A mite parasitoid,Pyemotes zhonghuajia,negatively impacts the fitness traits and immune response of the fall armyworm,Spodoptera frugiperda
2024-01-17YanfeiSongTaianTianYichaiChenKeshiZhangMaofaYangJianfengLiu
Yanfei Song ,Tai’an Tian ,Yichai Chen ,Keshi Zhang ,Maofa Yang, ,Jianfeng Liu#
1 Institute of Entomology,Guizhou Provincial Key Laboratory for Agricultural Pest Management of the Mountainous Region,Guizhou University/Scientific Observing and Experiment Station of Crop Pests in Guiyang,Ministry of Agriculture and Rural Affairs,Guiyang 550025,China
2 Administration of Guizhou Fanjingshan National Nature Reserve,Jiangkou 554400,China
3 Centre for Biodiversity and Biosecurity,School of Biological Sciences,The University of Auckland,Auckland 1072,New Zealand
4 College of Tobacco Science,Guizhou University,Guiyang 550025,China
Abstract Parasitoids are key regulators in ecological communities and widely used as agents in biocontrol programmes.The fall armyworm,Spodoptera frugiperda,recently invaded multiple continents and caused substantial economic losses in agriculture.Pyemotes zhonghuajia,a newly identified mite parasitoid,has shown potential for controlling various agricultural insect pests.Therefore,this study tested the performance of P.zhonghuajia in parasitising S.frugiperda.We also investigated the sublethal effects of parasitism by P.zhonghuajia on host fitness traits,transgenerational impacts,and cellular and humoral immunity.Our result showed that the fifth-instar larvae of S.frugiperda parasitised by 40 P.zhonghuajia were all dead (i.e.,a lethal effect),while parasitism by 5 or 10 P.zhonghuajia was considered sublethal since many S.frugiperda survived to adulthood and produced offspring after mating.The sublethal influences from parasitism by P.zhonghuajia resulted in reduced pupal weight,adult emergence rate and fecundity,but increased developmental time and longevity.Parasitism at both lethal (40 mites) and sublethal (10 mites) levels impaired the cellular and humoral immunity of S.frugiperda.This study presents the first empirical evidence that mite parasitoids can negatively influence host immunity.Moreover,it provides insights into the biocontrol potential of mite parasitoids and their interactions with hosts.
Keywords: Pyemotes zhonghuajia,Spodoptera frugiperda,immunity,parasitically-induced influence,biocontrol,parasitoid
1.lntroduction
The development of immature parasitoids requires nourishment from one individual of another species(i.e.,a single host) (Kuris 1974;de Rijketal.2013;Kim-Joetal.2019).Unlike parasites,some ectoparasitoids normally kill their hosts directly by feeding or through the development of immatures on the exterior of the host(Eggleton and Belshaw 1992;Okabe 2013;Abrametal.2019;Ouetal.2021).Depending on the timing of host death,parasitoids can be classified as idiobionts (i.e.,when hosts die shortly after parasitism) or koinobionts (i.e.,when hosts remain alive during parasitoid development)(Brodeur and Boivin 2004;Parry 2009).Aside from death,parasitoids induce developmental,behavioural,physiological,and morphological effects in their hosts(Vinson and Iwantsch 1980).Considerable attention has been given to insect parasitoids due to their ecological significance and importance in the biological control of agricultural pests (Eggleton and Belshaw 1992;Feener and Brown 1997;Parry 2009).
Due to their small size and remarkable plasticity,mites occupy an extensive range of habitats and lifestyles,including as ectoparasites of numerous hosts (Fain 1994).However,in contrast to the many studies on insect parasitoids,the focus on mite parasitoids is inadequate (Feener and Brown 1997).Members of Pyemotidae (Acari: Trombidiformes) are generalist parasitoids of various insect host species(Yu and Zhang 2019),which use venom proteins (i.e.,neurotoxins) to paralyse their insect hosts (Tomalskietal.1988,1989).The immobilisation of hosts is vital for successful feeding inPyemotesmites.Previous studies have revealed that an ovoviviparous pyemotid,PyemoteszhonghuajiaYu,Zhang &He,can paralyse and kill their hosts shortly after parasitism (Lindquist 1998;Heetal.2019;Liuetal.2020;Tianetal.2020a;Chenetal.2021).Pyemoteszhonghuajiafemales could kill hosts weighing about 680,000 times greater than themselves (Chenetal.2021).The high fecundity,short developmental time,and relatively strong lethality to hosts have encouraged studying the use ofP.zhonghuajiain biocontrol programs for various insect pests (Heetal.2019;Liuetal.2020;Tianetal.2020b;Chenetal.2021).
The fall armyworm (FAW),Spodopterafrugiperda(J.E.Smith) (Lepidoptera: Noctuidae),is a native crop pest in warmer regions of America but has spread to Africa,Asia,and Oceania since 2016,and causes significant economic losses in agriculture (Hruska 2019;Niassyetal.2021;Wanetal.2021).FAW has been reported to affect numerous plant species (Wanetal.2021).The biological characteristics of FAW,such as lack of diapause,short developmental time,high fecundity,wide food range,long migratory distance,and rapid development of insecticide resistance,have favoured its invasion and establishment in the introduced ranges (Zhaoetal.2020;Wanetal.2021).Hymenopteran and Dipteran parasitoids have been used as biocontrol agents against FAW worldwide(Delfín-Gonzálezetal.2007;Ordóñez-Garcíaetal.2015;Wanetal.2021;Winsouetal.2022).However,mites are rarely reported to be associated with FAW.Nevertheless,a parasitic miteTrombidiumsp.has been found on fieldcollected FAW in Nigeria,and it causes morphological and behavioural changes in hosts through feeding,and the rapid (<24 h) death of 1st instars (Ogunfunmilayoetal.2021).
