Isoliquiritigenin regulated ox-LDL through activating the PPAR-γ signaling pathway to stabilize atherosclerosis plaques
2023-12-29XUXinruiGAOZhaoZHANGQingyueYANGManfangSUNHaoFENGLuWANGTianyuLIYangLOULixiaWUAimingNIEBo
XU Xin-rui, GAO Zhao, ZHANG Qing-yue, YANG Man-fang, SUN Hao, FENG Lu,WANG Tian-yu, LI Yang, LOU Li-xia, WU Ai-ming, NIE Bo,✉
1.Key Laboratory of Chinese Internal Medicine of Ministry of Education and Beijing, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing 100700, China
2.Longhua Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai 200032, China
3.School of Traditional Chinese Medicine, Beijing University of Chinese Medicine, Beijing 100029, China
4.Department of Pathology, Dongzhimen Hospital Affiliated to Beijing University of Chinese Medicine, Beijing 100700, China
Keywords:Atherosclerosis mice Isoliquiritigenin Ox-LDL PPAR-γ Plaque stability
ABSTRACT
1.Introduction
Atherosclerosis (AS) is a lipid-driven chronic inflammatory response that is the main pathological basis of cardiovascular disease, which is characterized by disturbances in lipid metabolism,subendothelial oxidized low-density lipoprotein (ox-LDL) deposition and inflammatory response, foam cell accumulation and plaque formation[1].Ox-LDL is considered to be one of the main triggers for the progression of AS[2].Peroxisome proliferator-activated receptor γ (PPAR-γ) signaling pathway can regulate lipid metabolism and is a key target for the treatment of metabolic diseases.In the progression of AS, it plays an important role in stabilizing atherosclerotic plaque by improving the disorder of ox-LDL lipid metabolism, which is one of the important mechanisms for preventing and treating AS[3].The previous experiments confirmed that Simiao Yongan Decoction (SMYA) activated PPAR-γ, inhibited the expression of FABP-4, antagonized the ox-LDL lipid metabolism pathway, and thus stabilized plaque, and had an AS-protective effect[4].UHPLC-LTQ-Orbitrap technique was used to identify isoliquiritigenin in SMYA decoction and its blood components[5].Isoliquiritigenin was identified in the active part CS05 of SMYA in a screened PPAR-γ agonist model[6], and molecular docking verified that isoliquiritigenin had strong activity in activating PPAR-γ.Isoliquiritigenin was identified by network pharmacology as the main component of SMYA decoction for its anti-inflammatory effect[5].Studies of isoliquiritigenin have mainly focused on antitumor aspects[7], with few reports on the pharmacological effects and mechanisms of AS.Therefore, on the basis of preliminary verification of the anti-AS effect of isoliquiritigenin in previous experiments, this study explored the mechanism of isoliquiritigenin on ApoE-/- mice AS through the regulation of ox-LDL metabolism by PPAR-γ signaling pathway.
2.Materials and methods
2.1 Materials
2.1.1 Experimental animals
C57BL/6J mice, male, 7 weeks; ApoE-/- mice, male, 7 weeks,weight (20±2) g, provided by Beijing Vital River Laboratory Animal Technology Co., Ltd.[License No.SCXK (Beijing) 2021-0011].The animals were raised in the barrier class animal room of Dongzhimen Hospital, Beijing University of Chinese Medicine (NO.SYXK(Beijing) 2020-0013).The feeding environment was: constant temperature of 22-24 ℃, humidity of 50%, alternating light and dark once for 12 h.
The high-fat diet was composed of 15% fat, 0.25% cholesterol and 84.75% base feed.It was purchased from Beijing HFK Bioscience CO., Ltd.Science and Technology Co., Ltd.(NO.SCXK (Beijing)2014-0008).
