尿酸对果蝇幼虫生长发育的影响及机制分析
2024-01-01张睿迪邱洪斌王景涛关宝生白雪尹相林
摘要:目的 探讨尿酸(UA)对果蝇幼虫生长发育的影响及其机制。方法 收集刚孵化的野生型黑腹果蝇(W1118)1龄幼虫1350只,采用高嘌呤饮食构建高尿酸血症果蝇模型,分为对照组(标准玉米粉培养基)、低浓度干预组(含0.05%腺嘌呤的玉米粉培养基)和高浓度干预组(含0.10%腺嘌呤的玉米粉培养基),每组150只,另设2个平行实验组,观察各组幼虫的生长发育情况,测定果蝇体内UA和激素水平,以及生长发育相关基因的表达情况。结果 与对照组比较,低浓度干预组和高浓度干预组果蝇体内UA水平均显著增高(P均lt;0.001)、果蝇幼虫发育时间显著延长(P=0.024,Plt;0.001),高浓度干预组果蝇幼虫的生存率、蛹化率、羽化率均显著降低(P均lt;0.001),而果蝇幼虫体内保幼激素(JH)、20-羟基蜕皮甾酮(20E)水平均显著升高(P均lt;0.001)。PCR结果显示,与对照组比较,高浓度干预组果蝇幼虫体内活性氧(ROS)、叉头框O亚型(FOXO)、哺乳动物雷帕霉素靶蛋白(mTOR)mRNA的表达水平显著增高,应激诱导蛋白Sestrin、mTOR复合物1(mTORC1)、AMP活化的蛋白激酶mRNA的表达水平显著降低(P均lt;0.001)。结论 高浓度UA可能通过调节果蝇体内JH、20E的表达,促进ROS/FOXO/mTORC1/mTOR信号通路的表达水平,从而抑制果蝇幼虫的生长发育。
关键词:尿酸;果蝇;活性氧;叉头框转录因子;哺乳动物雷帕霉素靶蛋白复合物1
中图分类号: R589.7" 文献标识码: A" 文章编号:1000-503X(2024)05-0653-06
DOI:10.3881/j.issn.1000-503X.16013
Effect and Mechanism of Uric Acid in Regulating Larval Growth and Development of Drosophila Melanogaster
ZHANG Ruidi QIU Hongbin ,WANG Jingtao GUAN Baosheng ,BAI Xue4,YIN Xianglin
1School of Public Health,2Heilongjiang Province Key Laboratory of Gout Research,3
Department of Anatomy and Histoembryology,School of Basic Medicine Sciences,Jiamusi University,Jiamusi,Heilongjiang 154007,China
4Department of Pharmacy,The Second Affiliated Hospital of Harbin Medical University,Harbin 150086,China
Corresponding authors:YIN Xianglin Tel:13836655277,E-mail:yinxianglin@jmsu.edu.cn;
BAI Xue Tel:13803660659,E-mail:gbs@jmsu.edu.cn
ABSTRACT:Objective To explore the effect and mechanism of uric acid (UA) in regulating the larval growth and development of Drosophila melanogaster.Methods A total of 1350 newly hatched first-instar larvae of wild-type Drosophila melanogaster (W1118) were collected,and the Drosophila melanogaster model of hyperuricemia was constructed with a high purine diet.The larvae were assigned into three groups (n=150):control (standard corn meal medium),low-dose adenine (corn meal medium containing 0.05% adenine),and high-dose adenine (corn meal medium containing 0.10% adenine),and two parallel groups were set up.The growth and development of larvae in each group was observed,and the UA and hormone levels were measured.In addition,the expression levels of genes involved in growth and development were determined.Results Compared with the control group,the low- and high-dose adenine groups showed elevated UA levels (both Plt;0.001) and prolonged developmental period (P=0.024,Plt;0.001).The high-dose adenine group showed decreased survival rate,pupation rate,and eclosion rate and elevated levels of juvenile hormone (JH) and 20-hydroxyecdysone (20E) (all Plt;0.001).The PCR results showed that compared with the control group,high-dose adenine upregulated the mRNA levels of reactive oxygen species (ROS),forkhead box O (FOXO),and mammalian target of rapamycin (mTOR) while downregulating the mRNA levels of Sestrin,mTOR complex 1(mTORC1),and AMP-activated protein kinase (all Plt;0.001).Conclusion High concentrations of UA may promote the expression of ROS/FOXO/mTORC1/mTOR signaling pathway by regulating the levels of JH and 20E,thereby inhibiting the larval growth and development of Drosophila melanogaster.