The potential to control FAW using the parasitoid miteP.zhonghuajiahas been illustrated in previous studies(Liuetal.2020;Fengetal.2022).For instance,the presence ofP.zhonghuajiareduced the population FAW on sorghum plants (Fengetal.2022).The killing efficacy ofP.zhonghuajiais likely related to host size (Liuetal.2020).One adultP.zhonghuajiafemale can kill the smaller 1st to 3rd instar FAW larvae in a relatively short period,but the killing of larger instars and pupae requires parasitism at higher densities.
The sublethal influences ofP.zhonghuajiaparasitism have been shown to include reductions in the eclosion rate and consumption of FAW (Liuetal.2020;Fengetal.2022).Therefore,the toxins induced byP.zhonghuajiaat the sublethal level are likely to influence the growth and development of FAW.Additionally,sublethal influences of parasitic infestation can cause transgenerational impacts (Poulin and Thomas 2008;Pigeaultetal.2015),and parasitism byP.zhonghuajiaat the sublethal level in potato wormPhthorimaeaoperculellaparents reduces their offspring’s fecundity.Therefore,we examined the influence of parasitism byP.zhonghuajiaat the sublethal level on the developmental time and fecundity of FAW,and the transgenerational effects on their offspring.We hypothesise thatP.zhonghuajiaparasitism will prolong juvenile development and reduce the fecundity of both FAW parents and offspring.
Insects rely on their innate immunity against pathogens and parasites that enter their haemocoel(Vinson 1990;Carton and Nappi 1997;Hillyer 2016).The innate response entails the involvement of two interrelated mechanisms,cellular and humoral defences,to perform various tasks,including the synthesis and release of enzymes,melaninisation,phagocytosis,nodulation,and encapsulation (Abro 1982;Charles and Killian 2015;Hillyer 2016;Zhang and Zhang 2019).Parasitoids utilise complex strategies to survive the host’s immune defences (Brodeur and Boivin 2004).In previous studies,adultP.zhonghuajiafemales were able to penetrate the physical barrier (i.e.,cuticle) of their host to feed,paralyse,and kill their hosts (Heetal.2019;Liuetal.2020;Tianetal.2020a,b;Chenetal.2021).Therefore,we also examined the cellular and humoral immune responses of FAW against parasitism byP.zhonghuajia.This study provides a theoretical basis for the control of FAW and enhances our understanding of the insect immune response to infestation by mite parasitoids.
2.Materials and methods
2.1.lnsect source and rearing
Two hundred FAW mature larvae were collected from vegetation in Guiding County,Qiannan Buyei and Miao Autonomous Prefecture,Guizhou,China,in April 2019,and reared on corn seedlings (ca.15 cm in height) in a climate-controlled room at (28±1)°C,(70±5)% relative humidity,and a 14 h:10 h (L:D) photoperiod as described by Chenetal.(2022).The culture ofP.zhonghuajiawas obtained from Changli Institute of Pomology,Hebei Academy of Agriculture and Forestry Sciences,China in October 2019,and maintained onP.operculellain a climate chamber at (25±1)°C,(70±5)% relative humidity,and a 14 h:10 h (L:D) photoperiod (see Yeetal.2022 for details).
Fourth instar larvae of FAW were individually placed into enclosed round pudding containers (6 cm×3 cm:diameter×height) and fed corn seedlings.The container lids were punctured with small holes (ca.1 mm in diameter) to allow air exchange.Filter paper (6 cm in diameter) was placed at the bottom of the containers as bedding.Newly emerged fifth instars of FAW were used for experiments 1 and 2.
2.2.Experiment 1: lnfluence on fitness traits
lnfluence of P.zhonghuajia parasitism on the life history traits of FAWFifth instar larvae of FAW (<24 h old) were placed individually into the round pudding containers using a previously described setup (Tianetal.2020a).Newly emerged and matedP.zhonghuajiafemales (starved for 24 h) were placed on FAW using a fine brush at three densities: 5,10,and 40 individuals.WhenP.zhonghuajiafailed to kill FAWviaparasitism(i.e.,at low densities),the parasitoids would die four days later.Furthermore,at low densities,P.zhonghuajiacan be killed by FAW occasionally.The control group of FAW had noP.zhonghuajiaparasitism.Each treatment was replicated 150 times.The survival and development of FAW were checked daily,and the filter paper and corn seedlings were replaced daily during the observation period.The experimental units were placed in a climate chamber at the same conditions as described above for theP.zhonghuajiaculture.
After eclosion (<6 h),one adult FAW female and one male (from the same treatment) were paired for mating.They were placed in a 300 mL plastic container,and fed with a 10% (v/v) honey solution by saturating cotton balls.Thirty-eight,33,and 23 pairs of adult FAW females and males were observed in the control group,the five mites treatment group,and the 10 mites treatment group,respectively.The containers were sealed by mesh (96 μm mesh size) and rubber bands.Adult survival and fecundity of the FAW were observed daily until all the test moths died.FAW eggs collected during the daily observations were removed and used in the transgenerational influence experiment.
