无核葡萄胚珠发育过程中内源激素及多胺含量的变化
2023-12-28朱佩佩秦浩翔张剑侠
朱佩佩,秦浩翔,张剑侠
无核葡萄胚珠发育过程中内源激素及多胺含量的变化
朱佩佩,秦浩翔,张剑侠
西北农林科技大学园艺学院/旱区作物逆境生物学国家重点实验室/农业农村部西北地区园艺作物生物与种质创制重点实验室,陕西杨凌 712100
【目的】探究无核葡萄胚珠发育过程中内源激素和多胺含量变化对胚发育的影响,为花前喷洒外源激素及胚珠离体培养条件下培养基中添加外源激素以促进胚的发育提供理论依据。【方法】以欧亚种葡萄(L.)有核品种‘京秀’及其F1代种子败育型无核品种‘秦秀’为材料,采用高效液相色谱质谱(high performance liquid chromatography mass spectrometry,HPLCMS)和超高效液相色谱(ultra performance liquid chromatography,UPLC)分析方法,比较二者在果实不同发育时期胚珠中内源激素和多胺含量的变化规律。【结果】对于生长素(IAA)、玉米素(ZT)、玉米素核苷(ZR)和N6-异戊烯腺嘌呤(iPAS)含量,‘京秀’在花后39 d达到最高值,‘秦秀’在花后42 d达到最高值,但前者最高值均高于后者(1.5倍以上);对于茉莉酸(JA)和水杨酸(SA)含量,‘京秀’在花后36 d(2 000 ng∙g-1和6 500 ng∙g-1)开始急剧上升,至花后39 d 时均达到最高值(6 500 ng∙g-1和10 000 ng∙g-1),之后下降并在花后42—45 d维持在较高水平,而‘秦秀’自花后36 d(3 500 ng∙g-1和3 000 ng∙g-1)开始一直下降并处于较低的水平;对于氨基环丙烷羧酸(ACC)含量,‘京秀’在花后39 d时几乎为0,然后迅速上升,在花后45 d时达到最高值(1 200 ng∙g-1),而‘秦秀’在花后39 d时达到最高值(900 ng∙g-1),之后下降至花后42 d时几乎为0,并一直维持在极低的水平;对于脱落酸(ABA)含量,‘京秀’在花后36—45 d一直处于几乎为0的水平,而‘秦秀’自花后39 d从0开始急速上升到42 d的最高值(900 ng∙g-1);对于腐胺(Put)、精胺(Spm)和亚精胺(Spd)含量,2个品种变化趋势相似,但‘京秀’一直高于‘秦秀’,除精胺最高值均出现在花后42 d外,腐胺和亚精胺最高值在‘京秀’中为花后42 d,‘秦秀’为39 d。【结论】与有核品种‘京秀’相比,无核品种‘秦秀’胚珠中生长促进物质(IAA、CTK、GA3、ACC、JA、SA、Put、Spd和Spm)含量较低,而生长抑制物质(ABA)含量较高,(IAA+GA3)/ABA、(IAA+ZT+GA3)/ABA、Spm/多胺(PAs)、(Spd+Spm)/PAs和(Spd+Spm)/Put的比值较低,可能是导致其胚败育的主要原因之一。因此,在无核葡萄胚挽救过程中,可通过花前喷洒或者培养基中添加一定浓度的生长促进物质来抑制胚的败育。
种子败育型无核葡萄;胚珠;胚;内源激素;多胺
0 引言