Key words:uric acid;Drosophila melanogaster;reactive oxygen species;forkhead box transcription factor;mammalian target of rapamycin complex 1
Acta Acad Med Sin,2024,46(5):653-658
尿酸(uric acid,UA)是人体嘌呤代谢的终产物,当嘌呤代谢紊乱时可导致血液中UA水平的异常升高,进而可能诱发高尿酸血症,甚至痛风的形成。我国高尿酸血症患病率呈上升趋势[1-2]。有研究显示,摄入过多富含嘌呤的食物可以促进胰岛素样生长因子1的表达,进而改善特发性矮小症患儿的生长发育[3],提示UA影响生长发育可能与胰岛素/胰岛素样生长因子1信号通路有关,后者通过激活其下游转录因子叉头框O亚型(forkhead box O,FOXO)[4],上调应激诱导蛋白Sestrin的转录水平,间接激活AMP活化的蛋白激酶(AMP-activated protein kinase,AMPK),进而抑制哺乳动物雷帕霉素靶蛋白复合物1(mammalian target of rapamycin complex "mTORC1)的活性,从而减缓衰老过程[5-8]。此外,2型糖尿病合并高尿酸血症的患者血清中也可观察到FOXO3a表达量明显增加[9]。研究发现血UA增高能够诱发代谢紊乱和阻碍胰岛素信号传导,进一步激活巨噬细胞中AMPK/mTORC1通路[10]。但目前关于UA影响生长发育的具体机制尚不清楚。果蝇一直是动物发育和遗传学以及人类疾病研究的关键模式生物,越来越多的证据表明,黑腹果蝇能够被应用于研究进化保守的基本细胞机制以及复杂的人类疾病[11-12]。因此,本研究通过构建高尿酸血症果蝇模型,观察UA对果蝇生长发育以及生长激素含量的影响,进一步探索UA影响生长发育的具体机制,为高尿酸血症及相关疾病的治疗和预防提供理论依据。
1 材料和方法
1.1 果蝇品系及饲养条件
野生型黑腹果蝇(W1118)由佳木斯大学公共卫生学院尿酸生理功能研究团队提供。果蝇均采用标准玉米粉培养基饲养。在温度(25.0±0.5)℃、湿度(40.0±0.5)%、光照周期12 h的培养箱中培养。
1.2 高尿酸血症果蝇模型的构建及分组
