,, Yang WANG, Hubao A, Zhen HE, Kai WU, Lei WANG
Research Article
Stress relaxation properties of calcium silicate hydrate: a molecular dynamics study

1State Key Laboratory of Water Resources Engineering and Management, Wuhan University, Wuhan 430072, China2Key Laboratory of Advanced Civil Engineering Materials of Ministry of Education, School of Materials Science and Engineering, Tongji University, Shanghai 200092, China3College of Materials Science and Engineering, Xi’an University of Architecture and Technology, Xi’an 710055, China
The time-dependent viscoelastic response of cement-based materials to applied deformation is far from fully understood at the atomic level. Calcium silicate hydrate (C-S-H), the main hydration product of Portland cement, is responsible for the viscoelastic mechanism of cement-based materials. In this study, a molecular model of C-S-H was developed to explain the stress relaxation characteristics of C-S-H at different initial deformation states, Ca/Si ratios, temperatures, and water contents, which cannot be accessed experimentally. The stress relaxation of C-S-H occurs regardless of whether it is subjected to initial shear, tensile, or compressive deformation, and shows a heterogeneous characteristic. Water plays a crucial role in the stress relaxation process. A large Ca/Si ratio and high temperature reduce the cohesion between the calcium-silicate layer and the interlayer region, and the viscosity of the interlayer region, thereby accelerating the stress relaxation of C-S-H. The effect of the hydrogen bond network and the morphology of C-S-H on the evolution of the stress relaxation characteristics of C-S-H at different water contents was elucidated by nonaffine mean squared displacement. Our results shed light on the stress relaxation characteristics of C-S-H from a microscopic perspective, bridging the gap between the microscopic phenomena and the underlying atomic-level mechanisms.
Calcium silicate hydrate (C-S-H); Stress relaxation; Ca/Si ratio; Temperature; Water content; Atomic simulation
1 Introduction
Concrete is one of the most widely used materials in the world, and a better knowledge of its long-term behavior is essential for sustainability as well as for the damage prevention and optimal design of civil engineering structures (Gao et al., 2013; Giorla and Dunant, 2018; Fang and Zhang, 2020; Du and Pang, 2021). Its viscoelasticity plays a critical role in structures with a long service life such as hydropower plants, thus determining the long-term behavior of concrete. The viscoelasticity is referred to as creep and stress relaxation, which induces the redistribution of stress inside concrete (Brooks and Neville, 1976). The stress relaxation phenomenon can be described as the gradual reduction of stress under constant initial deformation. The stress relaxation plays a significant role in the reduction of tensile stress and stress rate of concrete, thus reducing the autogenous shrinkage and the cracking potential of concrete at an early age (Zhang and Qin, 2006; Fu et al., 2015; Atutis et al., 2018; Li ZM et al., 2020; Rong et al., 2021). In recent decades, the stress relaxation behavior of concrete has been extensively investigated. Li et al. (2018) used the finite element method to predict the long-term properties of cement paste and found that the stress-induced dissolution of solid constituents was associated with the stress/strain levels and the selected region. Frech-Baronet et al. (2017), through numerous micro-indentation tests on cement paste, found that in the 30%–85% relative humidity range, higher relative humidity led to a higher relaxation rate of cement paste. Giorla and Dunant (2018) showed by means of finite element simulation that certain micro cracks in cement paste could accelerate the stress relaxation of concrete. Despite extensive studies of the stress relaxation behavior of concrete, its underlying mechanism is not fully understood. It has been widely accepted that intrinsic time-dependent deformation within calcium silicate hydrate (C-S-H) gel, which is generally nearly amorphous, accounts for the main mechanism of stress relaxation of cement paste (Feldman, 1972; Tamtsia and Beaudoin, 2000; Vandewalle et al., 2002; Chen and Qian, 2017; Hou et al., 2018a; Ding and Chen, 2019). As the main binding phase and hydration product in cement paste, C-S-H is perhaps the most widely used amorphous material, exhibiting the ubiquitous characteristics of long-range disorder and short-range order. The Ca/Si ratio (molar ratio) of C-S-H in hydrated Portland cement is usually in the range of 1.5–2.0 with a mean value of about 1.7 (Richardson, 1999). However, to improve the durability of cement-based materials, various supplementary cementitious materials are used, resulting in a change in the Ca/Si ratio of C-S-H (Lothenbach et al., 2011). There have been many theories associated with C-S-H gel accounting for the stress relaxation of concrete, such as the viscous shear theory, the seepage theory, and the microprestress relaxation theory (Bažant et al., 1997; Maruyama et al., 2015; Liu et al., 2020). However, since some of these theories are based on assumptions and lack an appropriate understanding of the nano-structural characteristics of cement paste, most of them relate to the macro level, and it is still a challenge to describe where and how stress relaxation occurs. There are many other physical and chemical behaviors that occur during the process of stress relaxation, such as the nano-diffusion of water molecules in C-S-H gel, rearrangement of cement paste gel, and dissolution of load-bearing phases (Bazant and Chern, 1985; Jennings, 2004; Zhang et al., 2011; Li et al., 2015; Frech-Baronet et al., 2017). Therefore, it is difficult to investigate the stress relaxation behavior separately. In addition, some factors such as temperature, relative humidity, and water content, cannot be adequately explained by direct insights at the molecular level related to the stress relaxation behavior of C-S-H gel.
Molecular dynamics (MD) simulation is a very powerful method of computational chemistry for analyzing the physical motions of atoms or molecules within a system. It can be also used to investigate the structure, dynamics, and interfacial behavior of C-S-H gel (Scrivener and Kirkpatrick, 2008; Hou et al., 2018b). It is believed that MD can bridge simulation-based first-principle method and continuum method, helping gain a fundamental understanding of experimental phenomena at the atomic level, and providing valuable information that cannot be obtained from conventional experiments (Talebi et al., 2014; Shishegaran et al., 2020; Zhang et al., 2021). In recent decades, MD has been used to study the stress relaxation characteristics of numerous materials, such as vitrimers, polymers, and metals. Ciarella et al. (2018) proposed an MD coarse-grained model of vitrimers and proposed that some topological defects accelerated the stress relaxation. Chen et al. (2022) investigated the stress relaxation behavior of a SiO2/Si bilayer composite by nanoindentation with a finite indentation depth of 5.6 nm. Yang et al. (2016) studied the effect of size and temperature on the stress relaxation characteristics of copper using MD and experiments, and developed a formula to describe time, stress, and temperature-dependent deformation. For C-S-H gel, since Pellenq et al. (2009) proposed a realistic molecular structure of C-S-H with a Ca/Si ratio of 1.65, studies of C-S-H using MD have been further developed. Abdolhosseini Qomi et al. (2014) proposed a combinatorial approach to build the atomic structure of C-S-H with a Ca/Si ratio ranging from 1.1 to 2.1 to optimize the mechanical properties of cement paste at nanoscale. Due to its amorphous structure, it is quite hard to determine the morphology of C-S-H gel. However, at the atomic level, it is widely accepted that C-S-H presents seemingly a layered-like structure. In addition, to describe the interactions among atoms within the C-S-H model, the potential of force fields such as ClayFF, CSHFF, and ReaxFF has been developed (Cygan et al., 2004; Shahsavari et al., 2011; Manzano et al., 2012; Pitman and van Duin, 2012). CSHFF potential is a modified version of ClayFF, demonstrating a better prediction ability than ClayFF (Shahsavari et al., 2011). CSHFF potential has been widely accepted in the simulation of cement-based materials. For example, Kai et al. (2021) found that high temperature and interlayer water content could accelerate the creep of C-S-H, based on this force field. Youssef et al. (2011) studied the structural and dynamic properties of water ultra-confined in the nanopores of highly disordered C-S-H. Arayro et al. (2018) found that cesium ions had little effect on the elastic property of C-S-H according to the effect of cesium sorption on cement paste.
While there have been many studies of the viscoelasticity of C-S-H, most have focused on its creep behavior and few on its stress relaxation behavior. Therefore, there is still a huge gap in understanding the stress relaxation response of C-S-H at the molecular level and providing a reasonable explanation for the experimental phenomenon. In addition, some influencing factors of the stress relaxation of C-S-H, such as the applied initial deformation states (shear, tensile, and compressive modes), initial deformation magnitude, temperature, Ca/Si ratios, and water contents, have not been fully investigated at the atomic level.

2 Simulation method
2.1 Model construction
The C-S-H model was constructed based on the tobermorite model with an interlamellar spacing of 11 Å, and followed the procedures proposed by Pellenq et al. (2009) and Abdolhosseini Qomi et al. (2014). Firstly, the tobermorite 11 Å without water treated with supercell and orthogonalization was taken as the initial configuration, and the structure size was 22.32 Å×22.17 Å×22.77 Å (L×L×L). Next, the silicate chains were broken by randomly removing some charge-neutral SiO2groupsto match the experimental results obtained from nuclear magnetic resonance tests (Li J et al., 2019; Li B et al., 2020), thereby obtaining structures with different Ca/Si ratios (1.3, 1.7, and 1.9). Then, the water molecules were absorbed back into the dry structures above obtained from the previous step by grand canonical Monte Carlo (GCMC) method using CSHFF, and C-S-H molecular structures with different water contents (0%, 30%, 50%, 70%, and 100%) were obtained (Shahsavari et al., 2011). During the GCMC simulation, the chemical potential of the water was 0 eV, corresponding to the bulk water at room temperature. When the dry structures reached a saturated state, the water to silicon ratios were 1.02, 1.84, and 2.18 when the Ca/Si ratios were 1.3, 1.7, and 1.9, respectively, which were in accordance with those obtained from experiments (Allen et al., 2007). Then, a semi-classical MD simulation using ReaxFF potential with a time step of 0.25 fs was performed on the structures with adsorbed water molecules for 4 ns at room temperature, allowing water molecules to react with silica groups and interlayer calcium to form Si–OH and Ca–OH (Pitman and van Duin, 2012). The final equilibrated atomic configuration of C-S-H with a Ca/Si ratio of 1.7 is shown in Fig. 1.