2.1.2 Drugs and reagents
Isoliquiritigenin (NO.Y-008-181216), purchased from Chengdu Ruifensi Biotechnology Co., LTD.Mouse ox-LDL serum enzymelinked immunosorbent assay (ELISA) Kit purchased from Elabscience Biotechnology Co., Ltd.(No.E-EL-M0066C).Saturated Oil Red O dyeing solution (No.G1260) and improved Masson threecolor dyeing kit (item No.G1346-50) were purchased from Beijing Solaibao Technology Co., LTD.Antibody α-SMA (NO.ab124964),MOMA-2 (NO.ab33451), PPAR-γ (NO.ab45036), LXR-α (NO.ab106464), FABP-4 (NO.ab92501), MMP-2 (NO.ab37150),MMP-9 (NO.ab38898) and GAPDH (NO.ab9485) were purchased from Abcam.Goat anti-rabbit IgG/HRP antibody (No.C1309), BCA protein assay kit (No.P1511), RIPA lysate (NO.C1053) and skim milk powder (No.P1622) were purchased from Beijing Applygen Technologies Co., LTD.One-step PAGE Gel rapid preparation kit(No.PG212) was purchased from Shanghai Epizyme Biomedical Technology Co., LTD.
2.1.3 Instruments
AU5800 Automatic biochemical analyzer (Beckman-Coulter,USA); EG1150 tissue embedding machine (Arcadia, Germany);RM2135 Tissue slicer (Arcadia, Germany); MK3 automatic enzyme marker (Thermo, USA); LF-mini 3 small vertical electrophoresis tank, LF-ZY01 small transfer electrophoresis tank (Beijing Longfang Technology Co., LTD.); Tanon-5200 gel imaging system (Shanghai Tianneng Technology Co., LTD.).
2.2 Methods
2.2.1 Model preparation and administration
Referring to the literature [8], a model of AS was prepared using high-fat feed feeding combined with Perivascular Common Carotid artery (right) vessel Placement (PCCP).After 1 week of adaptive feeding 20 ApoE-/- mice were high-fat fed for 2 weeks and PCCP was performed.After anaesthesia, mice were depilated from the neck to the axilla, and an incision of about 2 cm was made in the right side of the neck to expose the right common carotid artery, and a silicone cannula (length: 2.5 mm, inner diameter: 0.3 mm) was placed around the carotid artery and fixed at the upper and lower ends of the cannula.After operation, the mice were randomly divided into model group and isoliquiritigenin group, with 10 mice in each group.Starting from the day after PCCP, mice in the isoliquiritigenin group were administered at a dosage of 40 mg/kg for 8 weeks, along with a high-fat chow diet.The model group was given the same dose of deionized water by gavage.10 C57BL/6J mice were set up as the normal group, fed with basic diet and the same dose of deionized water.The feeding cycle was the same as that of model mice.
2.2.2 Automatic biochemical analyzer for the detection of serum lipids detection
After the completion of modelling, mice were sampled, and blood was collected by removing the eyeballs, centrifuged at 4 ℃, 3 000 r/min, for 15 min, and then serum was collected, and total cholesterol(TC), triacylglycerol (TG), low-density lipoprotein cholesterol(LDL-C), and high-density lipoprotein cholesterol (HDL-C) levels were measured by automatic biochemical analyzer.
2.2.3 Enzyme-linked immunosorbent assay (ELISA) for the detection of ox-LDL levels in serum
Serum levels of ox-LDL were measured according to the ELISA kit instructions.
2.2.4 Pathological morphological characteristics of carotid plaque
After blood collection, the right carotid artery of mice was taken,fixed by 4% paraformaldehyde, and then underwent OCT embedding and paraffin embedding after dehydration.HE staining was used to observe the pathological morphology of plaques.Oil red O staining and Masson staining were used to analyze lipid and collagen content in plaques.MOMA-2 and α-SMA immunohistochemical staining were used for macrophage and smooth muscle cell analysis.Image Pro Plus 6.0 image processing software was applied to measure intima thickness (IT), media thickness (MT), plaque area (PA),and vascular lumen area (LA) of the vessels, IT/MT and PA/LA were calculated[9], and intra-plaque lipids, collagen, MOMA-2, and α-SMA content were measured to calculate the vulnerability index,vulnerability index (%) = (MOMA-2 positive area percentage +lipid positive area percentage)/(collagen positive area percentage +α-SMA positive area percentage) *100%.