将羽化后8 h内未交配的野生型雌雄黑腹果蝇转入产卵培养基(琼脂培养基)上培养,收集1350只1龄幼虫用于后续实验。采用高嘌呤饮食构建高尿酸血症果蝇模型,并分为对照组(标准玉米粉培养基)、低浓度干预组(含0.05%腺嘌呤的玉米粉培养基)和高浓度干预组(含0.10%腺嘌呤的玉米粉培养基),每组150只,另设2个平行实验组。本研究通过佳木斯大学动物伦理委员会批准(伦理审批编号:JMSU-2023120601)。
1.3 果蝇幼虫发育情况的测定
每组取100只果蝇卵,观察果蝇从卵发育至成虫所需的时间,每天同一时间记录各组果蝇的蛹化和羽化情况,并计算蛹化率和羽化率。
1.4 ELISA法检测果蝇幼虫UA以及生长发育激素水平
每组取果蝇3龄幼虫25只,每1 g幼虫与9 mL PBS溶液混合,放入玻璃匀浆器中制成组织匀浆,离心后取上清液,采用ELISA试剂盒检测各组果蝇幼虫体内UA、保幼激素(juvenile hormone,JH)、20-羟基蜕皮甾酮(20-hydroxyecdysone,20E)的水平。
1.5 果蝇幼虫发育相关基因mRNA的检测
每组取果蝇3龄幼虫25只,采用Trizol法提取总RNA,将总RNA逆转录为cDNA进行PCR扩增,测定果蝇幼虫FOXO、Sestrin、AMPK、mTORC1、活性氧(reactive oxygen species,ROS)、mTOR mRNA的表达水平,以rp49作为内参基因,表达水平以目的基因和内参基因的光密度比值表示。PCR引物序列见表1。
1.6 统计学处理
采用SPSS 29.0软件,符合正态分布的计量资料以均数±标准差表示,组间比较采用单因素方差分析,两两比较采用SNK-q检验。计数资料以率表示,组间比较采用卡方检验或卡方连续校正检验。P<0.05为差异有统计学意义。
2 结果
2.1 UA表达水平比较
低浓度干预组和高浓度干预组果蝇体内UA水平显著高于对照组[(235.71±4.29)μmol/L比(190.19±3.67)μmol/L,Plt;0.001和(247.12±2.04)μmol/L比(190.19±3.67)μmol/L,Plt;0.001],且高浓度干预组果蝇体内UA水平显著高于低浓度干预组(P=0.007)。
2.2 UA对果蝇幼虫发育情况的影响
与对照组比较,高浓度干预组果蝇幼虫的生存率显著下降(Plt;0.001),而低浓度干预组与对照组生存率差异无统计学意义(P=0.981)。低浓度干预组和高浓度干预组果蝇幼虫发育时间显著长于对照组[(10.61±1.01)d比(10.21±1.06)d,P=0.024和(14.71±1.86)d比(10.21±1.06)d,Plt;0.001]。与对照组比较,高浓度干预组果蝇幼虫的蛹化率及羽化率均显著降低(P均lt;0.001),而低浓度干预组与对照组的蛹化率及羽化率差异无统计学意义(P=0.11 P=0.293)。