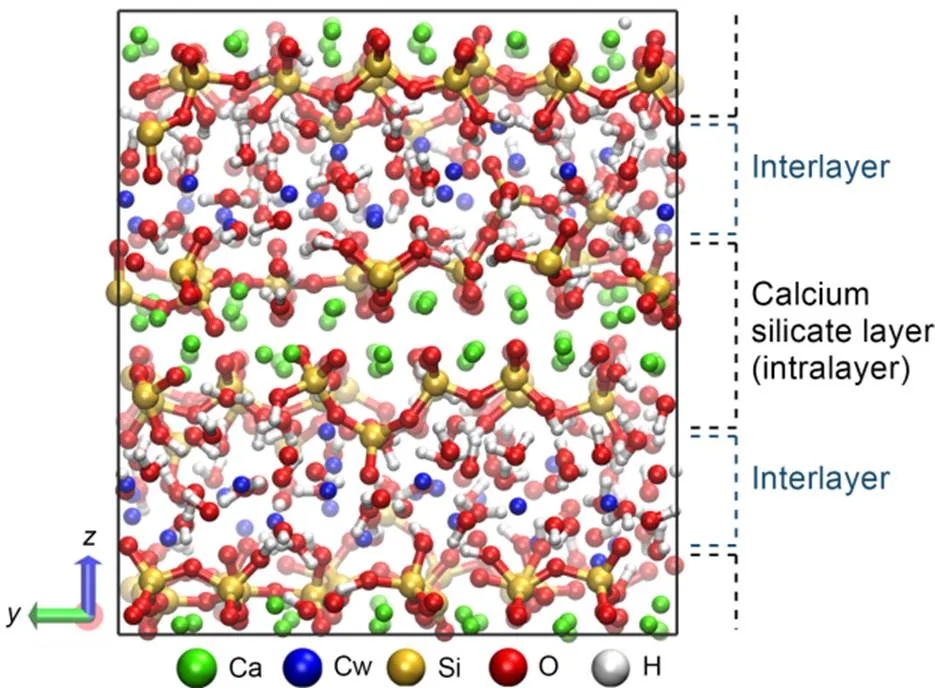
Fig. 1 Snapshot of the molecular model of C-S-H structure considered herein. Ca: intralayer calcium; Cw: interlayer calcium; Si: silicon; O: oxygen; H: hydrogen. References to color refer to the online version of this figure
2.2 Force field
CSHFF potential was used to simulate the interatomic interaction in the C-S-H system, except for the part handled by ReaxFF potential in the model building phase. By fitting ab initio-based lattice parameters and elastic constants of several minerals in cement-based materials, CSHFF potential has been widely and effectively used to predict the energy, structural information, and mechanical properties of cement-based materials, especially C-S-H. The force field parameters used in this simulation can be directly obtained from the related references (Pitman and van Duin, 2012; Abdolhosseini Qomi et al., 2014). To confirm the reliability of the force field, results obtained by the MD simulation are usually compared with experimental and first-principle calculation results. In recent years, an alternative routine based on machine-learning interatomic potentials was proposed by Mortazavi et al. (2021a, 2021b),which had first-principle calculation accuracy but MD efficiency to predict the mechanical response of different materials. Their studies made a great contribution to bridging first-principle-based modes to continuum models, and provided a broad perspective for generating reliable potentials.
2.3 Simulation procedures
Firstly, to remove the internal stress for further structural and dynamic investigation, a supercell (2×2×2) of C-S-H molecular structure constructed above was equilibrated using CSHFF potential for 4 ns in an isothermal-isobaric (NPT) ensemble at 298 K and 101.325 kPa to removal internal stress for subsequent structural and dynamic analysis (Abdolhosseini Qomiet al., 2014). The size of the equilibrated structures was around 4.4 nm×4.4 nm×4.6 nm. Secondly, uniaxial tension tests were conducted on the different directions in the NPT ensemble with constant strain rate of 0.08 ps-1to obtain the stress–strain relation of C-S-H. Then, shear loadings with a series of constant strain rates ranging from 0.8 to 0.0008 ps-1in canonical (NVT) ensembles using the SLLOD method (Todd and Daivis, 2017) were conducted in thedirection of C-S-H to clarify the effect of loading rate on the strength and stress relaxation of C-S-H.
To study the stress relaxation behavior of C-S-H subjected to different initial deformation states (shear, tensile, or compressive modes) with different deformation levels, different shear deformations (γandγ) and uniaxial deformations (ε,ε, andε) in opposite directions were applied individually to the equilibrated C-S-H structure. Four different initial shear deformations (shear angle2°, 4°, 8°, and 12°) were selected to study the effect of different deformation magnitudes on the stress relaxation behavior. A uniaxial strain (=0.04) was used to study the effect of direction on the stress relaxation behavior of C-S-H.
To study the effect of the Ca/Si ratio on stress relaxation behavior, C-S-H with different Ca/Si ratios (1.3, 1.7, and 1.9) was firstly equilibrated for 4 ns in the NPT ensemble at 298 K and 101.325 kPa. Then, a shear deformation (γ=4°), lower than the ultimate strain of the C-S-H molecular structure, was applied to the equilibrated C-S-H structure with different Ca/Si ratios. It was reported that the rupture strength of the C-S-H molecular structure was about 3 GPa by shear simulation (Pellenq et al., 2009). To study the effect of temperature on stress relaxation behavior, the C-S-H structure with a Ca/Si ratio of 1.7 was firstly heated from 298 to 373 or 423 K within 1 ns, and then allowed to relax in the NPT ensemble for 4 ns at the temperature to which it was heated. Secondly, the equilibrated C-S-H molecular structures were subjected toγ=4° to investigate the stress relaxation behavior of C-S-H under different temperatures. When studying the effect of different water contents on the stress relaxation behavior of C-S-H molecular structure, the C-S-H model (Ca/Si ratio is 1.7) with different water contents (0%, 30%, 50%, 70%, and 100%) was equilibrated for 4 ns at 298 K and 101.325 kPa, prior to the application ofγ=4°.
In conventional stress relaxation experiments, the specimen is loaded to a specified strain at a constant strain loading rate under specified temperature or humidity conditions, and then the strain is sustained for a period of time. During the stress relaxation process, directions other than the strain direction are not constrained. To mimic the conventional stress relaxation experiments, the C-S-H structure was deformed to the desired shear deformation at a constant strain rate of 0.08 ps-1in NVT using the SLLOD method for all the initial shear deformations mentioned above. For the uniaxial deformation, a constant strain rate of 0.08 ps-1was used to make the C-S-H structure deform to the desired deformation in the NPT ensemble. Then, the C-S-H structure was maintained at the specific strain for 4 ns with a Nosé- Hoover thermostat and a time step of 1 fs. CSHFF potential was used throughout the equilibrated and stress relaxation processes. Periodic boundary conditions were applied in all directions. Additionally, the NPT ensemble was implemented to keep the pressure in those undeformed directions constant (101.325 kPa) during the stress relaxation process so that the effect of Poisson could be considered. All MD simulations in this study were performed using the Large-scale Atomic/Molecular Massively Parallel Simulator (LAMMPS) package.
3 Results and discussion
3.1 Stress relaxation of C-S-H
3.1.1Effect of loading strain rate
To confirm the validity of the C-S-H model, we compared the mechanical parameters obtained in this study with those calculated from experiments and first-principles. In this study, several uniaxial tensile tests were performed on the C-S-H structure with a Ca/Si ratio of 1.7. Fig. 2 presents the stress–strain curves of C-S-H with a Ca/Si ratio of 1.7 under uniaxial tension tests, and Table 1 shows the related mechanical parameters obtained. In the- and-directions, Young's moduli in this simulation are in good agreement with those obtained from the nano-indentation tests (50–60 GPa), the ab initio calculation (55–68 GPa), and the MD simulation results (about 60 GPa) (Constantinides and Ulm, 2004, 2007; Pellenq et al., 2009; Hou et al., 2015b). The stress–strain curves in different directions can be divided into three stages: the elastic, yield, and damage stages (Fig. 2). In the elastic stage, the relation between stress and strain can be explained by Hooke's law. Note that the stress is lower in the-direction than in theanddirections, indicating that the mechanical performance of C-S-H in thedirection is the weakest. In addition, the stress in thedirection has a sudden drop at a strain of around 0.2 Å/Å, indicating the C-S-H structure suffers a brittle failure. The mechanical performance of C-S-H in this study was similar to that observed in other simulations (Hou et al., 2015b).

Fig. 2 Stress–strain curves of C-S-H with a Ca/Si ratio of 1.7 in different tensile loading directions
The effect of loading strain rate on the shear behavior of C-S-H in thedirection with a Ca/Si ratio of 1.7 and subsequent stress relaxation results are presented in Fig. 3. The loading rate has an obvious impact on the evolution of the stress–strain curves and a positive relationship with the slope of stress–strain curves and strain at failure. When the strain rate is 0.08 ps-1, the strain at failure is aroundγ=8°. Additionally, the stress–strain curves under different strain rates have a similar shape and can also be divided into three stages: the elastic, yield, and plastic stages. As expected, a large stress can be obtained by a large loading strain rate. Similar results have been reported from tension tests on cementitious materials (Rossi, 1997). The results indicate that a small loading rate can provide sufficient time to allow the rearrangement of atoms or molecules, so as to enhance the growth of cracks in the C-S-H gel and lead to early failure. In addition, water confined in the interlayer region plays an important role in the return force and delays the failure of C-S-H. This effect becomes profound with the increase of loading strain rate (Hou et al., 2014b).
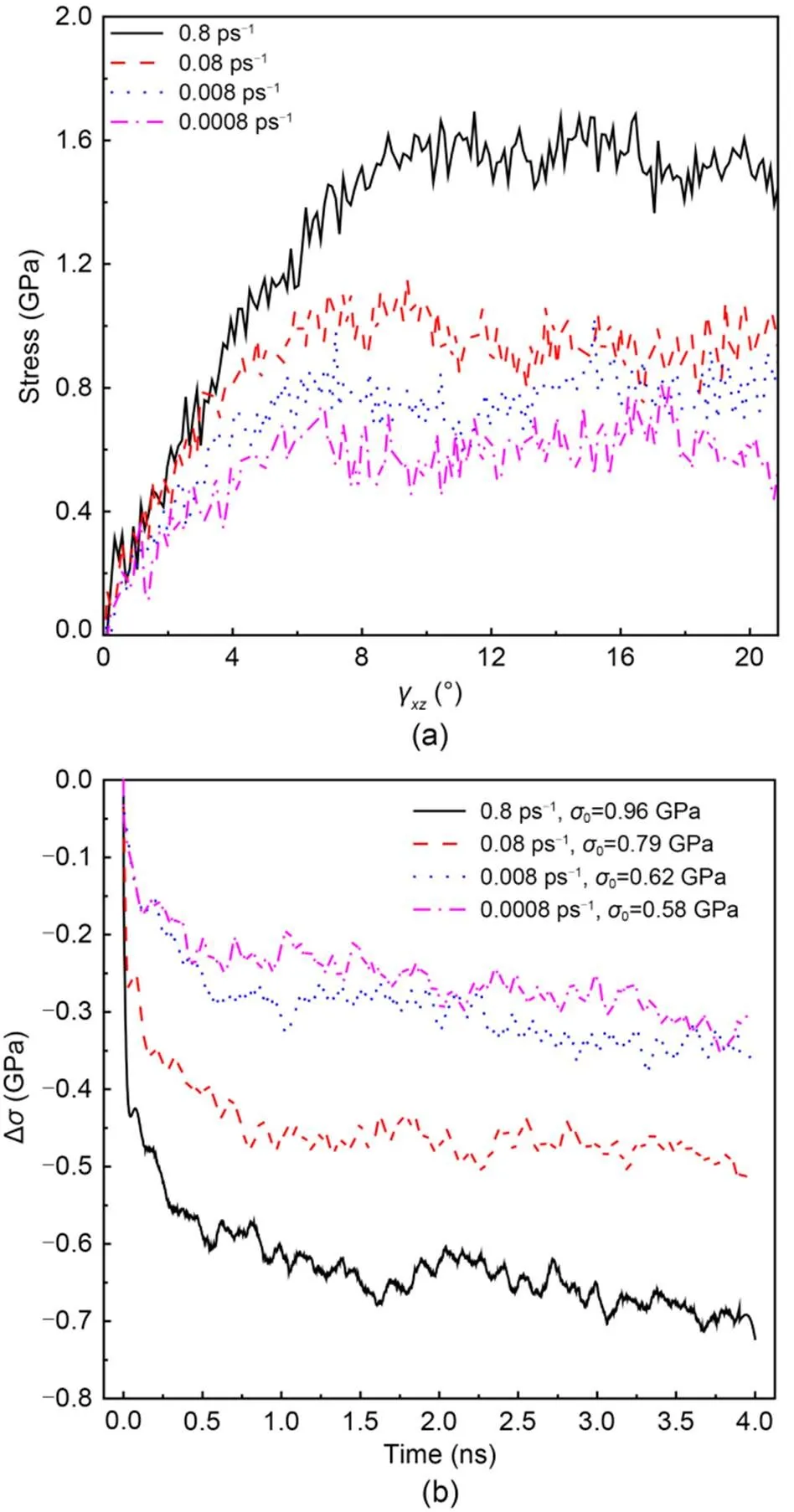
Fig. 3 Mechanical response of C-S-H with a Ca/Si ratio of 1.7 under different shear loading strain rates in the xz direction: (a) stress–strain curves; (b) evolution of the stress difference Δσ in the stress relaxation process under γxz=4° (σ0 is the initial stress)
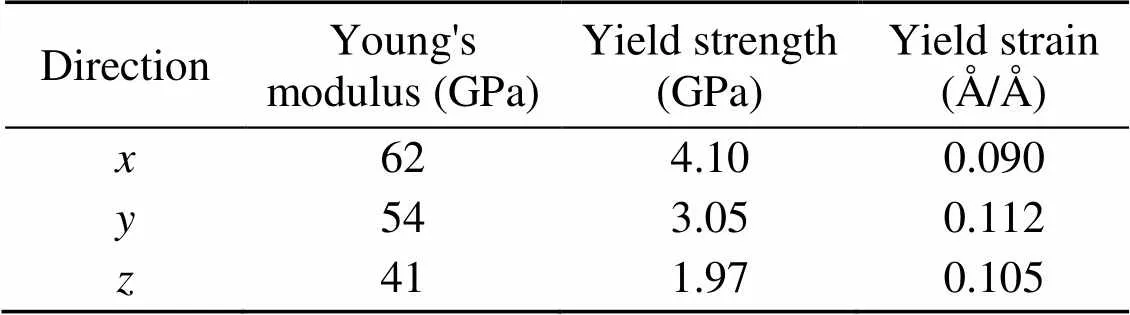
Table 1 Mechanical parameters of C-S-H with a Ca/Si ratio of 1.7 obtained from uniaxial tension tests
Under the same strain condition (γ=4°), the effect of loading strain rate on the subsequent stress relaxation process is shown in Fig. 3b. Due to the existence of thermal fluctuations in the process of MD simulation, the stress relaxation results obtained from MD simulation are not constant. To demonstrate the effect of thermal fluctuations on the stress relaxation results, 10 stress relaxation tests were carried out. C-S-H with a Ca/Si ratio of 1.7 was first shear deformed toγ=4° at a shear loading rate of 0.08 ps-1, then a stress relaxation test with a length of 4 ns was performed. The stress relaxation result under this condition was used throughout this study. For each stress relaxation simulation, the stress value at specific time, such as at 0.5 ns, was obtained by averaging the stress data within 10 ps before and after this time so as to ensure the accuracy and rationality of stress data. Then, the stress data of 10 tests at a specific time were processed. Compared with the overall mean and standard deviation values at each specific time, the data used for figure rendering are reliable (Table 2).