2.2.5 Western blot analysis of PPAR-γ, LXR-α, FABP-4,MMP-2, MMP-9 protein expression in aorta
The aorta was added with appropriate amount of protease inhibitor,broken by ultrasound, and the supernatant was collected by centrifugation, and the protein concentration was measured by BCA method.The same amount of protein was taken for electrophoresis,the protein was transferred to NC membrane, and 5% skim milk powder was enclosed at room temperature for 1 h.The blots were incubated overnight at 4 ℃ with first antibody PPAR-γ (1:1 000),LXR-α (1:1 000), FABP-4 (1:1 000), MMP-2 (1:1 000), MMP-9(1:1000), GAPDH (1: 1 000).The next day, after TBST washing, the membranes were probed with secondary anti-rabbit IgG (1:5000) for 1h at room temperature.After TBST washing of the membrane, ECL luminescent solution was used for colour development, exposed in the gel procedure system and photographed.Image J software was used to analyze strip gray values.
2.2.6 statistical analysis
SPSS 23.0 software was used to analyze the data, and the measurement information was expressed as mean ± standard deviation.The data were tested for normality and variance chisquared, one-way ANOVA was used to compare the samples between multiple groups that conformed to the normal distribution and had chi-squared variance, and the LSD method was used to compare between groups.Non-normal distribution using non-parametric tests.P < 0.05 and P < 0.01 indicate statistically significant differences.
3.Results
3.1 Effects of isolicoridine on the lipid of the AS mouse
Compared with the normal group, TC, TG, LDL-C and HDL-C in the model group were significantly increased (P < 0.01).Compared with model group, TC, TG and LDL-C in the isoliquiritigenin group were decreased (P < 0.05), and HDL-C was not significantly changed, the difference was not statistically significant (P> 0.05).Figure 1.
3.2 Effect of isoliquiritigenin on serum ox-LDL in mice with AS
Compared with the normal group, the serum ox-LDL in the model group was significantly increased (P < 0.01).Compared with the model group, ox-LDL in serum of mice was decreased in the isoliquiritigenin group (P < 0.01).Figure 2.
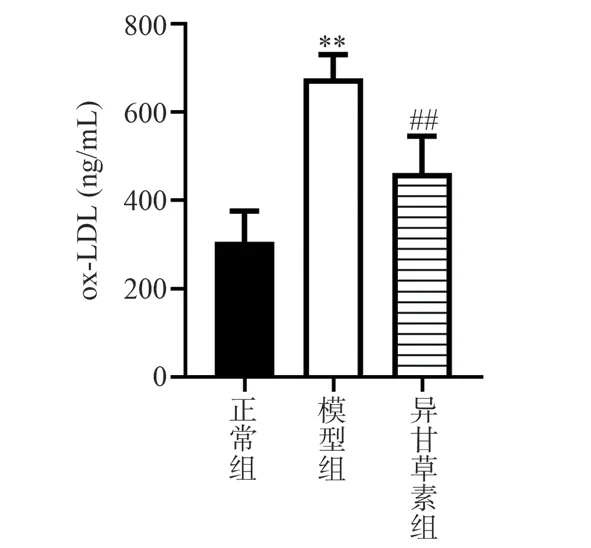
Fig 2 Effect of isoliquiritigenin on ox-LDL in AS mice ( n = 6)
3.3 Effects of isoliquiritigenin on pathological morphology of carotid artery in AS mice
In the normal group, the blood vessel wall was complete and smooth, the structure of the intima-media was intact, and no plaque was found.In the model group, the endothelium was not smooth,the thickness of the inner and media membrane was uneven, the arrangement of smooth muscle cells in the media membrane was seriously disordered, and the inner membrane bulge formed a plaque convex to the lumen, IT thickened (P < 0.01), IT/MT increased (P< 0.01), PA increased (P < 0.01), and PA/LA increased significantly(P < 0.01).Compared with the model group, in the isoliquiritigenin group, carotid intima was not smooth, IT decreased (P < 0.01),intima-media thickness was uneven, media thickness was thickened,IT/MT decreased (P < 0.01), luminal PA decreased significantly (P< 0.01), and luminal obstruction degree decreased.Figure 3 and 4.