【研究意义】在果实的生长发育过程中,欧亚种葡萄(L.)有核品种和无核品种的种子(胚珠)发育程度存在很大差异[1]。无核葡萄胚败育主要由基因型决定[2],还与胚珠中内源激素[3-6]以及树体营养状况[7]有关。通过研究无核葡萄内源激素及多胺含量的变化,可为花前喷洒外源激素及胚珠离体培养下培养基中添加外源激素以促进胚的发育提供理论依据。【前人研究进展】葡萄胚的发育和败育过程由多种植物激素共同调控,其中能够诱导单性结实的激素主要有生长素(indolyl-3-acetic acid,IAA)和赤霉素(gibberellic acid,GA3)[1,8]。内源IAA和GA3在无核葡萄中的含量高于有核葡萄,推测内源激素含量变化可能是导致胚败育的原因之一[3]。有研究表明,由于某种内源激素的含量或者不同内源激素之间的比值如(赤霉素+生长素)/脱落酸和玉米素核苷/脱落酸发生了变化导致无核葡萄胚败育[5]。不同发育阶段的种子对外源激素种类及水平要求也不同,外源激素的种类或浓度使用不当,会影响果实及种子中的激素含量,致使激素失衡,影响胚正常发育,最终导致败育,胚珠鲜重减少[4,9]。在已知的植物内源激素中,生长素主要在幼嫩的芽、叶和发育的种子中合成,除对植物的早期发育和形态建成具有重要作用外,还影响胚珠和胚的发育[10-13]。细胞分裂素(cytokinin,CTK)主要在根尖部位合成,在调控植物生长发育的过程中起关键作用,其中包括促进胚的发育[10,14]。植物体内的细胞分裂素主要有玉米素(trans-zeatin,ZT)、二氢玉米素(dihydrozeatin,DHZ)、N6-异戊烯腺嘌呤(iPAS)和玉米素核苷(trans-zeatin- riboside,ZR)等[15]。有研究表明,在花前喷洒外源细胞分裂素CPPU[16]、6-BA[17]、TDZ[18]均可促进无核葡萄胚发育。赤霉素在未成熟的种子、根尖和顶芽等部位合成,可有效打破种子休眠,促进胚发育和种子萌发以及细胞伸长[19]。研究表明,外源GA3一方面可使植物中苹果酸脱氢酶活性下降,导致植物呼吸作用减弱,能量供应不足,影响胚囊发育;另一方面促进珠心和子房壁的生长发育,使胚囊发育尚未成熟时就开花,从而影响授粉受精,形成无核果实[20-22]。葡萄果实经外源GA3处理后,差异表达基因主要集中在激素平衡调节、细胞凋亡、种皮发育、胚乳发育及胚珠发育等方面[20]。乙烯(ethylene,ETH)可在植物的各个部位合成,可促进胚和果实成熟及叶片衰老,打破植物种子和芽的休眠等[23-24]。氨基环丙烷羧酸(1-aminocyclopropane-1- carboxylic acid,ACC)是ETH合成的直接前体,通常诱导ETH响应,但在拟南芥()的生殖过程中,胚珠中的ACC作为非依赖ETH的信号,参与了花粉管的转动并有效地传递花粉,在ACC的存在下,种子数量几乎翻了一番[25]。茉莉酸(jasmonic acid,JA)与茉莉酸甲酯(methyl jasmonate,Me-JA)在调节植物生长发育、抗逆反应等方面起着重要的作用,可抑制非休眠种子的萌发但刺激休眠种子的萌发[26]。水杨酸(salicylicacid,SA)作为植物体内一种高效的抗氧化激素,参与调节气孔开放,促进开花结果并激活植物超敏反应[27]。SA生物合成相关基因和在欧亚种无核葡萄‘无核白’中的表达水平显著高于有核葡萄‘黑比诺’[28],暗示着无核葡萄胚败育可能与SA含量高有关。脱落酸(abscisic acid,ABA)主要在根冠和萎蔫的叶片中合成,可促使胚正常发育成熟并抑制过早萌发,但外源ABA施用浓度过高不利于胚的进一步发育[10,29]。多胺(PAs)是一类广泛存在于植物体内的脂肪族含氮碱,参与植物细胞增殖与分化,与胚胎发育、程序性死亡、休眠以及衰老等生命活动密切相关[30-33]。