Transgenerational influence of P.zhonghuajia parasitism on FAWOne hundred FAW eggs collected from the control and treatment groups were reared individually in the pudding containers described above.This experiment used the same rearing and observing procedures as the previous experiment.One newly emerged FAW female and one male (whose parents were from the same treatment) were paired for mating and reared using the plastic containers.Developmental time,longevity,and fecundity of the FAW were recorded until death.The transgenerational influences ofP.zhonghuajiaon FAW were examined for only one generation.Thus,any eggs found during the daily observations were removed but not reared.
2.3.Experiment 2: lnfluence on cellular and humoral immunity
Fifth instar FAW (<24 h old;weight=(0.11±0.03) g) were parasitised by either 10 or 40P.zhonghuajiaadults and individually reared in the round pudding containers with the same conditions and set up as in experiment 1.Successful parasitism was ensured by observing each FAW under a dissecting microscope for the occurrence of direct biting byP.zhonghuajia.Blood samples were taken from the FAW at 2,4,12,and 24 h after parasitism for both control (noP.zhonghuajiaparasitism) and treatment groups (parasitised byP.zhonghuajia).The fifth-instar host larvae were disinfected with 75% ethanol(v/v) followed by 0.1 mol L-1phosphate-buffered saline(PBS) (pH=7.4) and dried using filter paper.A pair of eye scissors was used to cut off the hind leg to allow natural bleeding.Haemolymph samples were taken from FAW using a pipette (Eppendorf,Germany) and tested using the following procedures.
Total haemocyte countThe sampled haemolymph of FAW (5 μL) was diluted three times in 1.5 mL centrifuge tubes with an anticoagulant buffer (10 μL).The anticoagulant buffer was made by dissolving 0.45 g NaCl,0.471 g KCl,0.041 g CaCl2,and 1 g EDTA in 50 mL deionised water.Total haemocytes were counted using a Neubauer haemocytometer under an optical microscope(Olympus,Japan).At 40× magnification,four views were randomly selected,and the number of haemocytes was counted in each view.The total haemocyte count (number of haemocytes per mL of haemolymph) was calculated following the haemocytometer manufacturer’s instructions.
Haemocyte spreading rateAccording to Lietal.(2011),10 μL of the collected FAW haemolymph was mixed with 90 μL of Grace’s insect medium and 2 μL of saturated phenylthiourea in 96-well treated tissue culture plates(Sangon Biotech,China).After 6 h at room temperature,the plates were examined under a microscope (40×magnification),and four views were randomly assigned for haemocyte observations.Haemocyte spreading rate(%)=(Number of spreading haemocytes/Number of blood cells observed)×100.
Apoptosis rateAfter examining the haemocyte spreading rate,an equal volume of trypan blue solution(0.04%) was added to each well of the culture plates,and allowed to react for 30 min at room temperature.The numbers of haemocytes stained blue (i.e.,dead)and unstained (i.e.,viable) were counted under the microscope to calculate the apoptosis rate,as described by Lietal.(2011).
In vitro phagocytic and encapsulation abilitiesThe phagocytic and encapsulation abilities of FAW in response to parasitism byP.zhonghuajiawere analysed according to Tojoetal.(2000).An aliquot of each FAW haemolymph sample (10 μL) was dispensed into a glass Petri dish,and 80 μL of Grace’s insect medium was added to the haemolymph for 1 h.Then,fluorescein isothiocyanate (FITC)-labelledEscherichiacoli(3.2×108cells mL-1) was added to the haemolymph and the sample was incubated in a climate chamber in the dark at 28°C for 2 h.Afterwards,the haemolymph was rinsed with PBS,and 100 μL of trypan blue solution (0.2%) was added to remove the fluorescence on unphagocytosedE.colicells.The treated haemolymph was collected using a pipette and observed under a fluorescence microscope (TS100,Nikon,Japan) to assess the presence of phagocytosis.
In the 96-well treated tissue culture plates,10 μL of FAW haemolymph,38 μL of Grace’s insect medium,1 μL of dextran microbeads (Sephadex A-50),and 1 μL of saturated PTU solution were added to each well,for a concentration of 15 beads per 100 μL.The mixture was incubated in a climate chamber at 27°C for 12 h to observe encapsulation,and the encapsulation index (%)was calculated as described by Tengetal.(2016).
MelanisationIn a 1.5 mL centrifuge tube,10 μL of FAW haemolymph was diluted with 10 μL PBS and placed in the climate chamber at 28°C for 20 min.The presence of melanisation or haemolymph blackening was then observed.The haemolymph changed from transparent or light green to light brown or dark brown,indicating blackening.If the haemolymph was not blackened,melanisation was likely suppressed.Blackening rate(%)=(Number of blackening reactions/Total number observed)×100.