3.4 Effects of isoliquiritigenin on lipid, collagen, MOMA-2 and α-SMA contents in carotid plaque of AS mice
Compared with normal group, the content of lipid deposition and MOMA-2 in carotid plaque in model group was higher (P <0.01), the content of collagen and α-SMA was lower (P < 0.01),the plaque vulnerability index was increased (P < 0.01).Compared with the model group, the contents of lipids and MOMA-2 in the isoliquiritigenin group were significantly decreased (P < 0.01), the contents of collagen and α-SMA were increased (P < 0.01), and the plaque vulnerability index was decreased (P < 0.01).Figure 5and 6.
3.5 Effects of isoliquiritigenin on the expression of lipid metabolism proteins PPAR-γ, LXR-α and FABP-4 in AS mice
Compared with the normal group, the expression of PPAR-γ and LXR-α in the model group were significantly reduced (P < 0.01).Compared with the model group, the expression of PPAR-γ and LXR-α in the isoliquiritigenin group were increased (P < 0.01).Compared with the normal group, the expression of FABP-4 protein in the model group was increased (P < 0.01).Compared with the model group, the expression of FABP-4 protein in the isoliquiritigenin group was significantly reduced (P < 0.01).Figure 7.

Fig 3 Effect of isoliquiritigenin on the pathological morphology of carotid artery in AS mice (HE staining, ×200)

Fig 4 Effects of isoliquiritigenin on carotid IT, PA, PA/LA and IT/MT in AS mice (, n = 5)
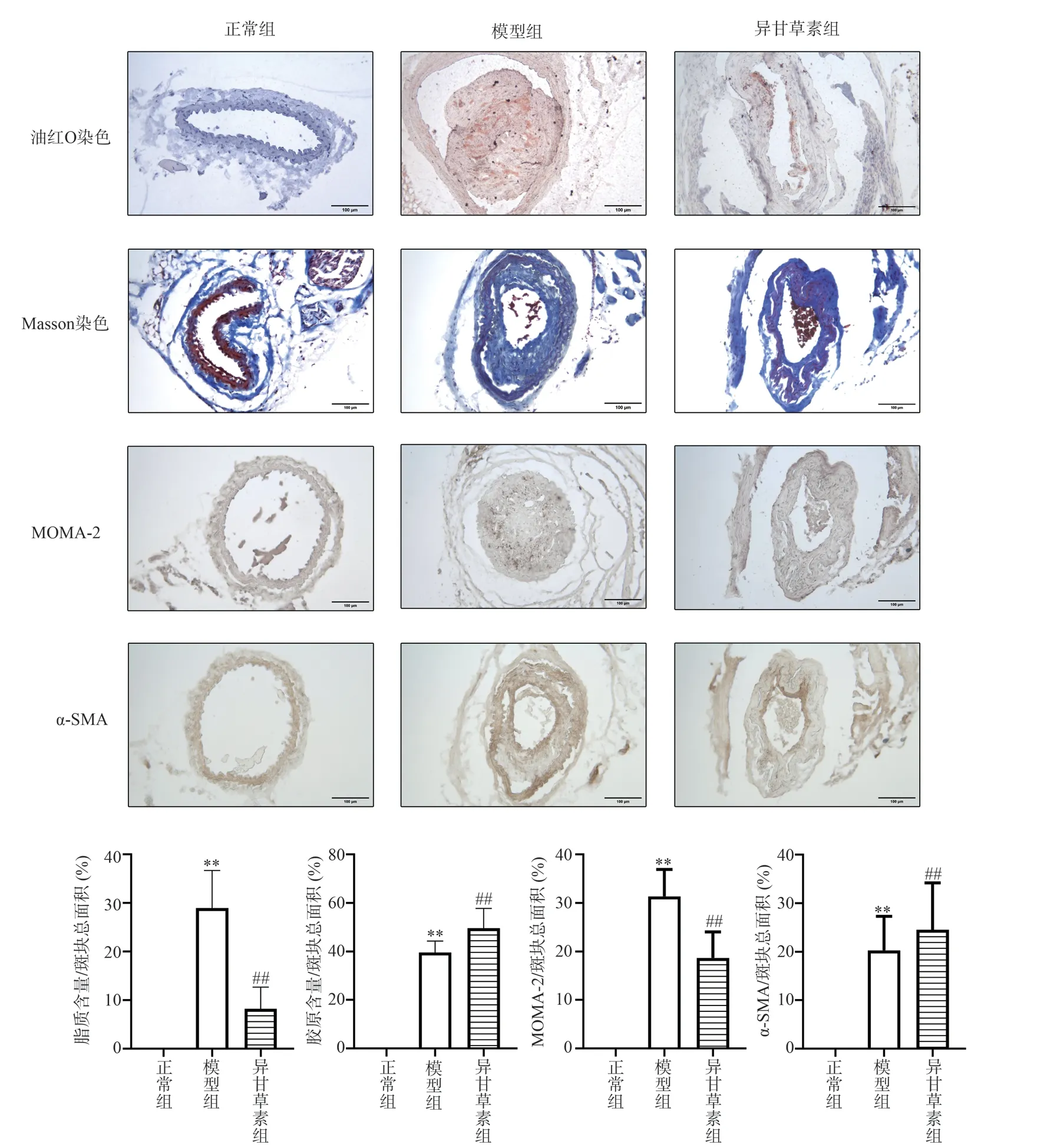