前人研究表明果皮及胚珠中内源多胺含量急剧下降可能是导致葡萄胚败育的原因之一[6,34]。近年来,对杏[35]和玉米[36]种子发育过程中内源多胺的动态变化研究也获得了相同的结果。无核葡萄是葡萄育种的重要目标之一。常规的无核葡萄杂交育种周期长,效率低。胚挽救技术以无核葡萄作母本,能极大提高无核葡萄的育种效率,因而被广泛用于无核葡萄育种[37-39]。由于目前对无核葡萄胚败育的机理尚不十分清楚,致使无核葡萄胚挽救的效率仍然较低。无核葡萄胚败育除了受基因控制外,还受营养状况及内源激素的影响[38]。前人研究表明,内源激素和多胺含量是影响克里曼丁橘[40]、杧果[33]、枣[41]、山茱萸[42]和甜樱桃[43]胚败育的重要因素之一。花前喷施或培养基中添加一定浓度的IBA、CPPU、6-BA、TDZ、腐胺或PP333均能促进种子败育型无核葡萄胚珠和胚的发育[16-18,30,39,44],从而提高胚挽救效率。但是,目前对种子败育型无核葡萄胚珠发育过程中内源激素的变化规律尚不完全清楚,探究这一变化规律对于花前喷施或培养基中添加何种外源激素、不同激素浓度及其比例以提高胚挽救效率十分重要。【本研究切入点】前人对无核葡萄浆果或胚珠中的IAA、GA3、ABA和ZR含量变化已有报道[1,3,5],但所测定的内源激素种类有限,且不同基因型的无核葡萄内源激素变化进程存在差异。【拟解决的关键问题】本研究以种子败育型无核葡萄新品种‘秦秀’(‘京秀’ב郑果大无核’)为研究对象,以其有核母本‘京秀’为对照,测定二者在胚发育过程中胚珠内源激素及多胺含量的变化,探讨内源激素和多胺对种子败育型无核葡萄胚发育的影响。
1 材料与方法
试验于2022年在西北农林科技大学进行。
1.1 植物材料
供试材料为有核葡萄品种‘京秀’[‘潘诺尼亚’×60-33(‘玫瑰香’ב红无籽露’)]及其F1代无核品种‘秦秀’(‘京秀’ב郑果大无核’),两个品种均为欧亚种二倍体早熟鲜食品种,开花期和果实成熟期基本一致,种植保存于西北农林科技大学葡萄种质资源圃,均为10年生以上大树,株行距1.0 m×2.5 m,采用单臂篱架和“T”字形整形,栽培管理同生产园。2022年6—8月采集2个品种不同时间点的幼果,在旱区作物逆境生物学国家重点实验室测定胚珠中内源激素及多胺的含量。
1.2 试验方法
1.2.1 分期取样 根据前期的研究结果,‘秦秀’的胚挽救最佳取样时期为花后(days after flowering,DAF)42 d[45],即胚发育程度最高但将要败育的拐点,以此为参考依据,对‘秦秀’及其母本‘京秀’分别在花后36、39、42、45和48 d共5个时间点分别采集幼果,均在清晨7:30—8:00进行。不同取样时期采集的果实贮于冰壶中,迅速带回实验室检测各项指标。
1.2.2 果粒大小及重量测定 取样后,随机选取同一品种同一时期150个果粒称取重量,之后进行果粒大小的测定。按照前人[46]的方法,将果粒的纵径和横径用数显游标卡尺测出,记录果粒大小(纵径×横径)并进行计算,包括3次生物学重复。
1.2.3 胚珠大小及重量的测定 随机选取同一品种同一时期50个果粒,对果粒小心横切,观察胚珠在子房中的分布及数量,之后迅速剥离胚珠进行称重,计算单个胚珠重量(鲜重),再使用数显游标卡尺测量胚珠的纵横径,计算胚珠大小(纵径×横径)并记录,进行3次生物学重复。之后随机选取10个胚珠进行称重并记录,装入预冷的2 mL棕色无酶离心管中,加入等质量的无菌钢珠,立即放入液氮中速冻,置于-80 ℃超低温冰箱中保存备用。