Phenoloxidase activityPhenoloxidase activity was measured due to its crucial role in activating the melanisation process in insect immunity (Hillyer 2016).For each FAW haemolymph sample,5 μL of haemolymph was placed in a 1.5 mL centrifuge tube with 90 μL of deionised water and centrifuged at 8,000 r min-1at 4°C for 5 min.The supernatant in each tube was removed and incubated in the climate chamber at 4°C for 1 h.Then,50 μL of the supernatant was placed in a new centrifuge tube with 50 μL of dopamine solution (125 mg mL-1) and incubated at room temperature for 10 min.The dopamine solution was prepared by dissolving 0.1 g dopamine and 0.055 g CaCl2in 100 mL deionised water.A modular multimode microplate reader (Bio Tek,USA) was used to determine the absorbance or optical density (OD490) of the samples,as described by Caietal.(2001).The changes in OD490per min during the 5-10 min interval for every 0.001 were equal to one enzyme catalytic activity unit (U).The dopamine solution (without haemolymph) was used as a blank.
2.4.Statistical analysis
The age-stage,two-sex life table (Chi and Liu 1985;Chi 1988) was used to analyse the FAW life history traits observed in experiment 1.The TWOSEX-MSChart computer program (Chi 2022) was used to perform the analysis.A paired bootstrap test was used to detect significant differences in the life history traits of the FAW parental and offspring generations.In experiment 2,SPSS (version 26.0) was used to analyse the immune response of FAW againstP.zhonghuajiaparasitism.A two-way ANOVA was used to evaluate the influences of experimental factors and their interactions on cellular and humoral immunity ofS.frugiperdaat the fifth instar larval stage as induced byP.zhonghuajiaparasitism.One-way analysis of variance (ANOVA) with Tukey’s honestly significant difference was used to determine the significance of the influence ofP.zhonghuajiaparasitism on total haemocyte count,haemocyte spreading rate,apoptosis rate,haemocyte phagocytosis rate,haemocyte encapsulation rate,melanisation rate,and phenol oxidase activity ofS.frugiperdaat the fifth instar larval stage.
3.Results
3.1.Experiment 1: lnfluence on fitness traits
FAW survivalThe mortality rate of immature FAW parasitised by fiveP.zhonghuajiawas statistically similar to the control for both parents and offspring (Table 1).However,parasitism at the density of 10P.zhonghuajiasignificantly increased the mortality of immature FAW compared to those either with no parasitism or parasitised by fiveP.zhonghuajiafor both parents and offspring.None of the 5th instar FAW larvae survived to the 6th instar when parasitised by 40P.zhonghuajia.
FAW development and fecundityFAW parasitised by either five or 10P.zhonghuajiaas fifth-instar larvae had significantly prolonged immature developmental time,slightly increased longevity,and considerably reduced fecundity compared to non-parasitised larvae (Table 2).The transgenerational influences ofP.zhonghuajiaparasitism on FAW were not significant,except for increased juvenile development and longevity.
The two-sex table parametersAfter being parasitised by 10P.zhonghuajia,the parents and their offspring had significantly reduced population growth as measured by the intrinsic rate of increase,finite rate of increase,and gross reproduction rate (Table 3).When parasitised by fiveP.zhonghuajia,the intrinsic rate of increase and finite rate of increase were significantly reduced only in the parents,but not in their offspring.The mean generation time of FAW was increased in FAW parasitised by either five or 10P.zhonghuajiain both the parents and their offspring.
3.2.Experiment 2: lnfluence on cellular and humoral immunity
Total haemocyte countThe total haemocyte counts were higher inP.zhonghuajiaparasitised groups than in the control group at all examined time intervals (one-way ANOVA:F=52.0,df=2,P<0.001 for 2 h;F=740.0,df=2,P<0.001 for 4 h;F=558.5,df=2,P<0.001 for 12 h;andF=921.5,df=2,P<0.001 for 24 h;Fig.1).The highest number of haemocytes (9.74×107cells mL-1) was found in FAW at 2 h after the parasitism by 40P.zhonghuajia.The total haemocyte count decreased with the increase in time after parasitism for individuals parasitised with 10 or 40P.zhonghuajia(F=100.4,df=3,P<0.001 for 10 mites;andF=24.0,df=3,P<0.001 for 40 mites;Table 4),but not for individuals in the control group (F=1.1,df=3,P=0.234).