Fig 5 Effects of isoliquiritigenin on pathologic morphology of carotid plaque in AS mice (×200)

Fig 6 Effects of isoliquiritigenin on plaque vulnerability index in AS mice
3.6 Effects of isoliquiritigenin on the expression of plaque stability proteins MMP-2 and MMP-9 in AS mice
Compared with the normal group, the protein expression of MMP-2 and MMP-9 in the model group were significantly increased (P <0.01, P < 0.05).Compared with the model group, the expression levels of MMP-2 and MMP-9 protein in the isoliquiritigenin group were decreased (P < 0.05).Figure 8.

Fig 7 Effects of isoliquiritigenin on the expression of PPAR-γ, LXR-α and FABP-4 proteins in AS mice (, n = 4)
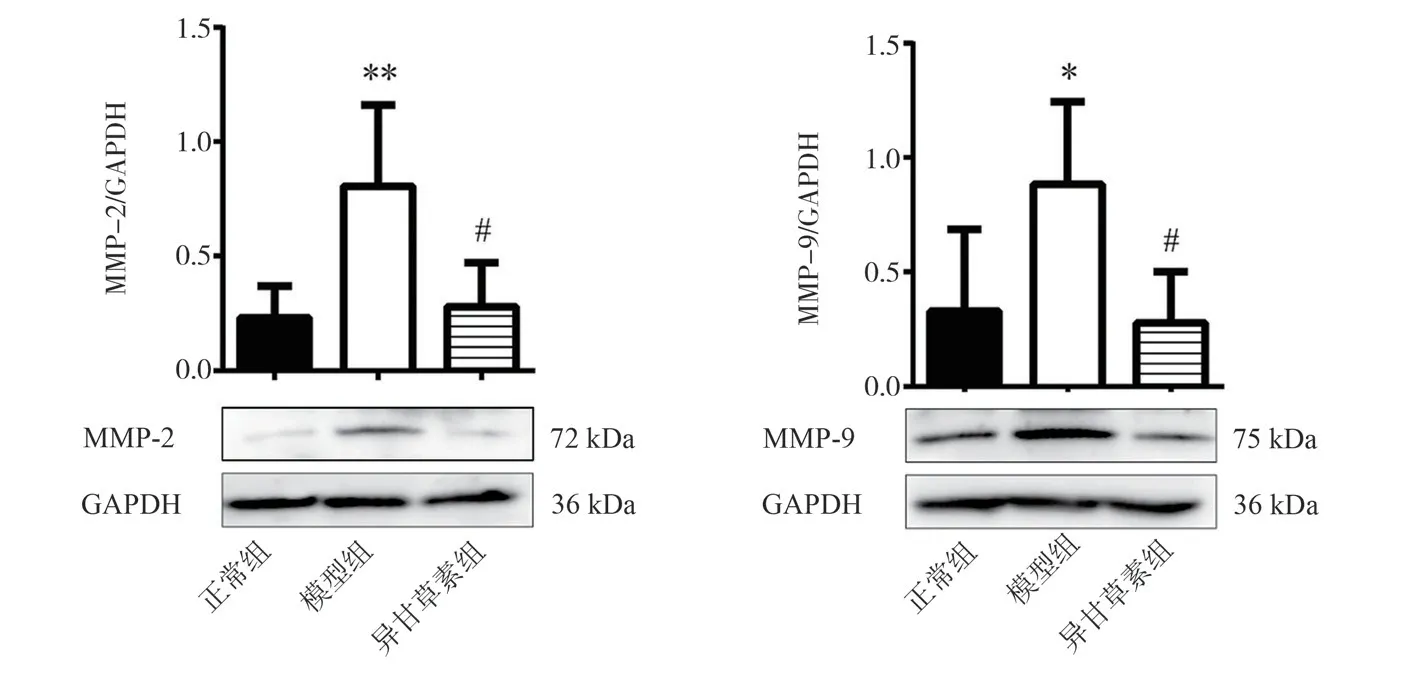
Fig 8 Effects of isoliquiritigenin on the expression of MMP-2 and MMP-9 in AS mice (, n = 4)
4.Discussions
AS is the leading cause of cardiovascular morbidity and mortality[10].The disorder of lipid metabolism is the pathological basis of AS.Isoliquiritigenin, the main flavonoid compound in glycyrrhiza[11],is the effective component in SMYA Decoction, which has antiinflammatory, antibacterial, antioxidant, immune regulation and heart protection effects[12].Studies have shown that isoliquiritigenin can modulate the lipid status of ApoE-/- mice[13].The results of this experiment showed that isoliquiritigenin could reduce the serum content of TC, TG, LDL-C and ox-LDL in AS mice, and modulate the lipid status of AS mice.Pathological results showed that the carotid arteries in the model group had uneven intima-media thickness and large plaque occlusion