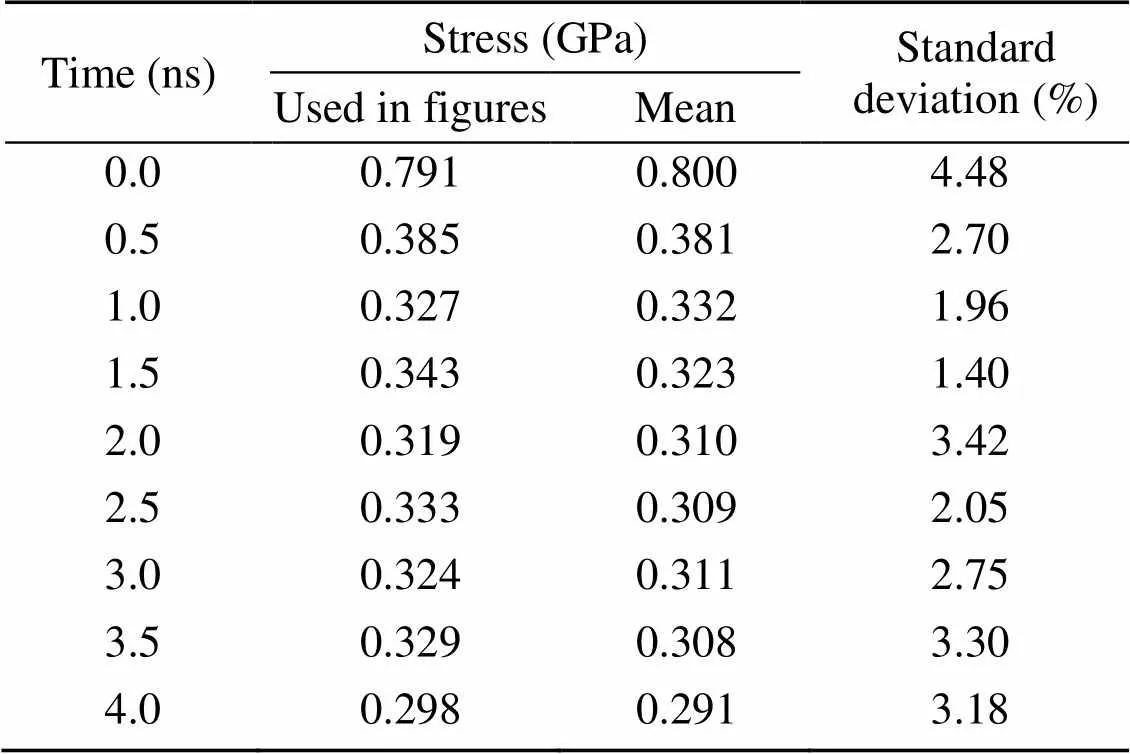
Table 2 Mean and standard deviation values of the stress relaxation simulation results of C-S-H (Ca/Si ratio is 1.7, γxz=4°, and strain rate is 0.08 ps-1)
It is clear from Fig. 3b that a large strain rate causes a large drop in stress in the early stage of the stress relaxation process, followed by a similar stress evolution stage. The stress difference ∆(calculated by Eq. (1)) is about 0.65 GPa at 1 ns when the applied strain rate is 0.8 ps-1. When the strain rate is not larger than 0.008 ps-1, this difference is not obvious. The effect of loading strain rate on the stress relaxation of C-S-H can be explained by the movement of water molecules confined in the interlayer region from the high-stress area to the low-stress area. The cracks expand further within a short time, resulting in rapid stress release in the early stage of the stress relaxation process. Considering the effect of water on the mechanical performance of the C-S-H gel, a loading strain rate of 0.08 ps-1is adopted in the following sections. The size effect plays a critical role in the evaluation of fractures and stress condition, especially at nanoscale. Talebi et al. (2014) gave a unique insight into using reasonable model sizes in simulation. In the presence of cracks at nanoscale, they proposed the following relation as the fundamental assumption, based on homogenization theories. For the existence of disparate length scales,Cr=RVE=Spec, whereCr,RVE, andSpecare the sizes of the crack, respective volume element, and specimen, respectively. Because most of the tests performed in this study are within the elastic range, the size of the crack is much smaller than that of the model. Moreover, the use of period boundary conditions can mitigate the effect of size and improve the convergence of simulation results so as to ensure the accuracy of MD results.
3.1.2Effect of initial deformation state
Different magnitudes of shear, tensile, or compressive deformation were applied to the equilibrated C-S-H system to reveal the stress relaxation behavior of C-S-H and its underlying mechanism. The stress evolution of C-S-H over time under different initial deformation states at a temperature of=298 K with a Ca/Si ratio 1.7 is shown in Figs. 4 and 5. Whether C-S-H is subjected to a shear, tensile, or compressive deformation, the stress evolution of the entire C-S-H system takes on a typical L-shape during the stress relaxation process. As shown in Figs. 4a and 4b, in a certain range of shear deformation (γandγ), the initial stress0increases with the increase of, indicating that the applied shear deformations are in the elastic or elastoplastic range. As the relaxation time passes, the stress gradually decreases, and a high initial shear angle corresponds to a large stress difference ∆:
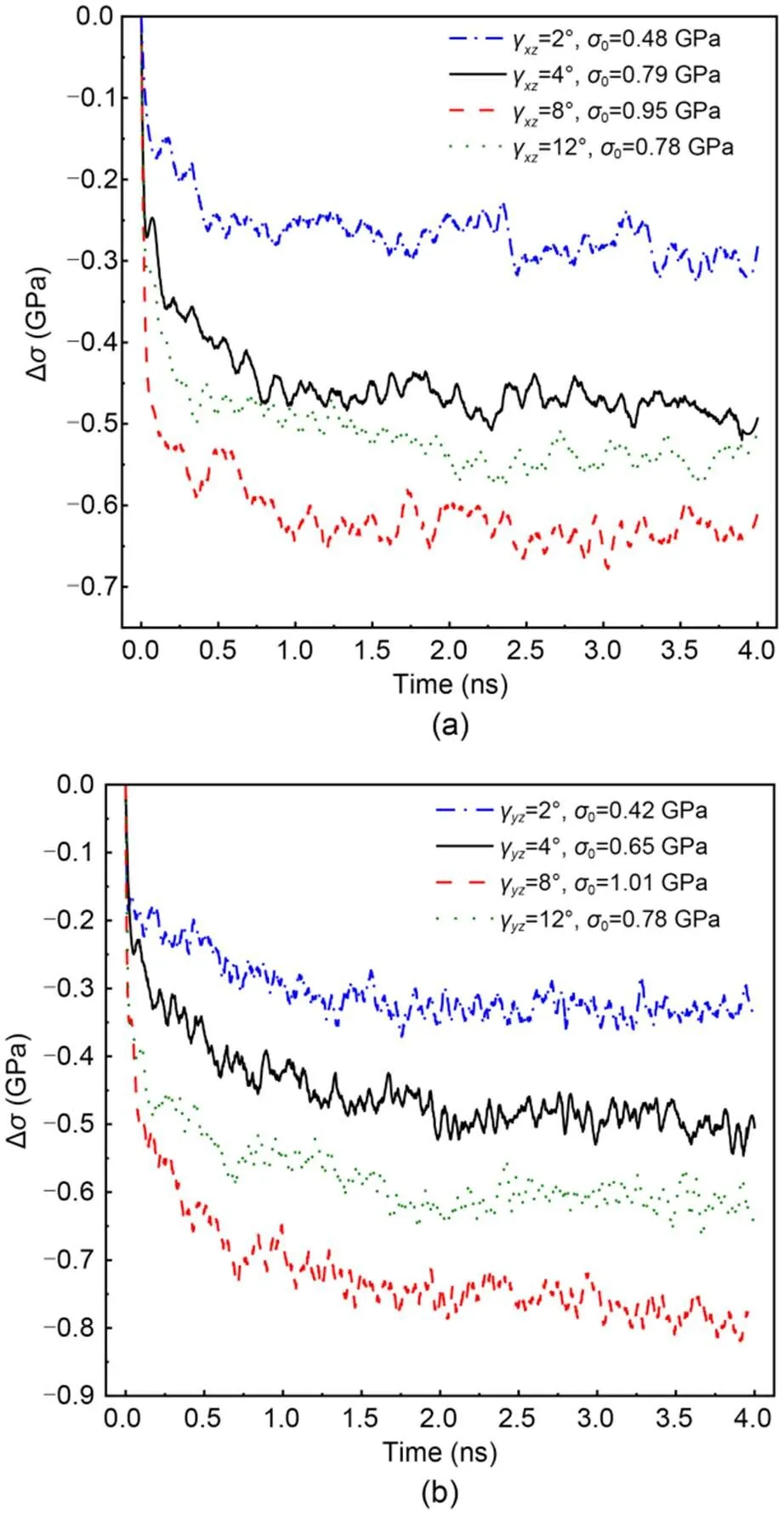
Fig. 4 Change in stress of C-S-H subjected to different shear deformations at T=298 K: (a) evolution of Δσ under γxz; (b) evolution of Δσ under γyz
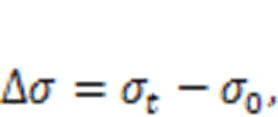
whereσis the stress of C-S-H at time. In addition, the stress declines slowly at a late stage. Such stress relaxation behavior under shear deformation is quite similar to that observed by experiment (Alizadeh et al., 2010). However, once the initial shear deformation exceeds the elastoplastic range, some micro cracks are probably generated within C-S-H systems, resulting in a relatively small ∆during the stress relaxation process. In addition, comparing Figs. 4a and 4b shows that the ∆of C-S-H under shear deformationγis slightly larger than that under shear deformationγ, within the same relaxation time. For example, ∆underγ=8° at 4 ns is about 0.78 GPa compared with 0.62 GPa underγ=8°. This can be attributed to the structural heterogeneity caused by the orientation of the silicate chains in the calcium-silicate layer (Manzano et al., 2013). Figs. 5a and 5b show the stress evolution of the C-S-H system under tensile or compressive deformation=0.04 with different directions, respectively. Due to the structural heterogeneity of C-S-H, the stress behaviors in different directions also show some differences. As shown in Figs. 5a and 5b, the maximum0and residual stress are in the-direction, followed by those in the-direction and-direction. Comparing Figs. 5a and 5b shows that the ∆of the C-S-H system under compressive deformation is slightly larger than that under tensile deformation. For instance, ∆is 0.91 GPa when C-S-H is subjected to compressive deformation and 0.83 GPa when subjected to tensile deformation. However, regardless of whether the initial deformation is tensile or compressive, the residual stress in the- or-direction is similar, except in the-direction. Compared to the other two directions, the-direction is more prone to brittleness due to the unstable hydrogen bonding, which weakens the interlayer cohesion (Hou et al., 2015a, 2015b). Under the same initial deformation conditions, micro cracks are more likely to occur within the C-S-H during the stress relaxation process, especially in the interlayer region, resulting in increased stress relaxation and low residual stress (Beushausen et al., 2012; Giorla and Dunant, 2018).