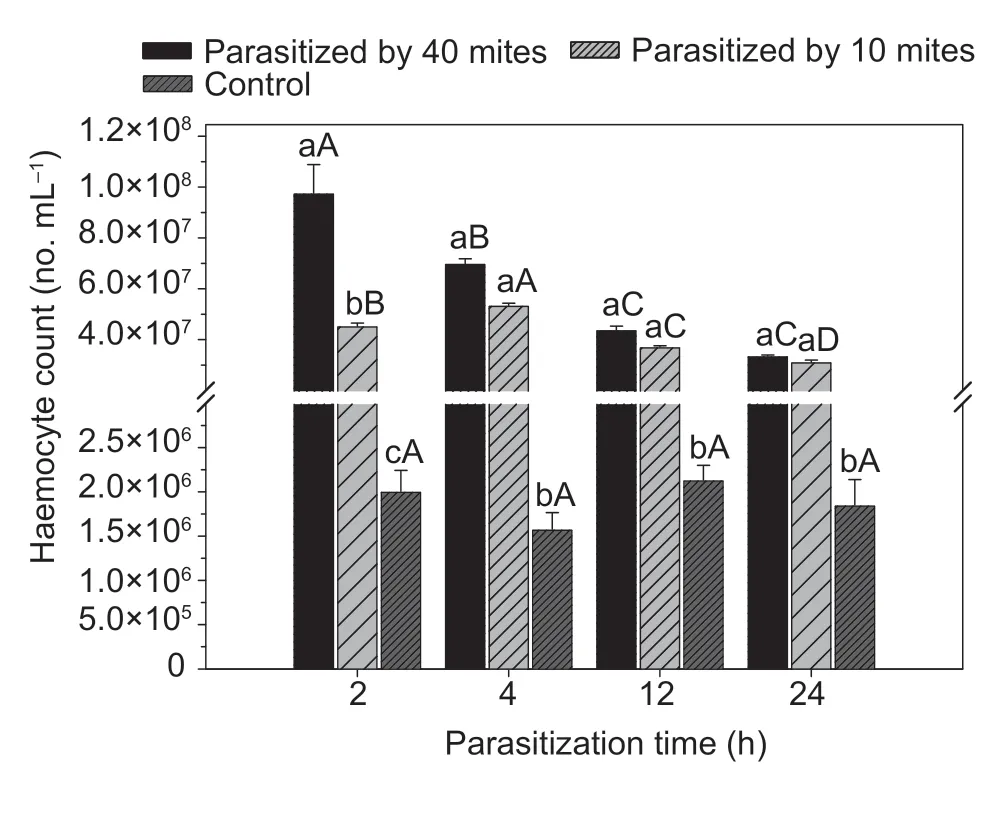
Fig.1 Total haemocyte count of Spodoptera frugiperda parasitised by Pyemotes zhonghuajia at different time intervals.Different lowercase letters above the bars indicate significant differences in the total haemocyte counts of S.frugiperda when exposed to 0,10 and 40 mites,respectively.Different capital letters above the bars indicate significant differences in the total haemocyte counts of S.frugiperda among different times after parasitism (error bars are SE,n=30;Tukey’s test: P<0.05).
Haemocyte spreading rateThe proportion of spreading haemocytes of FAW was largely reduced by parasitism byP.zhonghuajia(one-way ANOVA:F=177.5,df=2,P<0.001 for 2 h;F=108.1,df=2,P<0.001 for 4 h;F=482.4,df=2,P<0.001 for 12 h;andF=406.5,df=2,P<0.001 for 24 h;Fig.2;Table 4).The densities of 10 or 40P.zhonghuajiadid not exert a significant influence on the proportion of spreading haemocytes,except at 4 h after parasitism.The haemocyte spreading rate was relatively stable for the control over the examination period but showed a reduced trend for the parasitism groups (F=32.4,df=3,P<0.001 for 10 mites;andF=96.8,df=3,P<0.001 for 40 mites).
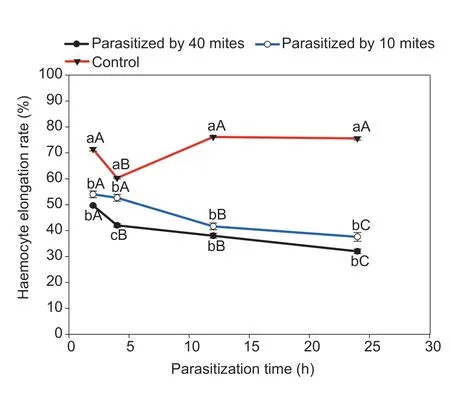
Fig.2 Haemocyte elongation rate of Spodoptera frugiperda parasitised by Pyemotes zhonghuajia at different time intervals.Different lowercase letters above the bars indicate significant differences in the haemocyte spreading rates of S.frugiperda when exposed to 0,10 and 40 mites,respectively.Different capital letters above the bars indicate significant differences in the haemocyte spreading rates of S.frugiperda among different times after parasitism (error bars are SE,n=30;Tukey’s test:P<0.05).
Apoptosis rateThe mortality rate of FAW haemocytes was significantly increased by parasitism byP.zhonghuajia(one-way ANOVA:F=48.4,df=2,P<0.001 for 2 h;F=306.5,df=2,P<0.001 for 4 h;F=248.3,df=2,P<0.001 for 12 h;andF=283.3,df=2,P<0.001 for 24 h;Fig.3;Table 4).The numbers of dead haemocytes in individuals parasitised by 10 (F=52.6,df=3,P<0.001) or 40P.zhonghuajia(F=100.4,df=3,P<0.001) increased with an increase in the time after parasitism but not for the control group (F=2.3,df=3,P=0.080).The haemocyte mortality rate of FAW was higher for individuals parasitised by 40P.zhonghuajiathan those parasitised by 10P.zhonghuajia.

Fig.3 Haemocyte apoptosis rates of Spodoptera frugiperda parasitised by Pyemotes zhonghuajia at different time intervals.Different lowercase letters above the bars indicate significant differences in the haemocyte mortality rates of S.frugiperda when exposed to 0,10 and 40 mites,respectively.Different capital letters above the bars indicate significant differences in the haemocyte mortality rates of S.frugiperda among different times after parasitism (error bars are SE,n=30;Tukey’s test:P<0.05).