in the lumen.Compared with the model group, after administration of isoliquiritigenin, IT, PA and PA/LA of carotid vessels were reduced, plaque area was reduced,lumen blockage was alleviated, and the pathological morphology of carotid arteries in AS mice was improved.Isoliquiritigenin can reduce blood lipid level, reduce ox-LDL content, improve vascular pathological morphology, and play a protective role in AS.
Vulnerable plaque formation, plaque bleeding, rupture and thrombosis are the main causes of acute cardiovascular events[14].Ox-LDL is associated with the formation of necrotic lipid cores and is an important feature of vulnerable plaques[15].Macrophage uptake of ox-LDL turned into foam cells[16].Foam cell formation,apoptosis and fusion evolved into a lipid necrotic core, reducing plaque stability[17].Removal of circulating ox-LDL could reduce necrotic core, increase plaque stability, and inhibit AS progression[18].Collagen is the main component of the extracellular matrix in the fiber cap.Plaque collagen content plays an important role in preventing plaque rupture and increases AS plaque stability[19].The results of this experiment showed that the plaque in model group contained more lipids and MOMA-2 content, less collagen and α-SMA content, which showed the characteristics of vulnerable plaque.Isoliquiritigenin was able to reduce ox-LDL content,decrease intraplaque lipid and MOMA-2 content, increase collagen and α-SMA content, decrease plaque vulnerability index, and prevent plaque rupture.