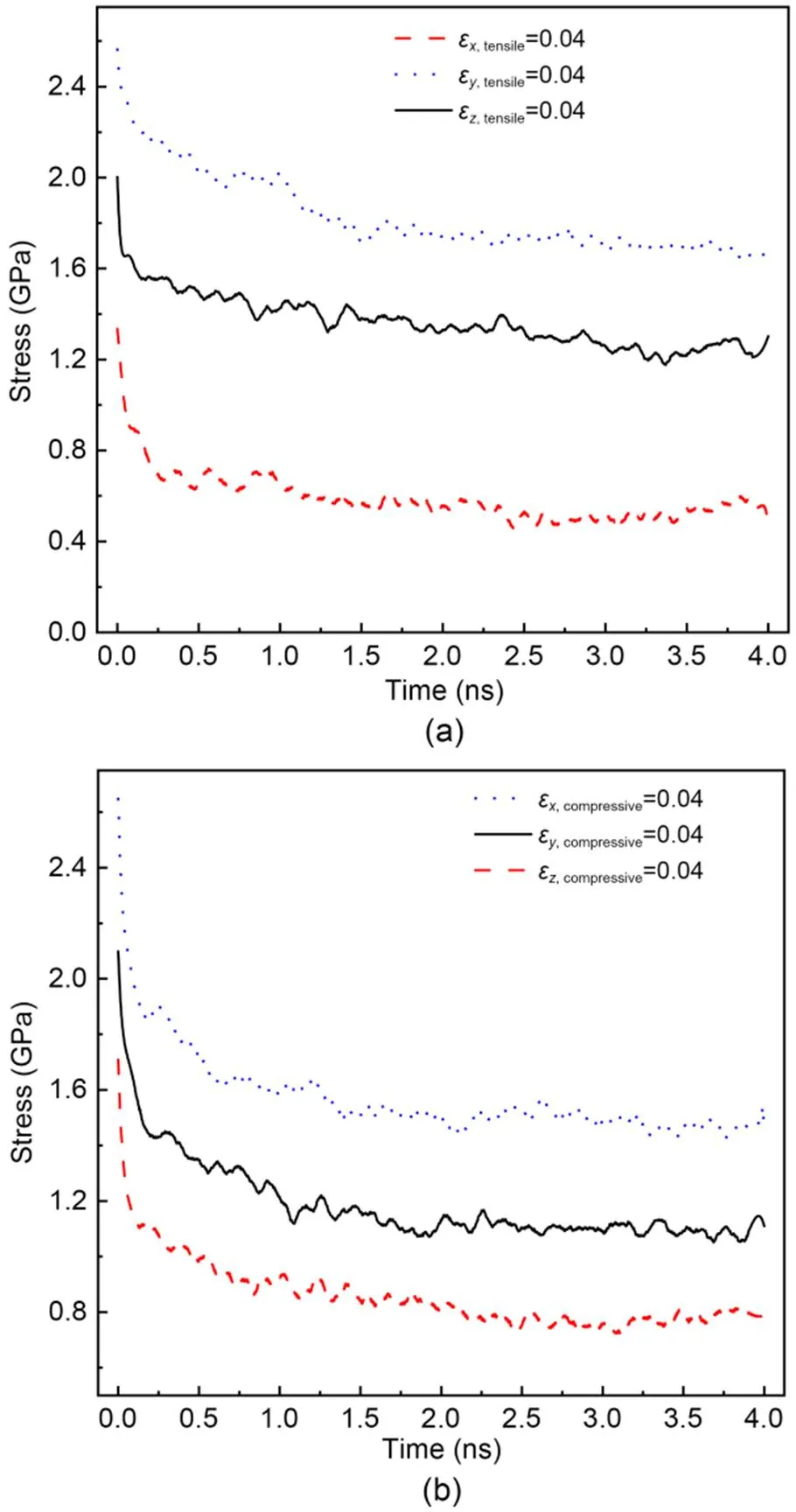
Fig. 5 Change in stress of C-S-H subjected to different uniaxial deformations at T=298 K: (a) evolution of stress under tensile deformation in different directions; (b) evolution of stress under compressive deformation in different directions
To better understand why the stress relaxation of C-S-H occurs, atomic displacement magnitude profiles are shown in Fig. 6. Figs. 6a and 6b show C-S-H subjected to two shear deformations with=4°, while Figs. 6c and 6d show C-S-H subjected to tensile and compressive deformation, respectively, with strain=0.04. Comparing Figs. 1 and 6 shows that whether the C-S-H is subjected to shear, tensile, or compressive deformation, the highlighted region in the C-S-H structure is concentrated mainly in the interlayer region composed of water molecules, hydroxyl groups, as well as interlayer calcium atoms. The dark blue region is referred to as the intralayer region where calcium-silicate layers exist. The stress relaxation of C-S-H is attributed mainly to atomic movement within the interlayer region.
To regain the equilibrium state, the movement of interlayer atoms causes some chemical events to occur in the interlayer region of C-S-H, especially the breakage and reformation of bonds, which is similar to what occurs during the process of creep. There are two main bonds associated with the process of stress relaxation: the Ca–O ionic bond and the hydrogen bond (H-bond). According to previous studies (Wang et al., 2004; Hou et al., 2014a), a Ca–O ionic bond is formed when the distance between the calcium and oxygen atoms is not larger than 3 Å. Two conditions are required for the formation of an H-bond: the first is that the distance between the donor hydrogen atom and the acceptor oxygen atom is not larger than 2.45 Å, and the second is that the angle between the donor oxygen-donor hydrogen vector and the donor oxygen-acceptor oxygen vector is not larger 30°. To evaluate the influence of initial deformation levels on the stress relaxation process of the C-S-H structure, the history-dependent() associated with the fluctuation of the bonds in the interlayer region is calculated (Youssef et al., 2011; Gowers and Carbone, 2015):
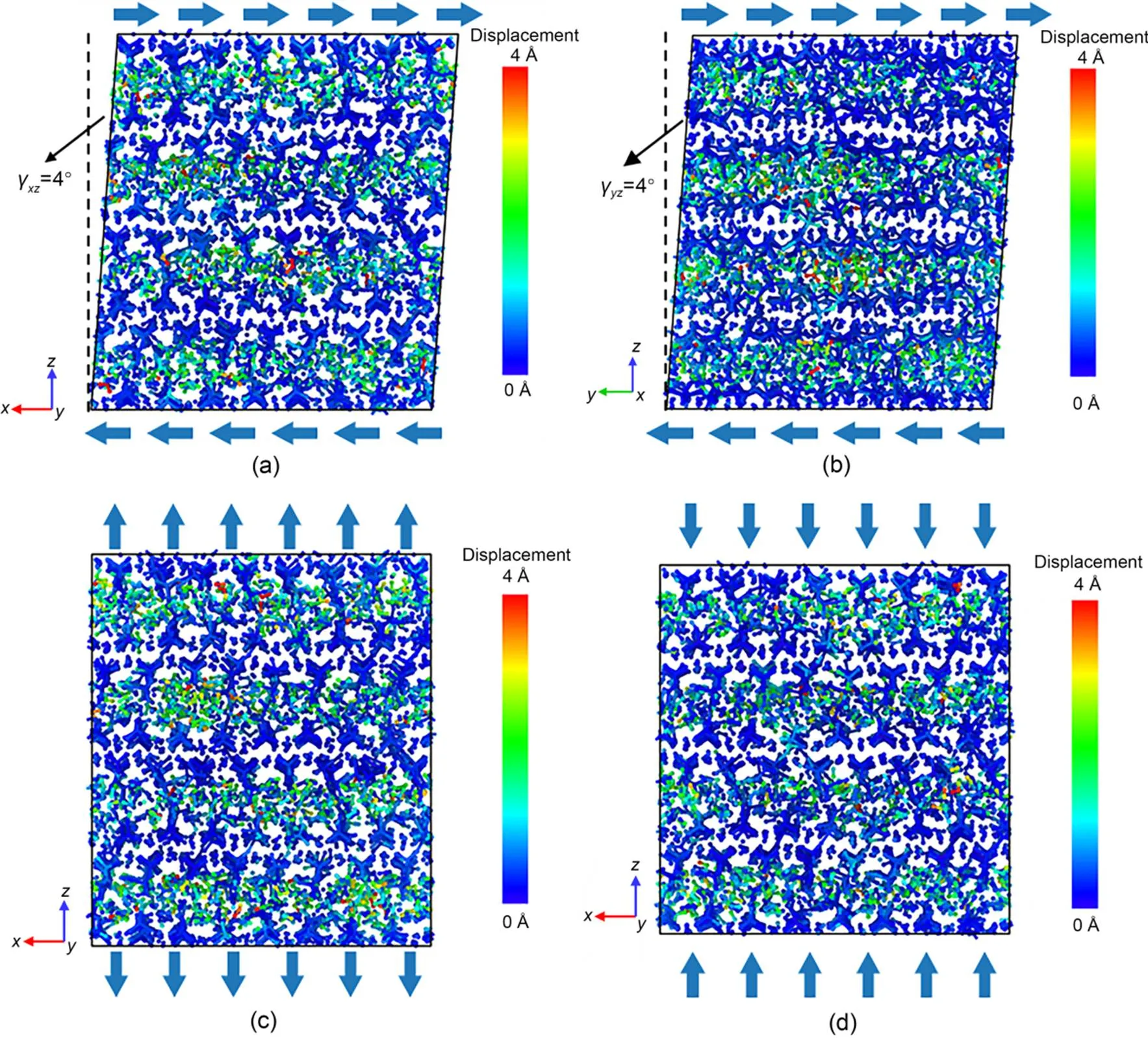
Fig. 6 Magnitude of the atomic displacement of the C-S-H structure under different initial deformation states: (a) γxz=4°; (b) γyz=4°; (c) εtensile=0.04; (d) εcompressive=0.04. References to color refer to the online version of this figure

To better understand the process of stress relaxation of the C-S-H structure, the potential energy of C-S-H systems subjected toγandγof different initial magnitudes is shown in Fig. 9. The stress relaxation process is accompanied by a decline in potential energy regardless of the magnitude and direction of the initial shear deformation. Additionally, the magnitude of initial shear deformation and the difference in potential energy (Δ), calculated by Eq. (3), have a positive relation.