2.3 UA对果蝇幼虫发育激素水平的影响
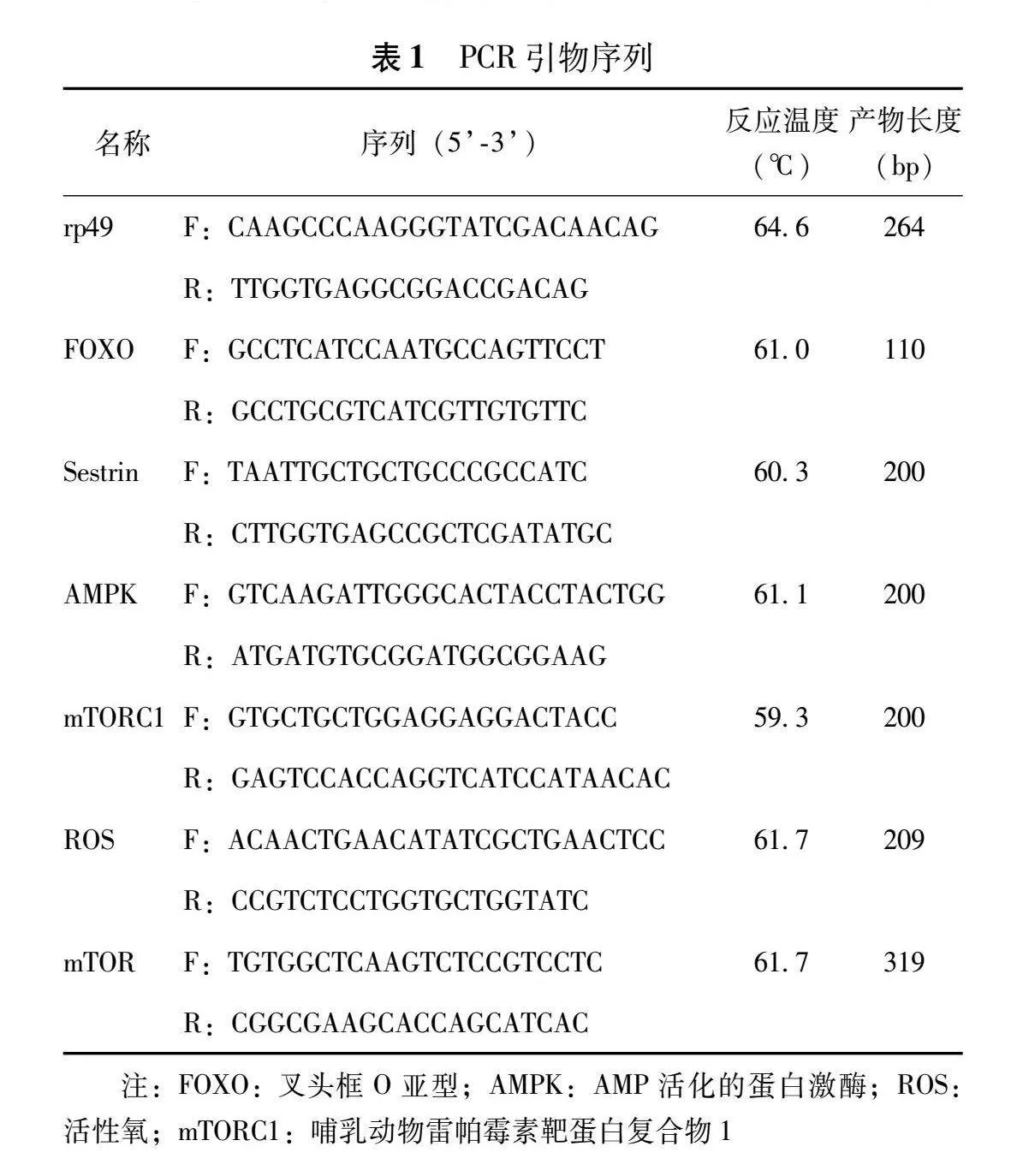
与对照组比较,低浓度干预组和高浓度干预组果蝇幼虫体内的JH水平显著增高(P=0.005,Plt;0.001),而低浓度干预组20E水平显著降低(Plt;0.001),高浓度干预组20E水平显著增高(Plt;0.001)(图1)。
2.4 UA对果蝇幼虫发育相关基因表达水平的影响
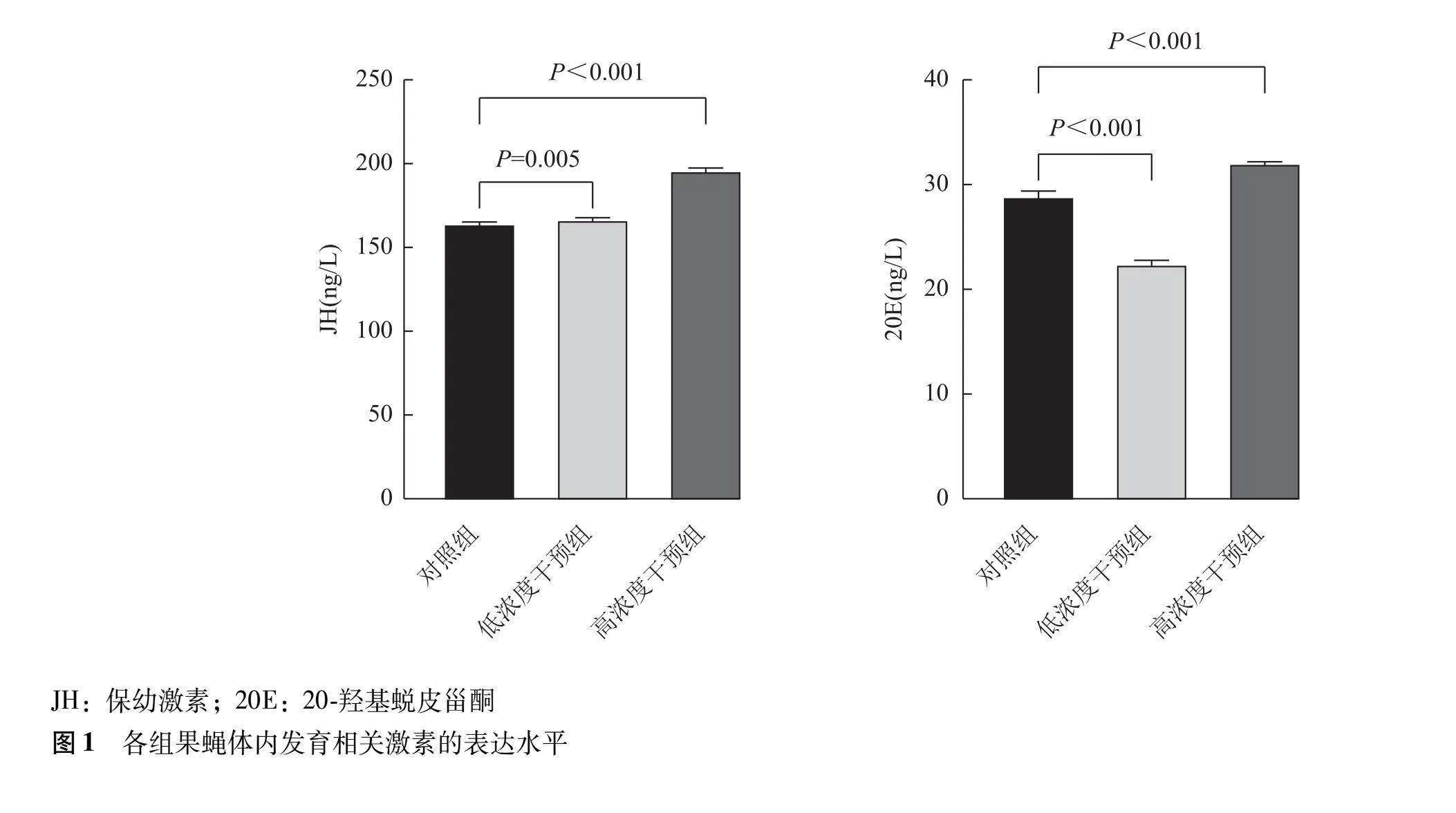
低浓度干预组果蝇幼虫体内ROS、 FOXO、mTOR mRNA表达量显著高于对照组(Plt;0.00 Plt;0.00 P=0.022),应激诱导蛋白Sestrin、mTORC1 mRNA表达量显著低于对照组(P均lt;0.001),AMPK mRNA表达量与对照组比较差异无统计学意义(P=0.136)。高浓度干预组果蝇幼虫体内ROS、 FOXO、mTOR mRNA表达量显著高于对照组(P均lt;0.001),应激诱导蛋白Sestrin、AMPK、mTORC1 mRNA表达量显著低于对照组(P均lt;0.001)(图2)。
3 讨论
如何延缓衰老一直是人类积极探索的问题,现有研究显示,ROS信号传导途径调节年龄依赖性细胞损伤,引起细胞老化[13-14]。UA本身在化学上被表征为抗氧化剂,在生理浓度下,UA能够抑制ROS的累积。同时,UA能够激活NADPH氧化酶并产生ROS,高浓度UA能够导致线粒体损伤和ROS的增加[15-16]。因此,探究UA与衰老之间的内在联系及其潜在的分子机制对于进一步理解UA与生长发育的关系至关重要。
UA在果蝇生长发育过程中起到重要的作用。兰榕榆等[17]研究显示UA能明显诱导果蝇体内氧化-抗氧化系统失衡,其可能是通过诱发氧化应激进而影响果蝇的生长发育。氧化应激是体内自由基产生的一种负面作用,ROS已被证实可以诱导不同类型的细胞衰老[18-19],但其潜在机制尚不清楚。本研究通过构建高尿酸血症果蝇模型,发现高浓度UA能够明显抑制果蝇幼虫的生长发育,降低其蛹化率和羽化率,并显著升高20E和JH水平,提示UA可能通过影响果蝇体内两种主要亲脂性激素JH与20E的相互作用调控幼虫的生长发育[20-22]。