Table 1 Mortality (%) of Spodoptera frugiperda (FAW) parasitised by Pyemotes zhonghuajia at different densities during the fifth instar larval stage
In vitro phagocytic and encapsulation abilitiesThe phagocytic abilities of FAW were significantly influenced by parasitism byP.zhonghuajia(one-way ANOVA:F=5.0,df=2,P<0.001 for 2 h;F=7.6,df=2,P<0.001 for 4 h;F=32.5,df=2,P<0.001 for 12 h;andF=23.1,df=2,P<0.001 for 24 h;Tables 4 and 5).Theinvitrophagocytic ability of FAW againstE.coliwas significantly reduced by parasitism by 40P.zhonghuajiaat all time intervals and with 10P.zhonghuajiaonly at 12 and 24 h after parasitism (Table 5).The phagocytosis rate showed a trend of reduction with the increase in time after parasitism for parasitised individuals (F=5.9,df=3,P<0.001 for 10 mites;andF=8.0,df=3,P<0.001 for 40 mites),but not for individuals of the control group (F=0.2,df=3,P=0.880).
Theinvitroencapsulation rates of FAW against dextran microbeads significantly differed between the treatments at all time intervals (one-way ANOVA:F=59.6,df=2,P<0.001 for 2 h;F=89.6,df=2,P<0.001 for 4 h;F=73.2,df=2,P<0.001 for 12 h;andF=83.1,df=2,P<0.001 for 24 h;Tables 4-6).A trend of reduction in the encapsulation rate was observed with the increase in time after parasitism for both parasitised groups (F=9.7,df=3,P<0.001 for 10 mites;andF=5.6,df=3,P=0.0012 for 40 mites) but not for the control (F=2.4,df=3,P=0.068).At each time interval,FAW individuals parasitised by 40P.zhonghuajiahad the lowest rates,while the control group had the highest rates.
MelanisationAll haemolymph samples of the control examined at different time intervals showed blackening(Fig.4).A significant reduction in the melanisation rate was caused by parasitism byP.zhonghuajiaat all intervals (one-way ANOVA:F=106.6,df=2,P<0.001 for 2 h;F=56.3,df=2,P<0.001 for 4 h;F=77.9,df=2,P<0.001 for 12 h;andF=189.5,df=2,P<0.001 for 24 h;Table 4).Individuals of FAW parasitised by 10 or 40P.zhonghuajiahad similar melanisation rates,except at 2 and 4 h after parasitism.
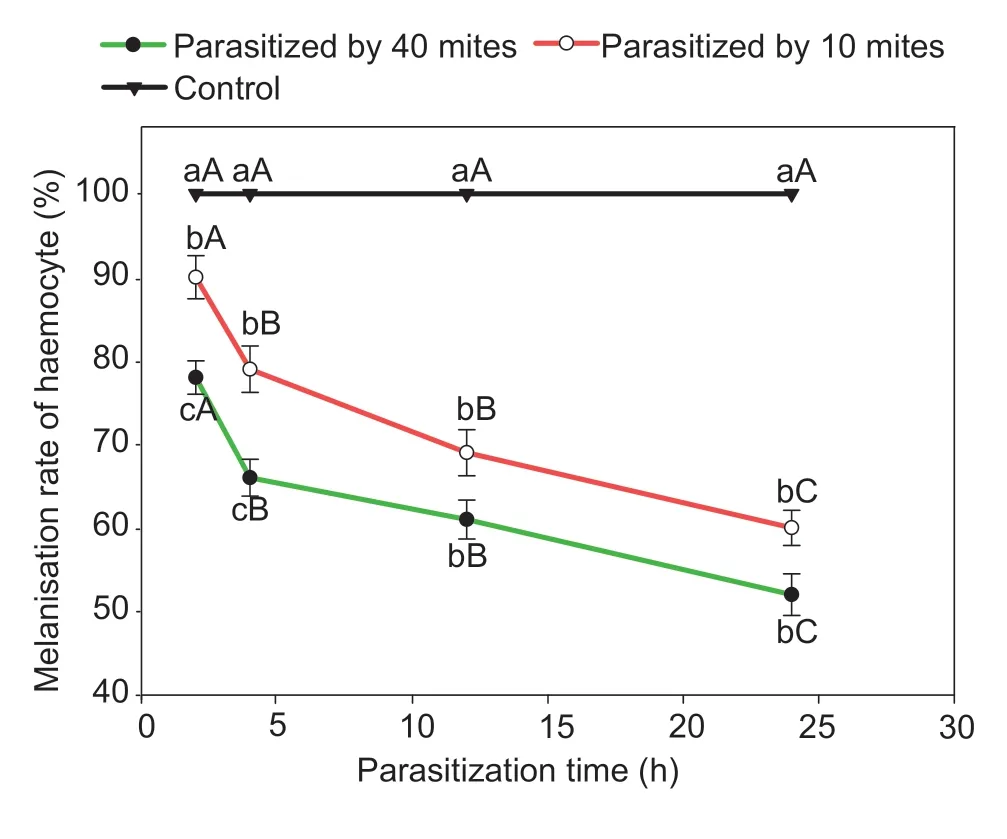
Fig.4 Influence of Pyemotes zhonghuajia parasitism on the melanisation rate of Spodoptera frugiperda.Different lowercase letters above the bars indicate significant differences in the melanisation rates of S.frugiperda when exposed to 0,10 and 40 mites,respectively.Different capital letters above the bars indicate significant differences in the melanisation rates of S.frugiperda among different times after parasitism (error bars are SE,n=30;Tukey’s test: P<0.05).