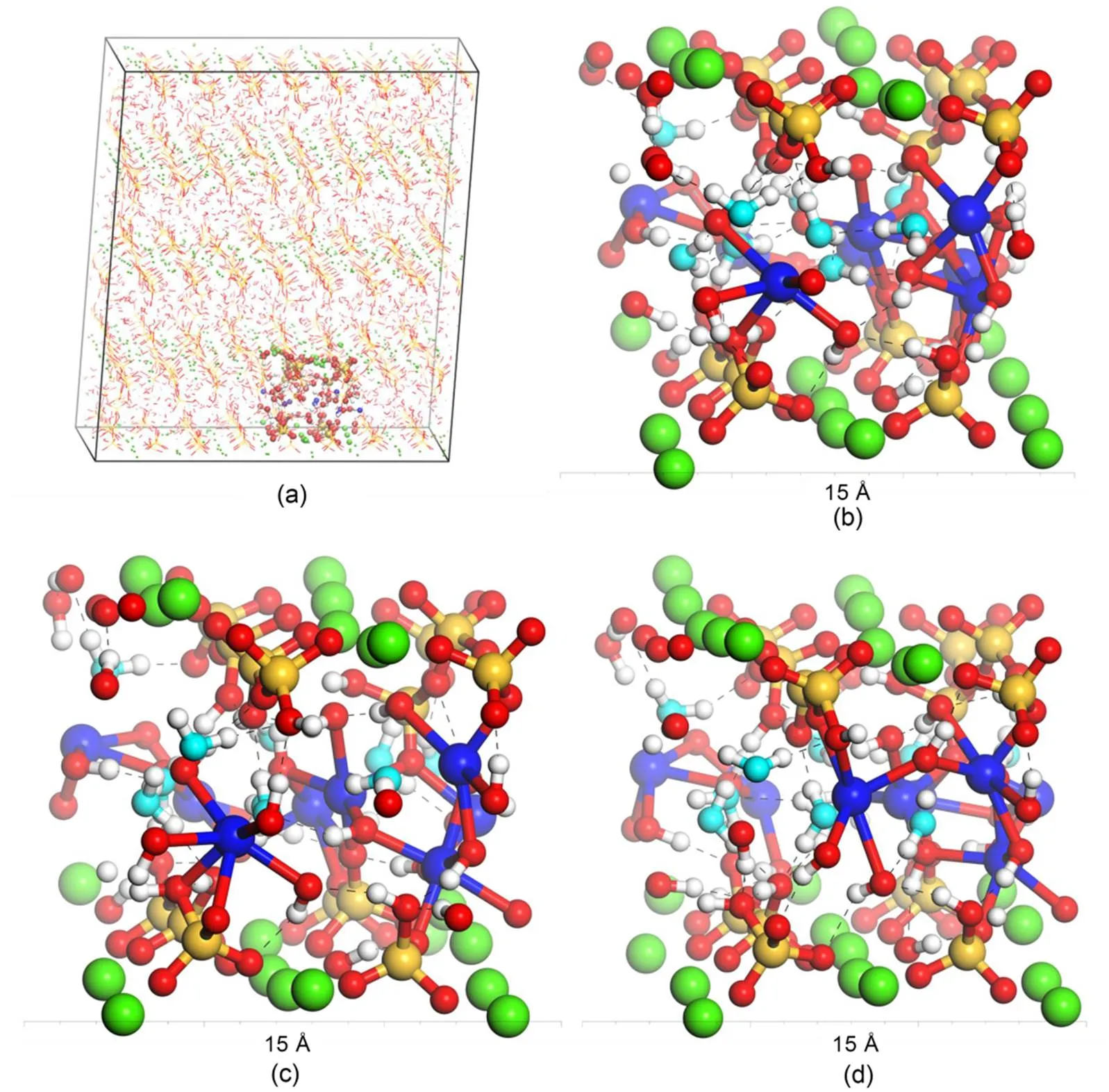
Fig. 8 Changes in local microstructure during the stress relaxation process at different time nodes with the breakage and reformation of interlayer Ca–O bonds and H-bonds: (a) molecular structure of C-S-H under γxz=4°, T=298 K, and t=0 ps (the local structure shown in ball-and-stick is the chosen region); (b) local molecular structure at t=100 ps (the dashed black line indicates the H-bonds); (c) local molecular structure at t=200 ps; (d) local molecular structure at t=4 ns. The Ca–O bonds and H-bonds defined herein refer to the criterion proposed by Hou et al. (2014a) and Wang et al. (2004), respectively. The atoms shown here are consistent with those shown in Fig. 1 except that cyan means oxygen in water molecules. References to color refer to the online version of this figure
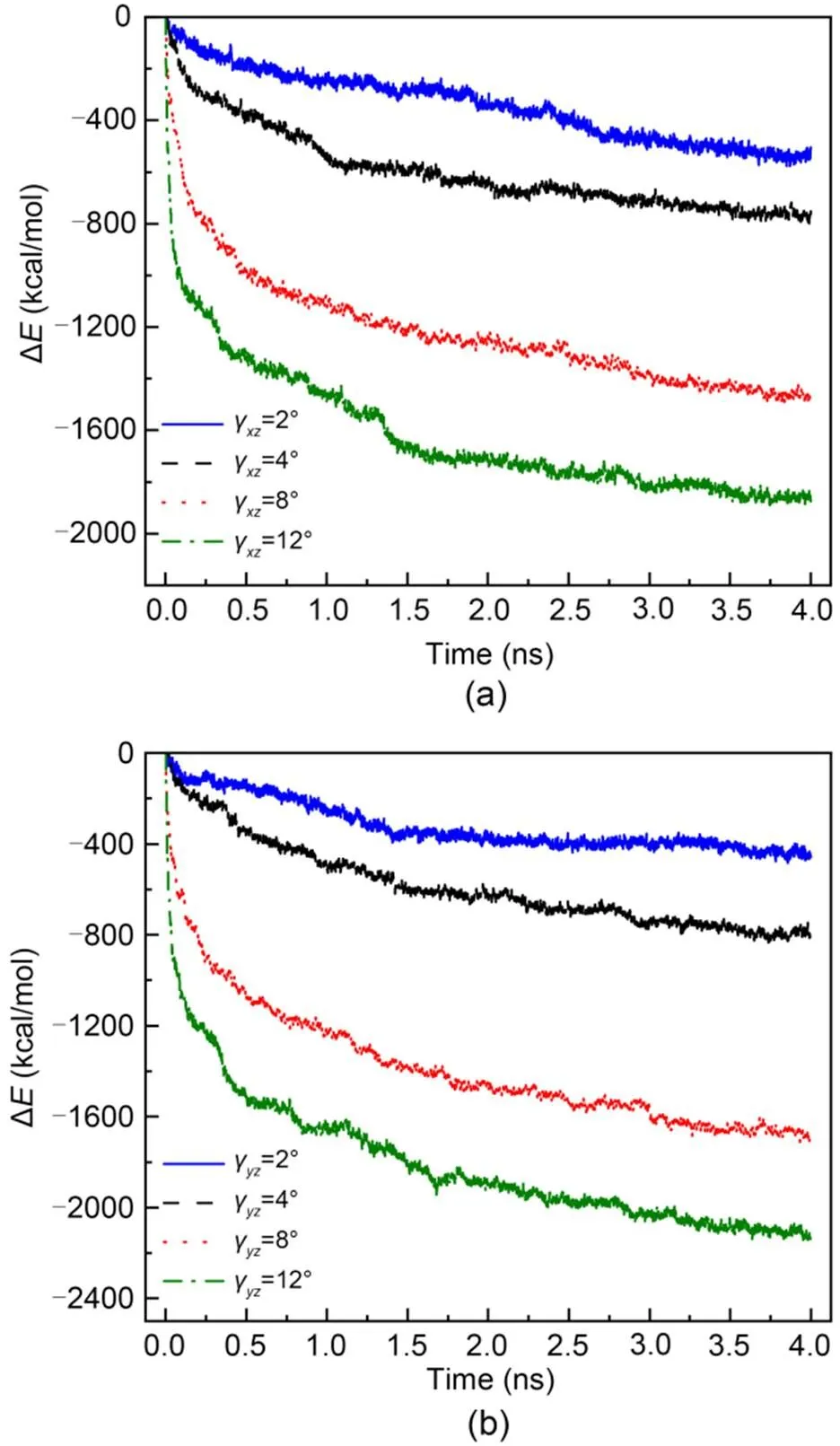
Fig. 9 Evolution of ΔE during the stress relaxation process subjected to two shear deformation states at T=298 K: (a) γxz; (b) γyz
3.2 Effect of Ca/Si ratio and temperature
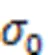

Fig. 10 Change in stress of C-S-H subjected to different Ca/Si ratios and temperatures under γxz=4°: (a) evolution of stress with different Ca/Si ratios at T=298 K; (b) evolution of stress at different temperatures with a Ca/Si ratio of 1.7
To further understand the mechanisms of the effects of Ca/Si ratio and temperature on the stress relaxation behavior of C-S-H, the viscosity of the interlayer region was considered. At the microscopic level, viscosity can be regarded as the internal friction among molecules as they move under external forces, which are determined mainly by the intermolecular interactions and temperature (Han et al., 2010; Feng et al., 2018). MSD enables changes in the viscosity of the interlayer region of C-S-H during the stress relaxation process to be considered (Tang and Wong, 2015). The magnitude of atomic movement in the interlayer region was evaluated by MSD (Youssef et al., 2011):
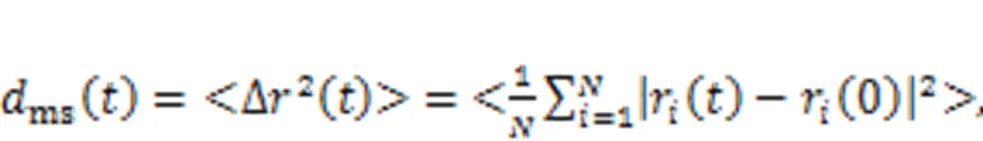

Fig. 11 MSD of constituent parts in the C-S-H system under γxz=4°: (a) MSD of each atom/molecule/group in C-S-H at T=298 K (Ob: bridge oxygen atom); (b) MSD of interlayer calcium; (c) MSD of hydroxyl group; (d) MSD of water molecule

Fig. 12 C(t) of the interlayer Ca–O bonds (a) and H-bonds (b) subjected to different Ca/Si ratios and temperatures under γxz=4°
3.3 Effect of water content
To reveal the effect of water content on the stress relaxation behavior of C-S-H, the evolution of Δ(calculated by Eq. (1)) of C-S-H with 0%, 30%, 50%, 70%, and 100% water content were studied underγ=4° at=298 K (Fig. 13). When there are no water molecules in the C-S-H, the stress inside C-S-H drops twice during the stress relaxation process: once in the first stage, by 0.52 GPa, and once again at 1.7 ns, by 0.27 GPa. The stress evolution of the C-S-H is changed after 30% water is added to the dry structure, reflected mainly in the first stage. The stress reduction is larger than that in the dry condition, while the final residual stress is basically the same as that in the dry condition. When the water content of the C-S-H structure increases to 50%, the stress relaxation of C-S-H becomes easier, and the secondary stress reduction that occurs in the cases of 30% water content and a dry state does not take place. However, when the water content exceeds 50%, Δdecreases with additional water absorbed. These results of stress evolution during the stress relaxation process are similar to those obtained by Liang et al. (2022) for the stress relaxation of C-S-H colloid at different humidities. In addition, the stress evolution of the C-S-H is seriously affected by the interlayer water content, which is in agreement with the findings of Alizadeh et al. (2010). They proposed a model for stress relaxation and creep by studying the viscoelastic nature of glass, C-S-H, and cement paste. This model indicated that the removal of a certain amount of water would increase the stress relaxation of C-S-H, and that some inconsistent changes in the stress relaxation after interlayer water removal may be due to the cross-linking of silicate tetrahedra and the interaction of calcium ions with silicate chains at different humidity levels.