进一步研究显示UA能够明显上调ROS、FOXO、mTOR和下调mTORC1的表达。UA通过调节ROS的表达水平,影响转录因子及相关激酶,诱导细胞周期进入停滞状态[23-25]。而ROS可以调节衰老过程关键因子FOXO的活性,并通过Sestrin间接抑制mTORC1表达[26-28],进而延长果蝇幼虫发育至成虫的时间。有研究报道,FOXO能够诱导Sestrin表达升高,提高AMPK活性,从而抑制mTORC1信号传导的能力[10,29-31]。但本研究结果显示,Sestrin和AMPK均与FOXO的表达量成反比,与mTORC1的表达量成正比。在果糖干预下,UA能够抑制AMPK活性,导致核苷酸结合寡聚化结构域样受体3炎症小体的激活和白细胞介素1β的产生,在加入别嘌呤醇后AMPK被重新激活[32-34]。但UA也可能通过诱导ROS产生激活AMPK[35-36]。
综上,本研究结果表明,高浓度UA能够促进果蝇体内JH、20E的表达,通过激活ROS上调FOXO表达,从而对mTORC1和mTOR等基因表达产生影响,最终导致果蝇幼虫的发育延迟。本研究为临床治疗高尿酸血症提供一定的理论支撑,但UA调控AMPK参与代谢过程中的具体机制还需更加深入的研究。
利益冲突 所有作者声明无利益冲突
作者贡献声明 张睿迪:研究设计、实施实验、论文撰写;邱洪斌:实验评估、实施实验;王景涛:数据收集、数据处理;关宝生:研究选题、论文修改;白雪、尹相林:研究设计、论文定稿
参 考 文 献
[1]Liu R,Han C,Wu D,et al.Prevalence of hyperuricemia and gout in mainland China from 2000 to 2014:a systematic review and meta-analysis[J].Biomed Res Int,2015,2015:762820.DOI:10.1155/2015/762820.
[2]Li Y,Shen Z,Zhu B,et al.Demographic,regional and temporal trends of hyperuricemia epidemics in mainland China from 2000 to 2019:a systematic review and mela-analysis[J].Clob Health Action,202 14(1):1874652.DOI:10.1080/16549716.2021.1874652.