1.2.4 胚珠内源激素含量的测定 植物激素的测定采用安捷伦三重四级杆高效液相色质联用仪(Agilent 1290 InfinityⅡ-6470,美国),IAA和CTK的提取方法参照XUE等[47],GA3的提取方法参照范建新等[48],ACC的提取方法参照MENCARELLI等[49],JA的提取方法参照WANG等[50],SA的提取方法参照YALPANI等[51],其中取胚珠材料重量0.7—1.0 g用于测定。
1.2.5 胚珠内源多胺含量的测定 植物多胺的测定使用安捷伦超高效液相色谱仪(Agilent 1290 Infinity-HDR-DAD,美国),参照FLORES和GALSTON[52]的方法,稍作修改,胚珠重量为0.7—1.0 g,4 ℃冰箱浸提1 h后取上清1 mL,添加1 mL 2 mol∙L-1NaOH溶液与10 µL苯甲酰氯,加入2 mL饱和NaCl和2 mL乙醚,于室温下6 000 r/min离心5 min;取1 mL上清液于10 mL离心管中,放入氮吹仪进行浓缩,然后加入1 mL乙醚并重复一次;随后溶于500 µL 60%甲醇,进行0.22 µm有机滤膜过滤,于棕色容量瓶中定容,以进一步测试。
组织样品中各激素及多胺的定量分析采用标准曲线法,定性分析采用外标法。植物激素和多胺的标样购于上海源叶生物科技有限公司。
1.2.6 数据统计与分析 利用IBM SPSS Statistics 22.0软件中的单因素方差ANOVA(one-way ANOVA)和检验进行试验数据统计与分析,数据表示为平均值(Mean)±标准误(SE)。激素及多胺含量测定数据的显著性分析采用Duncan’s法(<0.05)。‘京秀’与‘秦秀’成熟果实单粒重、单粒果实所含胚珠数及单粒胚珠重的差异性分析均采用检验(***<0.001)。
2 结果
2.1 成熟期两个品种果粒及胚珠重量
‘京秀’平均单粒重为3.57 g,胚珠众数为3个,‘秦秀’平均单粒重2.79 g,平均胚珠众数为2个,‘京秀’的平均单粒重和胚珠众数均显著高于‘秦秀’。‘京秀’平均胚珠重为60.31 mg,多为褐色且存在木质化,‘秦秀’平均胚珠重为24.80 mg,按照MAHANIL等[53]对无核葡萄的分类方法,‘京秀’属于有核品种,‘秦秀’属于无核品种(图1)。
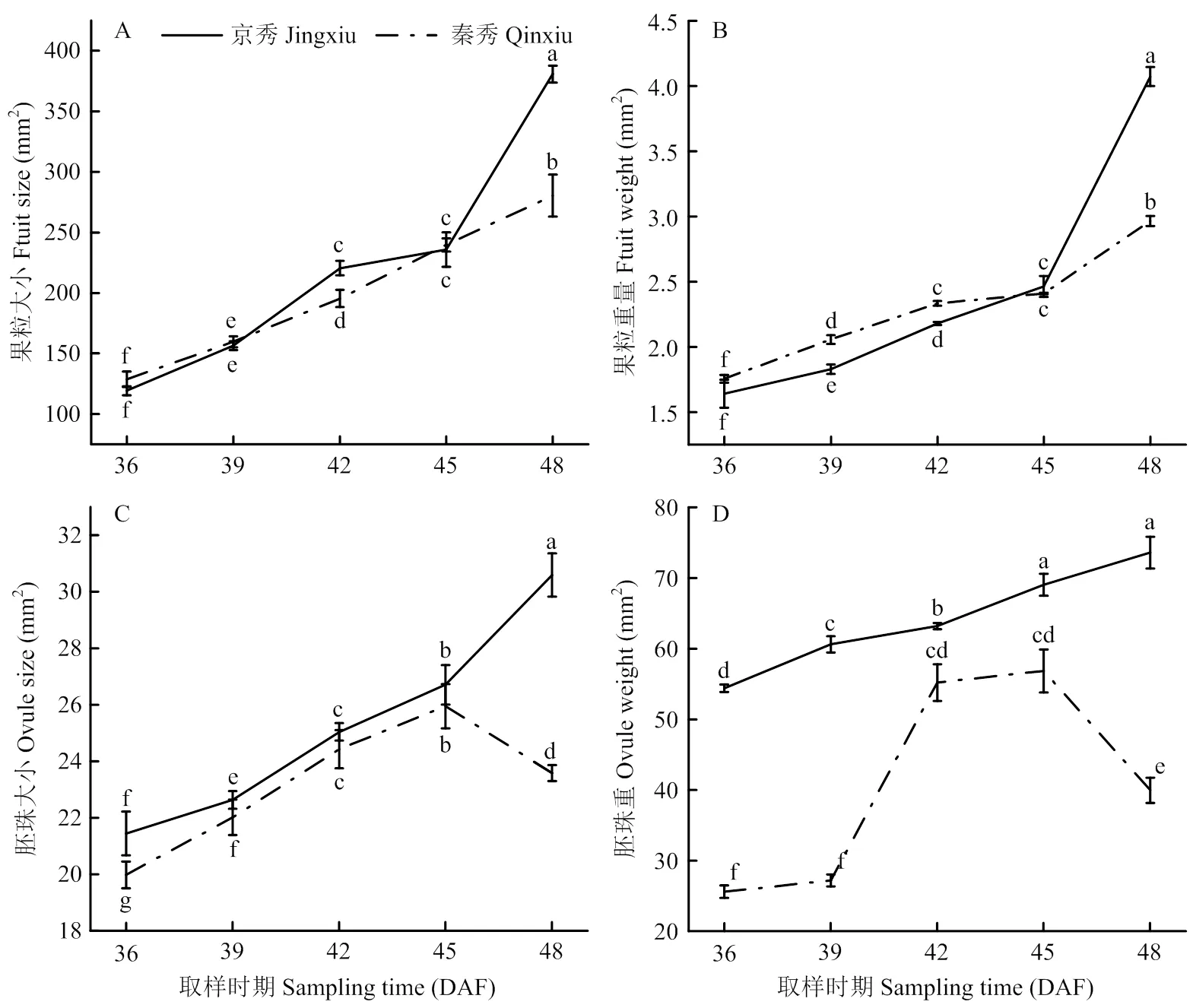
2.2 胚发育过程中果粒及胚珠大小和重量变化
两个品种的果粒大小和重量在生长发育过程中持续增加,花后48 d达到最大值,且‘京秀’的果粒大小与单粒重显著高于‘秦秀’(图2-A、B)。‘京秀’胚珠大小和重量在花后36—48 d持续增加,‘秦秀’胚珠大小和重量在花后42—45 d极缓慢增加,45 d时达到高峰,45 d后快速下降(图2-C、D),表明‘秦秀’胚此时开始败育。

***: P<0.001
2.3 胚发育过程中胚珠内源激素的变化
‘京秀’在花后36—39 d 的IAA含量急剧升高,于39 d达到顶点,42 d时下降到最低点,然后缓慢增加;‘秦秀’在花后39 d降到最低点,然后在花后42 d时迅速升高至最高点,之后急速下降后趋于平稳(图3-A)。