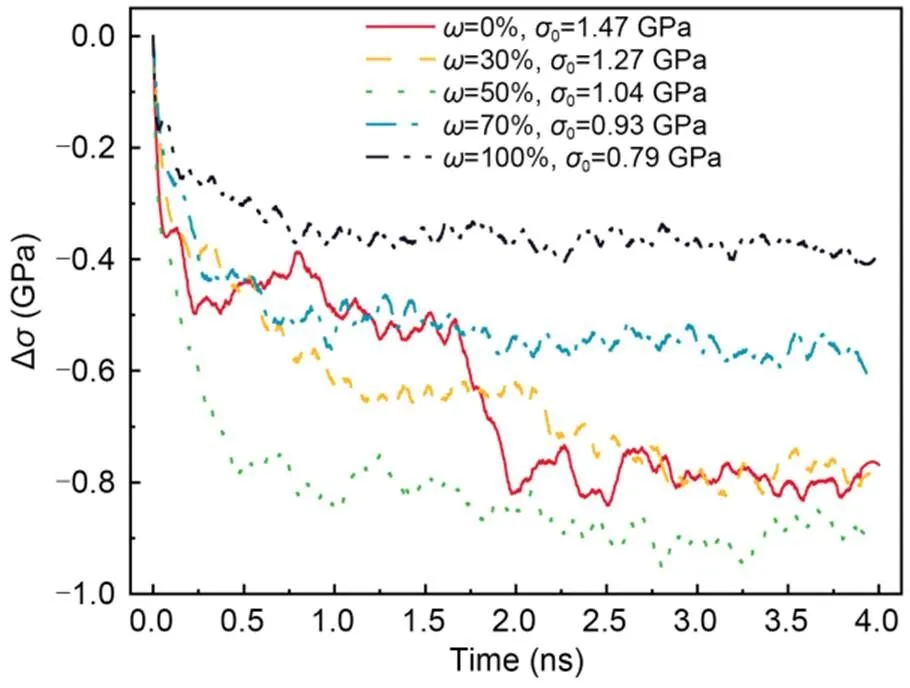
Fig. 13 Evolution of Δσ of C-S-H with different water contents (ω) under γxz=4° and T=298 K
A structural analysis was performed to reveal the structural transformation of C-S-H with the removal of water. The atomic number density profiles along the-direction for the intralayer atoms and interlayer atoms in four equilibrated atoms with different water contents as a function of the distance along the-direction are shown in Fig. 14. They show that the water content plays a quite important role in the distribution of interlayer atoms. As water molecules are removed from the interlayer region, the interlayer spacing decreases. However, the intralayer spacing is hardly affected by the water content and remains unchanged. Obviously, the cohesion between the calcium-silicate layer and the interlayer region is significantly affected by the interlayer water. The results also suggest that the removal of interlayer water molecules may enhance the probability of interaction between the silicate tetrahedron and the interlayer calcium (Alizadeh et al., 2010). As the water content declines, the number of interlayer Ca–O bonds increases while the number of H-bonds apparently decreases. In such case, the packing of water molecules transforms from a multi-layer to a monolayer configuration, leading to the evolution of the H-bond type, in turn affecting the bridging between nearby calcium-silicate layers (Hou et al., 2014a). Therefore, interactions between the interlayer atoms and the calcium-silicate layer atoms are responsible for the stress relaxation of C-S-H at different humidity levels.

Fig. 14 Atomic number density profiles of interlayer and intralayer atoms in equilibrated C-S-H along the z-direction with water contents of 0%, 30%, 70%, and 100% from top to bottom
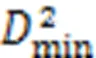
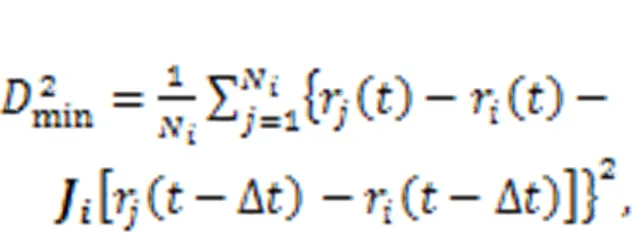
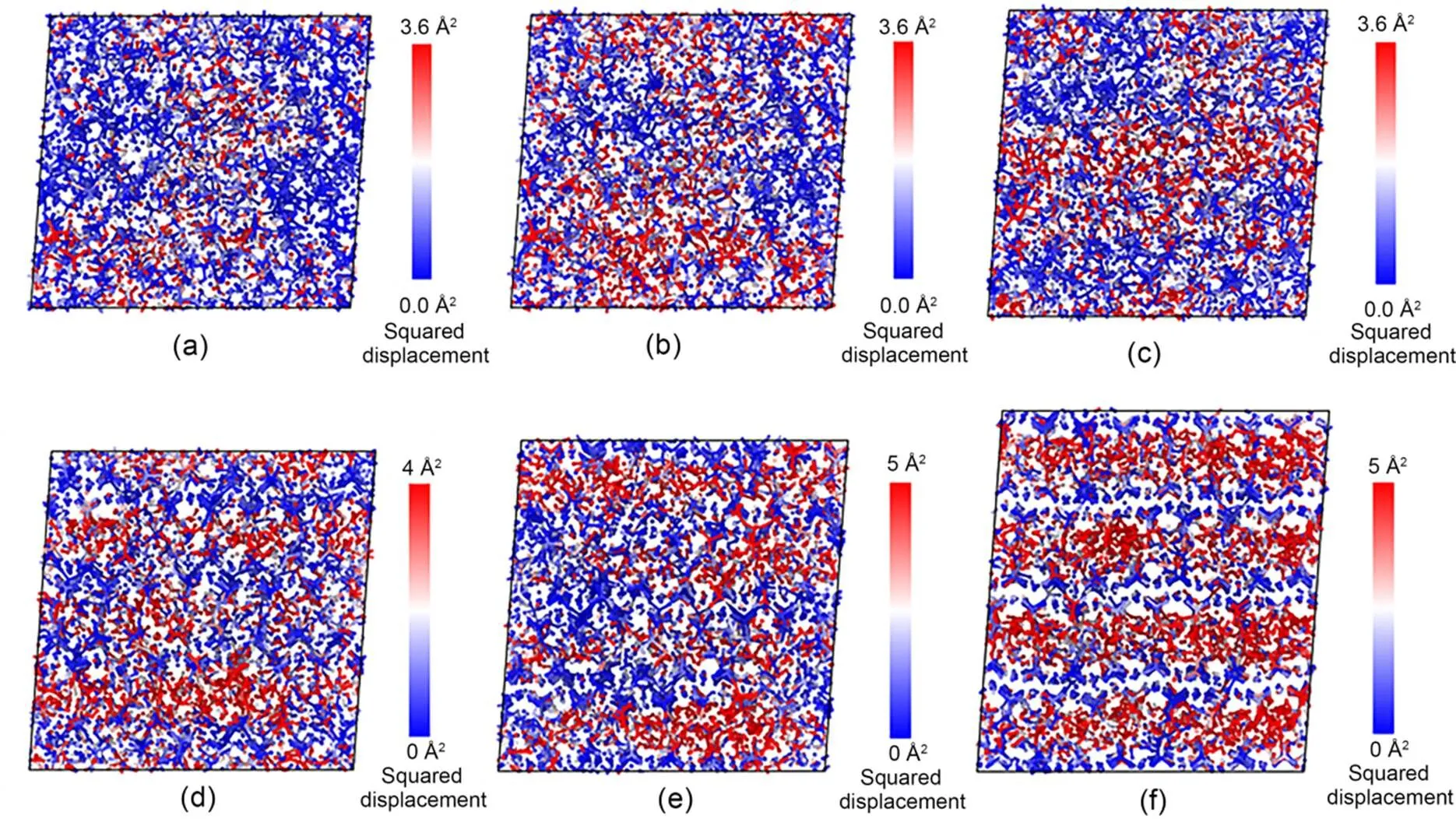
Fig. 15 Intensity plots of nonaffine squared displacement of C-S-H system with different water contents (rcut=3 Å): (a) ω=0%, t=300 ps, Δt=300 ps; (b) ω=0%, t=2.5 ns, Δt=2.5 ns; (c) ω=30%, t=300 ps, Δt=300 ps; (d) ω=50%, t=300 ps, Δt=300 ps; (e) ω=70%, t=300 ps, Δt=300 ps; (f) ω=100%, t=300 ps, Δt=300 ps
The effect of water molecules on the process of stress relaxation of C-S-H can be explained as follows. When the water content of C-S-H is lower than 50%, water has a prominent influence on the morphology of C-S-H. As the water content increases, the C-S-H structure transforms from a relatively disordered str ucture to a clearly layered one, remarkably affecting the stress relaxation behavior of C-S-H. However, when the water content is higher than 50%, the increasing water molecules lead to a weakening of the bonds between the calcium-silicate layers causing them to "slip" easily, thereby reducing the stress relaxation occurring in C-S-H as the water content increases.