[3]Panpan W,Baolan J,Qian S,et al.Association between insulin-like growth factor-1 and uric acid in Chinese children and adolescents with idiopathic short stature:a cross-sectional study[J].BioMed Res Int,2018,2018:4259098.DOI:10.1155/2018/4259098.
[4]Wan QL,Fu X,Dai W,et al.Uric acid induces stress resistance and extends the life span through activating the stress response factor DAF-16/FOXO and SKN-1/NRF2[J].Aging (Albany NY),2020,12(3):2840-2856.DOI:10.18632/aging.102781.
[5]Lee JH,Budanov AV,Park EJ,et al.Sestrin as a feedback inhibitor of TOR that prevents age-related pathologies[J].Science,2010,327(5970):1223-1228.DOI:10.1126/science.1182228.
[6]Peeters H,Debeer P,Bairoch A,et al.PA26 is a candidate gene for heterotaxia in humans:identification of a novel PA26-related gene family in human and mouse[J].Hum Genet,200 112(5-6):573-580.DOI:10.1007/s00439-003-0917-5.
[7]Budanov AV,Sablina AA,Feinstein E,et al.Regeneration of peroxiredoxins by p53-regulated sestrins,homologs of bacterial AhpD[J].Science,2004,304(5670):596-600.DOI:10.1126/science.1095569.