‘京秀’中ZT、ZR和iPAS含量在花后36—39 d急剧升高至顶点,39—42 d均显著降低。其中,ZT和ZR含量在花后42 d后趋于平稳,而iPAS含量在花后48 d时显著高于花后42 d(图3-B—D);‘秦秀’中ZR和iPAS含量则是在花后36 d之后下降,花后42 d时显著升高,之后显著下降后趋于平稳(图3-C、D)。说明花后39 d时较高含量的iPAS有利于胚珠进一步发育。而对于ZT含量变化,‘秦秀’在花后36 d下降至花后39 d,但差异不显著,花后42 d时显著升高,之后显著下降后趋于平稳(图3-B)。表明有核品种‘京秀’在花后39 d时细胞分裂素含量最高,无核品种‘秦秀’在花后42 d时细胞分裂素含量最高。
不同小写字母表示差异显著(P<0.05)。下同Different lowercase letters indicate significant difference (P<0.05 level). The same as below
与IAA相似,GA3在生长旺盛的组织中含量较高。‘京秀’在花后36—39 d的含量显著升高,之后显著下降后略有升高;‘秦秀’在花后36 d时GA3含量最高,之后显著降低,花后39—45 d表现为先升高再降低,之后趋于平稳(图3-E)。
ACC为乙烯生物合成的前体,‘京秀’在花后36—39 d时ACC含量无显著变化,花后39—45 d时显著升高到最高点,之后显著降低;‘秦秀’在花后36—39 d时,ACC含量显著升高到最高点,花后39—42 d时显著降低,之后趋于平稳。表明花后39 d之后较高的ACC含量对胚珠的生长发育有利(图3-F)。
‘京秀’在花后36—39 d时JA含量显著升高,花后39—45 d先降低再升高,之后显著降低;‘秦秀’在花后36—45 d一直降低,45—48 d时显著升高。说明花后39—45 d期间较高含量的JA有利于胚珠的进一步发育(图3-G)。
‘京秀’在花后36—39 d时SA含量显著升高,再显著降低至花后42 d,之后显著升高并趋于平稳;‘秦秀’在花后36—39 d降低,45—48 d时升高,且差异显著。此外,在相同的取样时期,‘京秀’的SA含量均显著高于‘秦秀’,说明较高含量的SA对胚珠的进一步发育具有促进作用(图3-H)。
‘京秀’在花后36—45 d的ABA含量保持相对稳定,而‘秦秀’在花后36–39 d显著降低,39—42 d时迅速上升并于42 d达到最高点,显著高于‘京秀’,花后42—45 d又迅速下降。在花后45 d之后,两个品种ABA含量均有所升高(图3-I)。
‘京秀’在花后36—39 d时(IAA+GA3)/ABA显著升高,之后逐渐降低,且差异显著;‘秦秀’在整个发育过程中无显著变化。说明在花后36—42 d时较高的(IAA+GA3)/ABA比例有利于胚珠的进一步发育(图4-A)。‘京秀’在花后36—39 d时(IAA+ZT+GA3)/ABA显著升高,之后逐渐降低,且差异显著;‘秦秀’在整个发育过程中无显著变化。说明在花后36—42 d时较高的(IAA+ZT+GA3)/ABA比例有利于胚珠发育(图4-B)。

图3‘京秀’与‘秦秀’不同发育时期胚珠激素含量变化
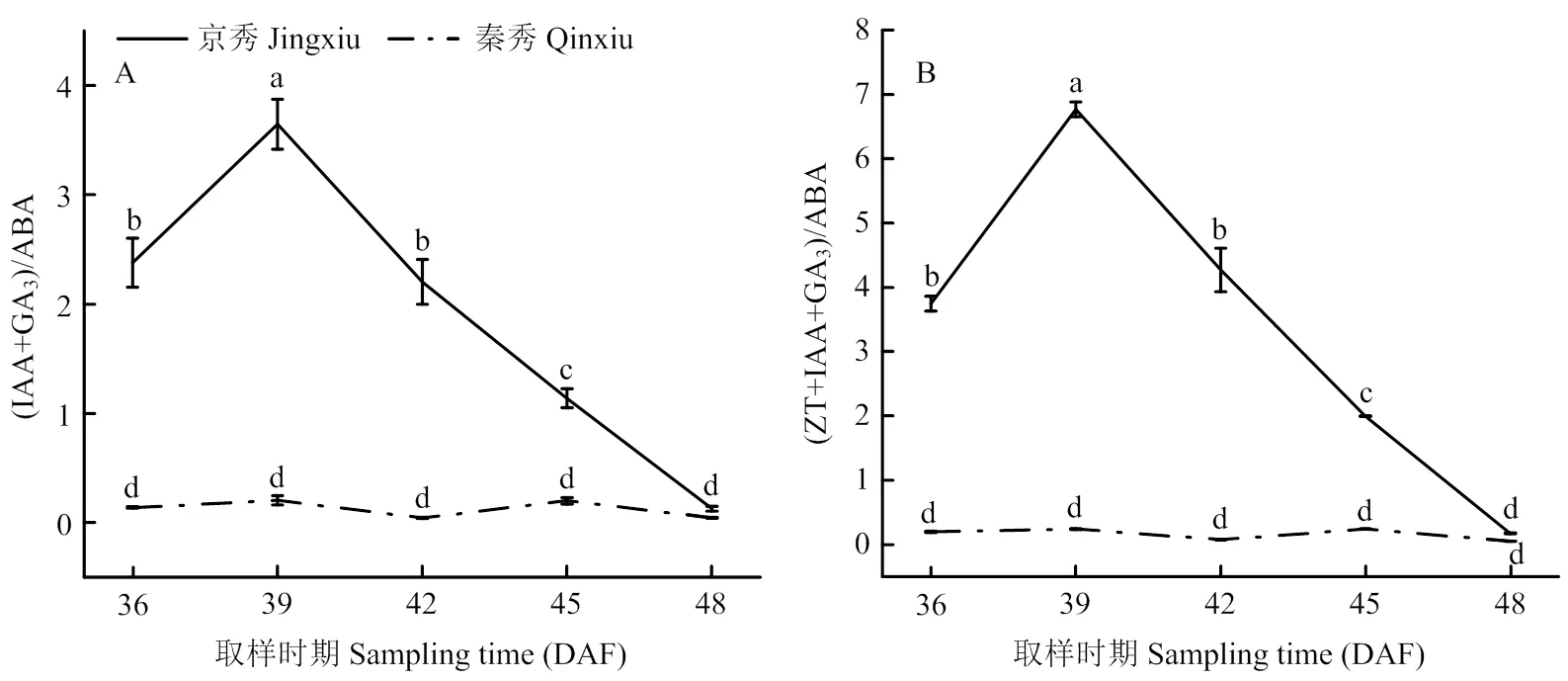
图4 ‘京秀’与‘秦秀’不同发育时期胚珠激素比值变化
2.4 胚发育过程中胚珠内源多胺的变化