Phenoloxidase activityThe parasitism of FAW byP.zhonghuajiasignificantly reduced the phenol oxidase activity (one-way ANOVA:F=13.9,df=2,P<0.001 for 2 h;F=13.0,df=2,P<0.001 for 4 h;F=12.1,df=2,P<0.001 for 12 h;andF=178.7,df=2,P<0.001 for 24 h;Fig.5;Table 4),except for individuals at 12 h after parasitism by 10 mites.The phenol oxidase activities were statistically similar between parasitised individuals (i.e.,10 or 40P.zhonghuajia) at 2,4,and 24 h after parasitism.Other than an increase at 12 h after parasitism with 10P.zhonghuajia,the phenol oxidase activities were similar between the different intervals.

Fig.5 Influence of Pyemotes zhonghuajia parasitism on the phenol oxidase activity of Spodoptera frugiperda.Different letters above the bars denote significant differences (Tukey’s test: P<0.05).Different lowercase letters above the bars indicate significant differences in the phenol oxidase activities of S.frugiperda when exposed to 0,10 and 40 mites,respectively.Different capital letters above the bars indicate significant differences in the phenol oxidase activities of S.frugiperda among different times after parasitism (error bars are SE,n=30;Tukey’s test: P<0.05).
4.Discussion
In this study,parasitism byP.zhonghuajiainduced acute death and long-term and transgenerational influences on several fitness traits of FAW.Parasitism at the high density (i.e.,40P.zhonghuajiafemales) killed all thetreated fifth instar FAW larvae.The earlier instars of FAW,oriental armywormMythimnaseparata,P.operculella,and tobacco cutwormSpodopteralituraare more vulnerable to parasitism byP.zhonghuajia,and can be killed at much lower mite densities (Yuetal.2010;Liuetal.2020;Tianetal.2020a;Fengetal.2022).This study found that several fitness traits,including survival,developmental time,pupal weight,longevity,and fecundity of FAW,were significantly influenced by parasitism byP.zhonghuajiaat sublethal levels.Various other helminth parasites have been shown to exert similar influences on their hosts(Songetal.2022).Furthermore,parasitism by five or 10P.zhonghuajiainduced transgenerational influences on the offspring’s immature survival,developmental time,and longevity.Comparable findings have been observed by Rasekhetal.(2022),where the aphid parasitoidLysiphlebusfabarumnegatively affected the fitness traits of offspring in the aphidAphisfabae.Therefore,parasitoids likely exert negative transgenerational effects on their hostsviaparasitism and feeding.However,whether this influence (i.e.,parasitism by parasitoids)can be passed on through multiple generations requires further investigation.

Table 4 Two-way ANOVA results for the influence of Pyemotes zhonghuajia parasitism on the cellular and humoral immunity of Spodoptera frugiperda at the fifth instar larval stage
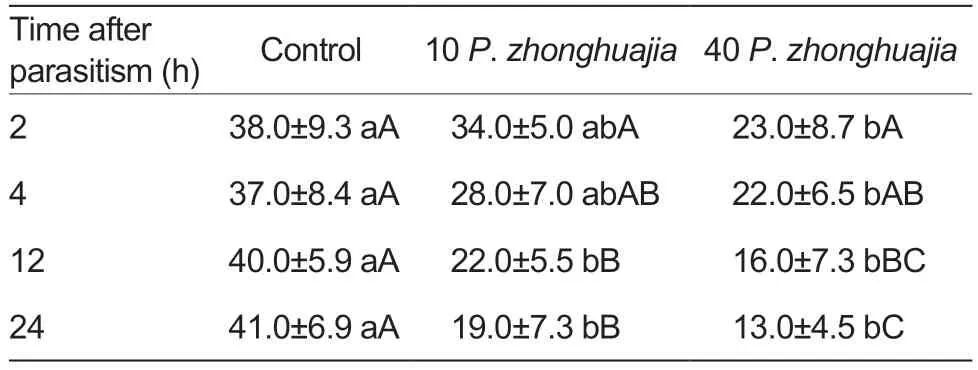
Table 5 Influence of Pyemotes zhonghuajia parasitism on the in vitro haemocyte phagocytosis rate (%) of Spodoptera frugiperda against Escherichia coli

Table 6 Influence of Pyemotes zhonghuajia parasitism on the in vitro haemocyte encapsulation rate (%) of Spodoptera frugiperda in response to dextran microbeads
In this study,parasitism byP.zhonghuajiasuppressed the cellular and humoral immunity of FAW at both sublethal (10 mites) and lethal (40 mites) levels,as indicated by increased haemocyte mortality and reduced haemocyte spreading,phagocytosis,encapsulation,and phenol oxidase activity.The parasitic waspCotesia marginiventrishas been shown to exert changes in the haemolymph proteins of FAWviaparasitism(Ferkovichetal.1983).Studies on the influences of mite parasitoids on host immunity are rather limited,except that infection byP.zhonghuajiahas been shown to reduce the haemolymph total protein content of the beetleSemanotusbifasciatus(Zhouetal.2010).Nonetheless,several studies have shown similar findings regarding parasitoid-induced immune suppression in insect hosts.These include the small white butterflyPierisrapaeparasitised by the endoparasitoid waspPteromaluspuparum(Caietal.2004;Shietal.2022),the greater wax mothGalleriamellonellaparasitised by the endoparasitoid waspPimplaturionellae(Eretal.2010),the mealworm beetleTenebriomolitorparasitised by the ectoparasitoid waspSclerodermaguani(Lietal.2018),and the fruit flyDrosophilamelanogasterparasitised by the ectoparasitoid waspPachycrepoideusvindemmiae(Yangetal.2019).Our results indicated that the mite parasitoidP.zhonghuajiacan alter host immunity just like these insect parasitoids.