[8]Budanov AV,Karin M.p53 target genes sestrin1 and sestrin2 connect genotoxic stress and mTOR signaling[J].Cell,2008,134:451-460.DOI:10.1016/j.cell.2008.06.028.
[9]桑艳红,饶小娟,焦培林.血清FOXO3a和IGF-1R表达水平与2型糖尿病并发高尿酸血症的相关性[J].热带医学杂志,202 22(5):652-655.DOI:10.3969/j.issn.1672-3619.2022.05.012.
[10]Zhao H,Lu J,He F,et al.Hyperuricemia contributes to glucose intolerance of hepatic inflammatory macrophages and impairs the insulin signaling pathway via IRS2-proteasome degradation[J].Front Immunol,202 13:931087.DOI:10.3389/fimmu.2022.931087.
[11]Arora S,Ligoxygakis P.Beyond host defense:deregulation of drosophila immunity and age-dependent neurodegeneration[J].Front Immunol,2020,11:1574.DOI:10.3389/fimmu.2020.01574.
[12]Vesala L,Hultmark D,Valanne S.Editorial:recent advance in drosophila cellular and humoral innate immunity[J].Front Immunol,2020,11:598618.DOI:10.3389/fimmu.2020.598618.
[13]Bouzid MA,Filaire E,McCall A,et al.Radical oxygen species,exercise and aging:an update[J].Sports Med,2015,45(9):1245-1261.DOI:10.1007/s40279-015-0348-1.
[14]Kwon MJ,Lee KY,Lee HW,et al.SOD3 variant,R213G,altered SOD3 function,leading to ROS-mediated inflammation and damage in multiple organs of premature aging mice[J].Antioxid Redox Signal,2015,23(12):985-999.DOI:10.1089/ars.2014.6035.
[15]Sautin YY,Nakagawa T,Zharikov S,et al.Adverse effects of the classic antioxidant uric acid in adipocytes:NADPH oxidase-mediated oxidative/nitrosative stress[J].Am J Physiol Cell Physiol,2007,293(2):C584-C596.DOI:10.1152/ajpcell.00600.2006.
[16]Sánchez-Lozada LG,Lanaspa MA,Cristóbal-García M,et al.Uric acid-induced endothelial dysfunction is associated with mitochondrial alterations and decreased intracellular ATP concentrations[J].Nephron Exp Nephrol,201 121(3-4):e71-e78.DOI:10.1159/000345509.
[17]兰榕榆,白雪,王景涛,等.尿酸对果蝇生长发育及寿命等的影响及机制[J].中国病理生理杂志,202 38(11):2038-2045.DOI:10.3969/j.issn.1000-4718.2022.11.015.
[18]Li Y,Lu J,Cao X,et al.A newly synthesized rhamnoside derivative alleviates alzheimer’s amyloid-beta-induced oxidative stress,mitochondrial dysfunction,and cell senescence through upregulating SIRT3[J].Oxid Med Cell Longev,2020,2020:7698560.DOI:10.1155/2020/7698560.
[19]Wang J,Zheng B,Yang S,et al.Olmesartan prevents oligomerized amyloid beta (abeta)-induced cellular senescence in neuronal cells[J].ACS Chem Neurosci,202 12(7):1162-1169.DOI:10.1021/acschemneuro.0c00775.
[20]Kozlova T,Thummel CS.Spatial patterns of ecdysteroid receptor activation during the onset of drosophila metamorphosis[J].Development,200 129(7):1739-1750.DOI:10.1242/dev.129.7.1739.
[21]Jindra M,Palli SR,Riddiford LM.The juvenile hormone signaling pathway in insect development[J].Annu Rev Entomol,201 58:181-204.DOI:10.1146/annurev-ento-120811-153700.