2.4.1 内源多胺含量 对于腐胺(Put),‘京秀’与‘秦秀’均呈现先升高后降低的变化趋势,但二者之间差异显著。两个品种分别在花后42 d和39 d 到达顶峰,随后大幅度降低(图5-A)。两个品种精胺(Spm)均呈现先升高后降低的变化趋势,且差异显著,均在花后 42 d时到达顶峰(图5-B)。亚精胺(Spd)在‘京秀’与‘秦秀’均呈现先升高后降低的变化趋势,且差异显著,分别在花后 42 d和 39 d时达到顶峰(图5-C)。‘京秀’与‘秦秀’的多胺(PAs)含量均呈现先升高后降低的变化趋势,分别在花后 42 d和 39 d达到顶峰,且差异显著(图5-D)。说明较高水平的胚珠内源腐胺、精胺、亚精胺及多胺含量有利于胚珠的进一步生长发育。

图5 ‘京秀’与‘秦秀’不同发育时期胚珠多胺含量变化
2.4.2 内源多胺比例 对于Spm/PAs,‘京秀’处于一直上升的趋势,而‘秦秀’在花后36—39 d先迅速下降,再在花后42 d时达到峰值且显著高于‘京秀’,之后又开始下降(图6-A)。对于(Spd+Spm)/PAs和(Spd+Spm)/Put,‘京秀’均处于一直上升的趋势,而‘秦秀’在花后36—39 d先迅速下降,然后上升至花后42 d时达到峰值,之后又开始下降,且在花后45 d以后,‘京秀’的多胺比例均显著高于‘秦秀’(图6-B、C)。花后45 d以后较高的Spm/PAs、(Spd+Spm)/PAs和(Spd+Spm)/Put比例有利于胚的进一步生长发育,而比例的迅速降低会导致胚的败育。
3 讨论
3.1 无核葡萄胚珠的败育
种子败育型无核葡萄的胚珠由珠被、珠心、珠孔、珠柄组成,珠心主要包括胚乳和胚[2,54]。葡萄开花后完成授粉受精形成受精卵,种子败育型无核葡萄胚的早期发育与有核葡萄胚的发育基本相似,在受精后15—20 d 开始分裂[54]。对于完成授粉受精的果实,一部分无核葡萄品种在花后15 d 左右,胚珠中胚乳核不分裂而逐渐退化,引起胚乳退化最终导致胚败育;一部分是在花后30 d左右,珠心组织不发达,珠被细胞逐渐解体退化最终导致胚败育[54]。不同的无核葡萄品种,其胚珠和胚败育的时间、速度和败育程度不同,且同一葡萄品种中胚珠和胚的发育与败育并非同步[17]。本研究中,有核葡萄‘京秀’胚珠大小及重量不断升高后趋于稳定,而无核葡萄‘秦秀’在花后45 d开始下降,即胚珠开始败育。
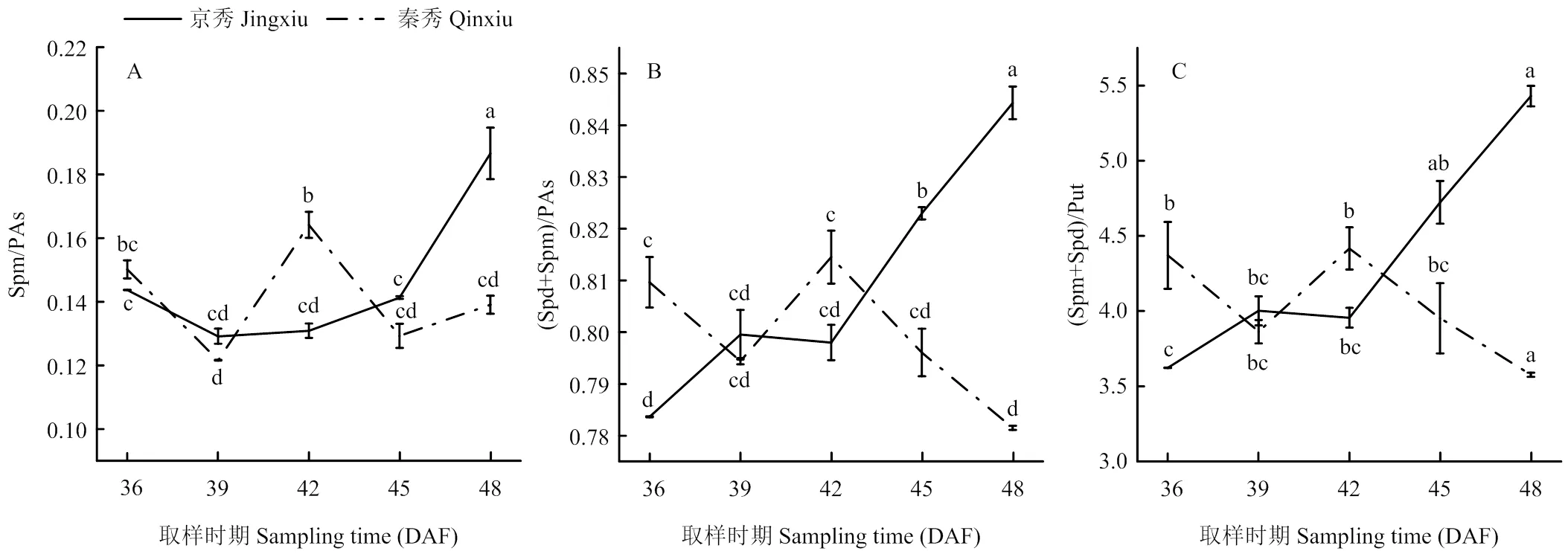
图6 ‘京秀’与‘秦秀’不同发育时期胚珠多胺比值变化
3.2 无核葡萄胚发育过程中内源激素含量及比例变化
胚珠内源激素含量的变化与胚珠及胚的生长发育密切相关,且不同发育时期胚珠内源激素含量及各激素间的比例不同[5,10-13]。CHEN等[10]认为,在正常胚珠中,IAA、GA和ABA浓度在荔枝品种‘兰珠’开花后第7天达到峰值,随后下降,而IAA和GA浓度在球形胚到鱼雷形胚期间再次升高,CTK在球形期之前达到峰值。在败育胚珠中,ABA始终保持较高浓度,IAA和GA下降至最低水平,CTK在败育胚珠中低于正常胚珠。本研究中,有核葡萄品种‘京秀’和无核葡萄品种‘秦秀’胚珠大部分激素在36—42 d时变化剧烈,特别是36—39 d,有核品种‘京秀’中IAA、ZT、ZR和iPAS急剧升高,GA3缓慢升高,且除了GA3外,其他激素在花后36—39 d时一直高于‘秦秀’;而无核品种‘秦秀’胚珠中IAA、ZT、ZR、iPAS和GA3在花后36—39 d下降,ABA含量在39—42 d急剧升高,之后又急剧下降,而ACC具有相似变化趋势,说明36—39 d是胚发育对生长素和细胞分裂素需求的关键时期,二者高含量促进胚发育,ABA和ACC维持在较低水平有利于胚发育。此外,花后39—42 d,‘秦秀’胚珠中ABA和GA3含量急速升高而JA和ACC含量降低,可能也是促进胚败育的重要因素。ROYO等[55]认为SA对促进‘克瑞森无核’F1代种子败育具有潜在作用,但本研究中,‘京秀’的SA含量始终高于‘秦秀’,而‘秦秀’的SA含量无显著变化,这与ROYO等[55]的结果不一致。