The protective mechanism of isoliquiritigenin against AS involved the PPAR-γ-dependent signaling pathway[20].PPAR-γ, a class of ligand-activated nuclear receptors, played an important role in the maintenance metabolic homeostasis and promoted intracellular cholesterol efflux[21].Macrophage PPAR-γ blocking increased cholesterol accumulation and induced foam cell formation[22].On the contrary, activation of PPAR-γ increased the transcription of downstream protein Liver X Receptor-α (LXR-α)[23], antagonized ox-LDL intake, enhanced cholesterol efflux, and reduced lipid accumulation[24], thereby increasing plaque stability.Fatty acid binding protein 4 (FABP-4) played a central regulatory role in lipid metabolism, promoted foam cell transformation by increasing ox-LDL-induced intracellular lipid accumulation[25].FABP-4 was a major target gene of PPAR-γ and negatively regulated PPAR-γ expression, producing opposite effects[26].The experimental results showed that in model group, PPAR-γ and LXR-α expression decreased, FABP-4 expression increased, plaque lipid increased,instability increased.Isoliquiritigenin could activate PPAR-γ,increase LXR-α expression, decrease FABP-4 expression, reduce ox-LDL uptake, and reduce the lipid content in plaques, thus stabilizing AS plaques.
Matrix metalloproteinases (MMPs) are an important index to evaluate plaque instability[27].The increase of Ox-LDL level aggravates vascular wall damage, increases the constant aggregation of foam cells, and increases the secretion of MMPs[28], increases plaque instability.MMP-2 and MMP-9 are the main proteases affecting plaque stability, degrading collagen in the extracellular matrix and making the fibrous cap weak and prone to rupture[29].PPAR-γ plays an important role in maintaining AS plaque stability,regulates ox-LDL and inhibits the expression of MMP-9 and MMP-2[30].The results of the present study showed that isoliquiritigenin was able to activate PPAR-γ and decrease the expression of MMP-2 and MMP-9, thereby increasing AS plaque stability.The results of the present study showed that isoliquiritigenin could activate PPAR-γ and decrease the expression of MMP-2 and MMP-9, and thus increase the stability of AS plaques.
In conclusion, isoliquiritigenin could exert anti-AS effects by activating PPAR-γ, up-regulating LXR-α, reducing FABP-4 expression, lowering lipid and ox-LDL levels, decreasing the expression of plaque instability-related proteins MMP-2 and MMP-9, and decreasing plaque vulnerability index.
Previous animal experiments, molecular docking and network pharmacology showed that isoliquiritigenin had PPAR-γ activation and played an anti-AS role.The main objective of the animal experiments was to further verify the pharmacodynamic effects of isoliquiritigenin against AS and to explore its mechanism.Further mechanism will be carried out in later cellular experiments to further clarify the anti-AS mechanism of isoliquiritigenin by activating PPAR-γ.
Authors’ contribution
NIE Bo: Experimental design, guidance and paper review; XU Xin-rui: Index detection, data analysis, paper writing; GAO Zhao:Experimental modeling, drug intervention; SUN Hao: Pathological index detection; ZHANG Qing-yue, FENG Lu, YANG Man-fang, LI Yang, WANG Tian-yu: Experimental materials and index detection;WU Ai-ming, LOU Li-xia: Technical guidance of pathological experiment and Western Blot experiment.
All authors declare no conflict of interest.
杂志排行
Journal of Hainan Medical College的其它文章
- Study on the mechanism of action of Feng-Liao-Chang-Wei-Kang combined with 5-fluorouracil in the treatment of colitis-associated colon cancer
- Inhibitory effect of water soluble propolis on oxidative damage in rats with ulcerative colitis
- Effect of hepatocyte growth factor on inflammatory factors associated with CCL4-induced hepatocyte injury
- The expression of TUSC3 in Preeclampsia and the function in trophoblast cell
- Effects of intravenous infusion of esketamine on analgesia and postpartum antidepressant after cesarean section
- Expression and significance of ADAM12 in bladder cancer