[22]Mitchell NC,Lin JI,Zaytseva O,et al.The ecdysone receptor constrains wingless expression to pattern cell cycle across the drosophila wing margin in a cyclin B-dependent manner[J].BMC Dev Biol,201 13:28.DOI:10.1186/1471-213X-13-28.
[23]Lim JM,Lee KS,Woo HA,et al.Control of the pericentrosomal H2O2 level by peroxiredoxin I is critical for mitotic progression[J].J Cell Biol,2015,210(1):23-33.DOI:10.1083/jcb.201412068.
[24]Fong CS,Temple MD,Alic N,et al.Oxidant-induced cell-cycle delay in saccharomyces cerevisiae:the involvement of the SWI6 transcription factor[J].FEMS Yeast Res,2008,8(3):386-399.DOI:10.1111/j.1567-1364.2007.00349.x.
[25]Owusu-Ansah E,Yavari A,Mandal S,et al.Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint[J].Nat Genet,2008,40(3):356-361.DOI:10.1038/ng.2007.50.
[26]Ding R,Wang X,Chen W,et al.WX20120108,a novel IAP antagonist,induces tumor cell autophagy via activating ROS-FOXO pathway[J].Acta Pharmacol Sin,2019,40(11):1466-1479.DOI:10.1038/s41401-019-0253-5.
[27]Toshniwal AG,Gupta S,Mandal L,et al.ROS Inhibits cell growth by regulating 4EBP and S6K,independent of TOR,during development[J].Dev Cell,2019,49(3):473-489.e9.DOI:10.1016/j.devcel.2019.04.008.
[28]Chen CC,Jeon SM,Bhaskar PT,et al.FOXOs inhibit mTORC1 and activate Akt by inducing the expression of Sestrin3 and Rictor[J].Dev Cell,2010,18(4):592-604.DOI:10.1016/j.devcel.2010.03.008.
[29]Hay N.Interplay between FOXO,TOR,and Akt[J].Biochim Biophys Acta,201 1813(11):1965-1970.DOI:10.1016/j.bbamcr.2011.03.013.
[30]Rhee SG,Bae SH.The antioxidant function of sestrins is mediated by promotion of autophagic degradation of Keap1 and Nrf2 activation and by inhibition of mTORC1[J].Free Radic Biol Med,2015,88(Pt B):205-211.DOI:10.1016/j.freeradbiomed.2015.06.007.
[31]Budanov AV,Lee JH,Karin M.Stressin’ sestrins take an aging fight[J].EMBO Mol Med,2010,2(10):388-400.DOI:10.1002/emmm.201000097.
[32]Lanaspa MA,Cicerchi C,Garcia G,et al.Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver[J].PLoS One,201 7(11):e48801.DOI:10.1371/journal.pone.0048801.
[33]Kimura Y,Yanagida T,Onda A,et al.Soluble uric acid promotes atherosclerosis via AMPK (AMP-activated protein kinase)-mediated inflammation[J].Arterioscler Thromb Vasc Biol,2020,40(3):570-582.DOI:10.1161/ATVBAHA.119.313224.
[34]García-Arroyo FE,Monroy-Sánchez F,Muoz-Jiménez I,et al.Allopurinol prevents the lipogenic response induced by an acute oral fructose challenge in short-term fructose fed rats[J].Biomolecules,2019,9(10):601.DOI:10.3390/biom9100601.
[35]Zhang Y,Yamamoto T,Hisatome I,et al.Uric acid induces oxidative stress and growth inhibition by activating adenosine monophosphate-activated protein kinase and extracellular signal-regulated kinase signal pathways in pancreatic β cells[J].Mol Cell Endocrinol,201 375(1-2):89-96.DOI:10.1016/j.mce.2013.04.027.
[36]Luo C,Lian X,Hong L,et al.High uric acid activates the ROS-AMPK pathway,impairs CD68 expression and inhibits OxLDL-induced foam-cell formation in a human monocytic cell line,THP-1[J].Cell Physiol Biochem,2016,40(3-4):538-548.DOI:10.1159/000452567.
(收稿日期:2024-01-22)