4 Conclusions
The effects of different initial deformation states, Ca/Si ratios, temperatures, and water contents on the stress relaxation behavior of C-S-H, as well as the underlying stress relaxation mechanisms at the atomic-level, were studied using MD simulation with a CSHFF. The results of our simulations were in good agreement with those published from experiments. The simulations explained the stress relaxation characteristics at the atomic level and bridged the gap between atomic simulation and experimentation. The following findings were obtained:
1. The stress relaxation response of C-S-H occurs regardless of whether it is under initial shear, tensile, or compressive deformation, and shows a heterogeneous characteristic. The evolution of stress during the stress relaxation process originates mainly from the breakage and reformation of the H-bond and Ca–O bond network in the interlayer region, accompanied by the reduction of potential energy, thus reaching a relatively low energy state. A large initial deformation results in a short interlayer Ca–O and H-bond lifetime and a large drop in potential energy.
2. A large Ca/Si ratio or high temperature leads to low viscosity in the interlayer region and low cohesion between the calcium-silicate layer and the interlayer region, resulting in high atomic dynamics, especially for water molecules, hydroxyl groups, and interlayer calcium atoms. The initial stress and residual stress of C-S-H in the stress relaxation process decrease as the Ca/Si ratio or temperature increases.
3. Water molecules play a critical role in the stress relaxation performance of C-S-H. When the water content of C-S-H decreases from saturation state to 50%, the morphology of interlayer water molecules gradually transforms from a multi-layer to a monolayer configuration. Nonaffine deformation occurs mainly in the interlayer region, but gradually increases in the calcium-silicate layers, and the stress relaxation process of C-S-H also gradually speeds up. As more and more water molecules are removed from the interlayer region, a longitudinal shear band gradually replaces the transverse band in the nonaffine deformation, and the stress relaxation process of C-S-H gradually slows down. In addition, when the water content of C-S-H lowers to a certain extent, some small adjacent nonaffine deformations connect with each other to form a transverse shear band when the stress relaxation time is long. This accounts for the secondary stress drop in the stress relaxation process.
This work is supported by the National Natural Science Foundation of China (Nos. 51602229 and U2040222), the Opening Project of Key Laboratory of Advanced Civil Engineering Materials of Ministry of Education (Tongji University), and the Water Conservancy Science and Technology Project of Hunan Province (No. XSKJ2021000-15), China.
Zhicheng GENG: investigation, methodology, data curation, formal analysis, visualization, writing–original draft. Shengwen TANG: conceptualization, funding acquisition, supervision, validation, writing–review & editing. Yang WANG: visualization, formal analysis, project administration. Hubao A: validation, writing–review & editing. Zhen HE: resources, methodology. Kai WU: supervision, data curation. Lei WANG: writing–review & editing.
Zhicheng GENG, Shengwen TANG, Yang WANG, Hubao A, Zhen HE, Kai WU, and Lei WANG declare that they have no conflict of interest.
Abdolhosseini Qomi MJ, Krakowiak KJ, Bauchy M, et al., 2014. Combinatorial molecular optimization of cement hydrates., 5:4960. https://doi.org/10.1038/ncomms5960
Alizadeh R, Beaudoin JJ, Raki L, 2010. Viscoelastic nature of calcium silicate hydrate., 32(5):369-376. https://doi.org/10.1016/j.cemconcomp.2010.02.008
Allen AJ, Thomas JJ, Jennings HM, 2007. Composition and density of nanoscale calcium–silicate–hydrate in cement., 6(4):311-316. https://doi.org/10.1038/nmat1871
Arayro J, Dufresne A, Zhou TT, et al., 2018. Thermodynamics, kinetics, and mechanics of cesium sorption in cement paste: a multiscale assessment., 2(5):053608. https://doi.org/10.1103/PhysRevMaterials.2.053608
Atutis M, Valivonis J, Atutis E, 2018. Experimental study of concrete beams prestressed with basalt fiber reinforced polymers. Part II: stress relaxation phenomenon., 202:344-354. https://doi.org/10.1016/j.compstruct.2018.01.109
Bazant ZP, Chern JC, 1985. Concrete creep at variable humidity: constitutive law and mechanism., 18(1):1-20. https://doi.org/10.1007/BF02473360
Bažant ZP, Hauggaard AB, Baweja S, et al., 1997. Microprestress-solidification theory for concrete creep. I: aging and drying effects., 123(11):1188-1194. https://doi.org/10.1061/(asce)0733-9399(1997)123:11(1188)
Beushausen H, Masuku C, Moyo P, 2012. Relaxation characteristics of cement mortar subjected to tensile strain., 45(8):1181-1188. https://doi.org/10.1617/s11527-012-9825-2
Brooks JJ, Neville AM, 1976. Relaxation of stress in concrete and its relation to creep., 73(4):227-232. https://doi.org/10.14359/11070
Chen J, Fang L, Chen HQ, et al., 2022. The loading speed facilitating stress relaxation behaviors of surface-modified silicon: a molecular dynamics study., 28(6):160. https://doi.org/10.1007/s00894-022-05136-5
Chen JK, Qian C, 2017. Loading history dependence of retardation time of calcium-silicate-hydrate., 147:558-565. https://doi.org/10.1016/j.conbuildmat.2017.04.183
Ciarella S, Sciortino F, Ellenbroek WG, 2018. Dynamics of vitrimers: defects as a highway to stress relaxation., 121(5):058003. https://doi.org/10.1103/PhysRevLett.121.058003
Constantinides G, Ulm FJ, 2004. The effect of two types of C-S-H on the elasticity of cement-based materials: results from nanoindentation and micromechanical modeling., 34(1):67-80. https://doi.org/10.1016/S0008-8846(03)00230-8
Constantinides G, Ulm FJ, 2007. The nanogranular nature of C-S-H., 55(1):64-90. https://doi.org/10.1016/j.jmps.2006.06.003
Cuesta A, Santacruz I, de la Torre AG, et al., 2021. Local structure and Ca/Si ratio in C-S-H gels from hydration of blends of tricalcium silicate and silica fume., 143:106405. https://doi.org/10.1016/j.cemconres.2021.106405
Cygan RT, Liang JJ, Kalinichev AG, 2004. Molecular models of hydroxide, oxyhydroxide, and clay phases and the development of a general force field., 108(4):1255-1266. https://doi.org/10.1021/jp0363287
Ding H, Chen JK, 2019. Research on the resistivity attenuation law of cementitious conductive composites induced by stress relaxation., 206:347-354. https://doi.org/10.1016/j.conbuildmat.2019.02.075
Du HJ, Pang SD, 2021. Long-term influence of nanosilica on the microstructures, strength, and durability of high-volume fly ash mortar., 33(8):04021185. https://doi.org/10.1061/(asce)mt.1943-5533.0003822
Falk ML, Langer JS, 1998. Dynamics of viscoplastic deformation in amorphous solids., 57(6):7192-7205. https://doi.org/10.1103/PhysRevE.57.7192
Fang GH, Zhang MZ, 2020. Multiscale micromechanical analysis of alkali-activated fly ash-slag paste., 135:106141. https://doi.org/10.1016/j.cemconres.2020.106141
Feldman RF, 1972. Mechanism of creep of hydrated Portland cement paste., 2(5):521-540. https://doi.org/10.1016/0008-8846(72)90107-X
Feng D, Li XF, Wang XZ, et al., 2018. Capillary filling under nanoconfinement: the relationship between effective viscosity and water-wall interactions., 118:900-910. https://doi.org/10.1016/j.ijheatmasstransfer.2017.11.049
Frech-Baronet J, Sorelli L, Charron JP, 2017. New evidences on the effect of the internal relative humidity on the creep and relaxation behaviour of a cement paste by micro-indentation techniques., 91:39-51. https://doi.org/10.1016/j.cemconres.2016.10.005
Fu Q, Xie YJ, Long GC, et al., 2015. Temperature sensitivity and model of stress relaxation properties of cement and asphalt mortar., 84:1-11. https://doi.org/10.1016/j.conbuildmat.2015.03.064
Gallucci E, Zhang X, Scrivener KL, 2013. Effect of temperature on the microstructure of calcium silicate hydrate (C-S-H)., 53:185-195. https://doi.org/10.1016/j.cemconres.2013.06.008
Gao Y, Zhang J, Han P, 2013. Determination of stress relaxation parameters of concrete in tension at early-age by ring test., 41:152-164. https://doi.org/10.1016/j.conbuildmat.2012.12.004
Giorla AB, Dunant CF, 2018. Microstructural effects in the simulation of creep of concrete., 105:44-53. https://doi.org/10.1016/j.cemconres.2017.12.001
Gowers RJ, Carbone P, 2015. A multiscale approach to model hydrogen bonding: the case of polyamide., 142(22):224907. https://doi.org/10.1063/1.4922445
Han GZ, Fang ZK, Chen MD, 2010. Modified Eyring viscosity equation and calculation of activation energy based on the liquid quasi-lattice model., 53(10):1853-1860. https://doi.org/10.1007/s11433-010-4096-9
Hou DS, Ma HY, Yu Z, et al., 2014a. Calcium silicate hydrate from dry to saturated state: structure, dynamics and mechanical properties., 67:81-94. https://doi.org/10.1016/j.actamat.2013.12.016
Hou DS, Zhu Y, Lu YY, et al., 2014b. Mechanical properties of calcium silicate hydrate (C-S-H) at nano-scale: a molecular dynamics study., 146(3):503-511. https://doi.org/10.1016/j.matchemphys.2014.04.001
Hou DS, Zhao TJ, Ma HY, et al., 2015a. Reactive molecular simulation on water confined in the nanopores of the calcium silicate hydrate gel: structure, reactivity, and mechanical properties., 119(3):1346-1358. https://doi.org/10.1021/jp509292q
Hou DS, Zhang JR, Li ZJ, et al., 2015b. Uniaxial tension study of calcium silicate hydrate (C-S-H): structure, dynamics and mechanical properties., 48(11):3811-3824. https://doi.org/10.1617/s11527-014-0441-1
Hou DS, Li ZJ, Zhao TJ, et al., 2015c. Water transport in the nano-pore of the calcium silicate phase: reactivity, structure and dynamics., 17(2):1411-1423. https://doi.org/10.1039/C4CP04137B
Hou DS, Yu J, Jin ZQ, et al., 2018a. Molecular dynamics study on calcium silicate hydrate subjected to tension loading and water attack: structural evolution, dynamics degradation and reactivity mechanism., 20(16):11130-11144. https://doi.org/10.1039/C7CP08634B