此外,前人研究发现植物激素间的比例和平衡关系对胚败育的发生比单一激素更为重要[5]。LI等[5]对‘无核白’葡萄内源激素含量的两个比值(IAA+GA3)/ABA和ZR/ABA进行测定,发现在36 DAF后显著降低。经过石蜡切片进行细胞学观察后发现,37—42 DAF胚珠中很少或没有胚胎,推测这两种激素比例的降低对胚败育具有一定影响。本研究结果表明,无核葡萄‘秦秀’的(IAA+GA3)/ABA和(IAA+ZT+GA3)/ABA比值在相同取样时期均低于有核葡萄‘京秀’,说明生长类激素含量相对于抑制类激素的比率下降,破坏了激素平衡,从而导致胚败育,这与前人研究结果一致[5,56-57]。此外,笔者课题组前期分别在‘秦秀’花后36、38、40、42、44和46 d取样进行胚挽救,调查胚的发育率、萌发率和成苗率,发现‘秦秀’的最佳胚挽救取样时间为42 DAF[45]。本研究中,‘秦秀’的(IAA+GA3)/ABA和(IAA+ZT+GA3)/ABA比值在42 DAF均显著降低,预示着胚败育的开始,这与前期对‘秦秀’胚挽救确定的最佳取样时间相吻合。
3.3 无核葡萄胚发育过程中多胺含量及比例变化
多胺含量随子房发育而变化,不同品种的变化趋势不同[35]。潘学军等[6]研究认为,欧亚种葡萄品种的胚珠重量与内源PAs、Spd和Spm极显著正相关,欧美杂种的胚珠重量与Spm显著正相关。‘红宝石无核’的(Spd+Spm)/PAs和Spm/PAs比值表现为下降趋势,‘火星无核’表现为先升后降,而有核对照均呈上升趋势。胚珠内较低的多胺含量及其胚胎发育过程中多胺含量的大幅度下降是导致种子败育型葡萄胚败育的主要因素[6]。本研究中,有核品种‘京秀’胚珠中的Spm/PAs、(Spd+Spm)/PAs和(Spd+Spm)/Put比值均显著升高,而无核品种‘秦秀’胚珠中的两种比值在花后42 d时显著降低,预示着胚败育的开始。
3.4 外源多胺影响无核葡萄胚发育
在无核葡萄胚挽救过程中,PONCE等[30]认为花前喷施腐胺可提高‘Emperatriz’和‘Fantasy’的胚挽救成苗率。TANG等[58]发现花前14 d喷施20 mg·L-1腐胺可促进无核葡萄胚发育。EBADI等[59]采用花前施用0.34 mmol∙L-1腐胺,在NN培养基中添加1.0 mmol∙L-1精胺、0.5 mmol∙L-1亚精胺或1.0 mmol∙L-1腐胺均能提高无核葡萄胚发育率和萌发率。JIAO等[60]认为,添加3 mmol∙L-1腐胺、0.5 mmol∙L-1亚精胺或0.3 mmol∙L-1精胺可显著提高‘红宝石无核’ב紫香无核’和‘红宝石无核 ’ב火焰无核’的胚胎发育率和萌发率。这些研究结果均说明外源多胺在胚的发育过程中起重要作用。
4 结论
无核葡萄‘秦秀’胚珠中低含量的生长促进物质(IAA、GA3、CTK、ACC、SA、JA、Put、Spd和Spm)、高含量的生长抑制物质(ABA)及较低的(IAA+GA3)/ABA、(IAA+ZT+GA3)/ABA、Spm/PAs、(Spd+Spm)/PAs和(Spd+Spm)/Put比值是导致其胚败育的主要原因之一。因此,在无核葡萄胚挽救过程中,可通过花前喷洒或者在培养基中添加一定浓度的生长促进物质来抑制胚的败育。