In addition to the paralytic effect,the venom of parasitoids can induce cytotoxic and cytolytic influences on hosts (Eretal.2010).For example,the venom of parasitoids comprises a combination of biologically active components,which manipulates host physiology by suppressing the activation of haemocytes and humoral immunity (Shietal.2022).The venom of waspP.puparumresulted in a round configuration of haemocytes and reduced pseudopod extension (i.e.,haemocyte spreading)in the hostP.rapae(Caietal.2004).Due to the similar effects on host immunity,the venom ofP.zhonghuajiais likely to contain neurotoxins or proteins similar to those insect parasitoids (Tomalskietal.1989).
Parasitoids can alter the host’s physiology and behaviour (Abrametal.2019).In addition to the influences on host fitness traits and immunity,P.zhonghuajiaincreased the consumption rate of 5th instar FAW larvae on maize leaves (Fengetal.2022).This study found that the influence of parasitism byP.zhonghuajiaon the immunity of FAW can persist for 24 h after the parasitism.Similarly,the relatively longterm influence ofP.puparumon the immunity ofP.rapaeexisted after five days (Caietal.2004).However,determining whether the parasitoid-induced negative impacts and transgenerational influences on FAW fitness traits result from impaired immunity or long-term physiological effects requires further investigation.
Parasitoid wasps are frequently used for controlling FAW (Hay-Roeetal.2013;Kenisetal.2019).However,most parasitoid wasps are koinobionts,meaning that they develop inside their hosts (Molina-Ochoaetal.2003).Factors such as differences in host conditions and diets can influence the defence mechanisms of the hosts,thereby affecting the survival of the parasitised wasps (Barreto-Barrigaetal.2021).For example,FAW fed on stargrass may ingest different allelochemicals that increase the larval mortality rate of the parasitoid waspEuplectrusplatyhypenae(Hay-Roeetal.2013).In contrast,parasitism byP.zhonghuajiacan cause the direct death of FAW,which can reduce crop losses compared to the use of koinobiont insect larval parasitoids (Salazar-Mendozaetal.2020).Additionally,P.zhonghuajiacan infest more life stages of FAW compared to parasitoid wasps,such asCotesiaicipe(in which oviposition only occurred in the first to fourth instar larvae) (Mohamedetal.2021).
Pyemoteszhonghuajiais a potential biological control agent against FAW,and the nonlethal attacks from this mite parasitoid can result in altered fitness traits with transgenerational influences on the host.The biocontrol efficacy ofP.zhonghuajiashould be examined in future studies at larger scales,such as in greenhouses and field trials.As a generalist parasitoid,the non-target effects ofP.zhonghuajiaalso require consideration and investigation.To the best of our knowledge,this is the first study that examines the nonlethal and transgenerational influences of ectoparasitoid mites on the fitness traits and immunity of their host.This study provides insights into the mechanisms underlying the nonlethal impacts of parasitoid-induced influences by mite parasitoids,and encourages the use of mite parasitoids in the biocontrol of insect pests.
5.Conclusion
In the study,we tested the performance ofP.zhonghuajiain parasitisingS.frugiperda,and investigated the effects of sublethal parasitism byP.zhonghuajiaon host fitness traits and cellular and humoral immunity.The results showed that 40P.zhonghuajiafemales could kill all the parasitised fifth instar larvae ofS.frugiperda.Five or 10P.zhonghuajiareduced the pupal weight,adult emergence rate,and fecundity ofS.frugiperda,but delayed the developmental time and longevity.In addition,40 mites or 10 mites suppressed the cellular and humoral immunity of FAW,as indicated by increased haemocyte mortality,and reduced haemocyte spreading,phagocytosis,encapsulation,and phenol oxidase activity.
Acknowledgements
This study was funded by the National Natural Science Foundation of China (32060637 and 32260708),the Highlevel Talent Innovation and Entrepreneurship Funding Project in Guizhou Province,China ((2021)01),the Guizhou Provincial Science and Technology Innovation Talent Team Project,China (Qian Ke He Pingtai Rencai-CXTD (2021)004),the Systematic and Applied Acarology Society International Joint Project,England (2022(01)),the Growth Project of Youth Talent in Ordinary Universities in Guizhou Province,China ((2021)079),and the Natural Science Special Project in Guizhou University,China((2020)02).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Advances in DNA methylation and its role in cytoplasmic male sterility in higher plants
- Assessment of molecular markers and marker-assisted selection for drought tolerance in barley (Hordeum vulgare L.)
- 3D genome organization and its study in livestock breeding
- SUPER WOMAN 2 (SPW2) maintains organ identity in spikelets by inhibiting the expression of floral homeotic genes OsMADS3,OsMADS58,OsMADS13,and DROOPING LEAF
- Genetic dissection and validation of a major QTL for grain weight on chromosome 3B in bread wheat (Triticum aestivum L.)
- Coupling of reduced inorganic fertilizer with plant-based organic fertilizer as a promising fertilizer management strategy for colored rice in tropical regions