Hou DS, Jia YT, Yu J, et al., 2018b. Transport properties of sulfate and chloride ions confined between calcium silicate hydrate surfaces: a molecular dynamics study., 122(49):28021-28032. https://doi.org/10.1021/acs.jpcc.8b07484
Jana R, Pastewka L, 2019. Correlations of non-affine displacements in metallic glasses through the yield transition., 2(4):045006. https://doi.org/10.1088/2515-7639/ab36ed
Jennings HM, 2004. Colloid model of C-S-H and implications to the problem of creep and shrinkage., 37(1):59-70. https://doi.org/10.1007/BF02481627
Kai MF, Zhang LW, Liew KM, 2021. New insights into creep characteristics of calcium silicate hydrates at molecular level., 142:106366. https://doi.org/10.1016/j.cemconres.2021.106366
Li B, Li N, Brouwers HJH, et al., 2020. Understanding hydrogen bonding in calcium silicate hydrate combining solid-state NMR and first principle calculations., 233:117347. https://doi.org/10.1016/j.conbuildmat.2019.117347
Li J, Yu QJ, Huang HL, et al., 2019. Effects of Ca/Si ratio, aluminum and magnesium on the carbonation behavior of calcium silicate hydrate., 12(8):1268. https://doi.org/10.3390/ma12081268
Li X, Grasley ZC, Garboczi EJ, et al., 2015. Modeling the apparent and intrinsic viscoelastic relaxation of hydrating cement paste., 55:322-330. https://doi.org/10.1016/j.cemconcomp.2014.09.012
Li XD, Grasley ZC, Bullard JW, et al., 2018. Creep and relaxation of cement paste caused by stress-induced dissolution of hydrated solid components., 101(9):4237-4255. https://doi.org/10.1111/jace.15587
Li ZM, Zhang SZ, Liang XH, et al., 2020. Cracking potential of alkali-activated slag and fly ash concrete subjected to restrained autogenous shrinkage., 114:103767. https://doi.org/10.1016/j.cemconcomp.2020.103767
Liang CY, Zheng Q, Jiang JY, et al., 2022. Calcium silicate hydrate colloid at different humidities: microstructure, deformation mechanism, and mechanical properties., 228:117740. https://doi.org/10.1016/j.actamat.2022.117740
Liu WH, Zhang LW, Liew KM, 2020. Modeling of crack bridging and failure in heterogeneous composite materials: a damage-plastic multiphase model., 143:104072. https://doi.org/10.1016/j.jmps.2020.104072
Lothenbach B, Scrivener K, Hooton RD, 2011. Supplementary cementitious materials., 41(12):1244-1256. https://doi.org/10.1016/j.cemconres.2010.12.001
Manzano H, Pellenq RJM, Ulm FJ, et al., 2012. Hydration of calcium oxide surface predicted by reactive force field molecular dynamics., 28(9):4187-4197. https://doi.org/10.1021/la204338m
Manzano H, Masoero E, Lopez-Arbeloa I, et al., 2013. Shear deformations in calcium silicate hydrates., 9(30):7333-7341. https://doi.org/10.1039/C3SM50442E
Maruyama I, Igarashi G, Nishioka Y, 2015. Bimodal behavior of C-S-H interpreted from short-term length change and water vapor sorption isotherms of hardened cement paste., 73:158-168. https://doi.org/10.1016/j.cemconres.2015.03.010
Masoumi S, Zare S, Valipour H, et al., 2019. Effective interactions between calcium-silicate-hydrate nanolayers., 123(8):4755-4766. https://doi.org/10.1021/acs.jpcc.8b08146
Morshedifard A, Masoumi S, Abdolhosseini Qomi MJ, 2018. Nanoscale origins of creep in calcium silicate hydrates., 9(1):1785. https://doi.org/10.1038/s41467-018-04174-z
Mortazavi B, Javvaji B, Shojaei F, et al., 2021a. Exceptional piezoelectricity, high thermal conductivity and stiffness and promising photocatalysis in two-dimensional MoSi2N4family confirmed by first-principles., 82:105716. https://doi.org/10.1016/j.nanoen.2020.105716
Mortazavi B, Silani M, Podryabinkin EV, et al., 2021b. First-principles multiscale modeling of mechanical properties in graphene/borophene heterostructures empowered by machine-learning interatomic potentials., 33(35):2102807. https://doi.org/10.1002/adma.202102807
Pellenq RJM, Kushima A, Shahsavari R, et al., 2009. A realistic molecular model of cement hydrates., 106(38):16102-16107. https://doi.org/10.1073/pnas.0902180106
Pitman MC, van Duin ACT, 2012. Dynamics of confined reactive water in smectite clay–zeolite composites., 134(6):3042-3053. https://doi.org/10.1021/ja208894m
Priezjev NV, 2017. Collective nonaffine displacements in amorphous materials during large-amplitude oscillatory shear., 95(2):023002. https://doi.org/10.1103/PhysRevE.95.023002
Richardson IG, 1999. The nature of C-S-H in hardened cements., 29(8):1131-1147. https://doi.org/10.1016/S0008-8846(99)00168-4
Rong H, Dong W, Zhao XY, et al., 2021. Investigation on multi-cracks initiation and propagation of fiber reinforced concrete in restrained shrinkage ring tests., 111:102856. https://doi.org/10.1016/j.tafmec.2020.102856
Rossi P, 1997. Strain rate effects in concrete structures: the LCPC experience., 30(1):54-62. https://doi.org/10.1007/BF02539277
Scrivener KL, Kirkpatrick RJ, 2008. Innovation in use and research on cementitious material., 38(2):128-136. https://doi.org/10.1016/j.cemconres.2007.09.025
Shahsavari R, Pellenq RJM, Ulm FJ, 2011. Empirical force fields for complex hydrated calcio-silicate layered materials., 13(3):1002-1011. https://doi.org/10.1039/C0CP00516A
Shimizu F, Ogata S, Li J, 2007. Theory of shear banding in metallic glasses and molecular dynamics calculations., 48(11):2923-2927. https://doi.org/10.2320/matertrans.MJ200769
Shishegaran A, Khalili MR, Karami B, et al., 2020. Computational predictions for estimating the maximum deflection of reinforced concrete panels subjected to the blast load., 139:103527. https://doi.org/10.1016/j.ijimpeng.2020.103527
Talebi H, Silani M, Bordas SPA, et al., 2014. A computational library for multiscale modeling of material failure., 53(5):1047-1071. https://doi.org/10.1007/s00466-013-0948-2
Tamtsia BT, Beaudoin JJ, 2000. Basic creep of hardened cement paste: a re-examination of the role of water., 30(9):1465-1475. https://doi.org/10.1016/S0008-8846(00)00279-9
Tang C, Wong CH, 2015. Effect of atomic-level stresses on local dynamic and mechanical properties in CuZr100-xmetallic glasses: a molecular dynamics study., 58:50-55. https://doi.org/10.1016/j.intermet.2014.11.006
Tian HW, Stephan D, Lothenbach B, et al., 2021. Influence of foreign ions on calcium silicate hydrate under hydrothermal conditions: a review., 301:124071. https://doi.org/10.1016/j.conbuildmat.2021.124071
Todd BD, Daivis PJ, 2017. Nonequilibrium Molecular Dynamics: Theory, Algorithms and Applications. Cambridge University Press, Cambridge, UK. https://doi.org/10.1017/9781139017848
Vandewalle L, Nemegeer D, Balazs L, et al., 2002. Design of steel fibre reinforced concrete using the-method: principles and applications., 35:262-278. https://doi.org/10.1007/BF02482132
Venkovic N, Sorelli L, Martirena F, 2014. Nanoindentation study of calcium silicate hydrates in concrete produced with effective microorganisms-based bioplasticizer., 49:127-139. https://doi.org/10.1016/j.cemconcomp.2013.12.003
Wang JW, Kalinichev AG, Kirkpatrick RJ, 2004. Molecular modeling of water structure in nano-pores between brucite (001) surfaces., 68(16):3351-3365. https://doi.org/10.1016/j.gca.2004.02.016
Wang XF, Bao YW, Liu XG, et al., 2015. The stress relaxation of cement clinkers under high temperature., 10(4):413-417. https://doi.org/10.1007/s11465-015-0357-7
Wang ZP, Chen YT, Xu LL, et al., 2022. Insight into the local C-S-H structure and its evolution mechanism controlled by curing regime and Ca/Si ratio., 333:127388. https://doi.org/10.1016/j.conbuildmat.2022.127388
Yang XS, Wang YJ, Wang GY, et al., 2016. Time, stress, and temperature-dependent deformation in nanostructured copper: stress relaxation tests and simulations., 108:252-263. https://doi.org/10.1016/j.actamat.2016.02.021
Youssef M, Pellenq RJM, Yildiz B, 2011. Glassy nature of water in an ultraconfining disordered material: the case of calcium–silicate–hydrate., 133(8):2499-2510. https://doi.org/10.1021/ja107003a
Zhang MZ, Ye G, van Breugel K, 2011. Microstructure-based modeling of water diffusivity in cement paste., 25(4):2046-2052. https://doi.org/10.1016/j.conbuildmat.2010.11.042
Zhang T, Qin WZ, 2006. Tensile creep due to restraining stresses in high-strength concrete at early ages., 36(3):584-591. https://doi.org/10.1016/j.cemconres.2005.11.017
Zhang Y, Zhou Q, Ju JW, et al., 2021. New insights into the mechanism governing the elasticity of calcium silicate hydrate gels exposed to high temperature: a molecular dynamics study., 141:106333. https://doi.org/10.1016/j.cemconres.2020.106333
Zheng Q, Jiang JY, Li XL, et al., 2021. In situ TEM observation of calcium silicate hydrate nanostructure at high temperatures., 149:106579. https://doi.org/10.1016/j.cemconres.2021.106579
水化硅酸钙应力松弛特性的分子动力学研究
耿志成1,汤盛文1,2,汪洋1,阿胡宝1,何真1,吴凯2,王磊3
1武汉大学,水资源工程与调度全国重点实验室,中国武汉,430072;2同济大学,先进土木工程材料教育部重点实验室,中国上海,200092;3西安建筑科技大学,材料科学与工程学院,中国西安,710055
水化硅酸钙(C-S-H)是波特兰水泥的主要水化产物,是影响水泥基材料粘弹性机制的主要成分之一。然而,人们还未能在原子层面上完全理解水泥基材料在外加变形作用下随时间变化的粘弹性响应。本文旨在通过建立不同钙硅比的C-S-H模型,以分子动力学模拟的方式系统研究不同因素对水化硅酸钙应力松弛性能的影响。
1. 基于分子动力学模拟,获得C-S-H的应力松弛特性;2. 研究应变状态、钙硅比和内部水含量对C-S-H应力松弛的影响,揭示其在应力松弛过程中所涉及的内部结构及能量变化。
1. 通过各原子基团的均方位移在应力松弛过程中考虑C-S-H层间区域的粘度变化;2. 基于时间相关函数,在不同应变状态、钙硅比以及温度的条件下研究C-S-H层间区域涉及到的化学键断裂与重组;3. 阐明氢键网络和C-S-H形态对不同含水量下C-S-H应力松弛特性演变的影响。
1. 在不同的初始变形条件下,C-S-H应力松弛响应均会发生,并显示出非均质特征;2. 钙硅比的增大以及温度的提高会导致水分子、羟基和层间钙原子的运动加快,从而引起C-S-H层间区域的粘度降低,进而导致C-S-H的初始应力及残余应力降低;3. 由于水分子会影响C-S-H的形貌以及层间氢键网络,所以C-S-H在不同水含量时展现出不同的应力松弛特性。
水化硅酸钙;应力松弛;钙硅比;温度;水含量;原子模拟
https://doi.org/10.1631/jzus.A2300476
https://doi.org/10.1631/jzus.A2300476
Received Sept. 19, 2023;
Revision accepted Nov. 27, 2023;
Crosschecked Dec. 28, 2023
© Zhejiang University Press 2024
猜你喜欢
杂志排行
Journal of Zhejiang University-Science A(Applied Physics & Engineering)的其它文章
- Transfer relation between subgrade frost heave and slab track deformation and vehicle dynamic response in seasonally frozen ground
- Influence of ground effect on flow field structure and aerodynamic noise of high-speed trains
- Constitutive modelling of concrete material subjected to low-velocity projectile impact: insights into damage mechanism and target resistance
- Effect of coral sand on the mechanical properties and hydration mechanism of magnesium potassium phosphate cement mortar