[1] COOMBE B G. Relationship of growth and development to changes in sugars, auxins, and gibberellins in fruit of seeded and seedless varieties of. Plant Physiology, 1960, 35(2): 241-250.
[2] 刘巧, 张立华, 王跃进, 张剑侠. 两个无核葡萄品种胚及胚乳败育的细胞学研究. 北方园艺, 2016(3): 31-35.
LIU Q, ZHANG L H, WANG Y J, ZHANG J X. Cytological study of embryo and endosperm abortion in two seedless grape varieties. Northern Horticulture, 2016(3): 31-35. (in Chinese)
[3] BAYDAR N G, HARMANKAYA N. Changes in endogenous hormone levels during the ripening of grape cultivars having different berry set mechanisms. Turkish Journal of Agriculture and Forestry, 2005, 29(3): 205-210.
[4] 陶建敏, 庄智敏, 章镇, 邵宏干, 蔡斌华. 几种生长调节剂对火星无核葡萄种子形成的影响. 果树学报, 2006, 23(4): 534-537.
TAO J M, ZHUANG Z M, ZHANG Z, SHAO H G, CAI B H. Effects of auxins and cytokinins on seed trace development of stenospermic grape cultivar Mars. Journal of Fruit Science, 2006, 23(4): 534-537. (in Chinese)
[5] LI S S, LIU K K, YU S S, JIA S S, CHEN S, FU Y H, SUN F, LUO Q W, WANG Y J. The process of embryo abortion of stenospermocarpic grape and it develops into plantletusing embryo rescue. Plant Cell, Tissue and Organ Culture, 2020, 143(2): 389-409.
[6] 潘学军, 李顺雨, 张文娥, 刘崇怀. 种子败育型葡萄胚珠中内源多胺含量与胚珠发育及败育的关系. 果树学报, 2011, 28(5): 770-775.
PAN X J, LI S Y, ZHANG W E, LIU C H. Endogenous polyamines in stenospermocarpic grape ovules and their relationship with ovule development and abortion. Journal of Fruit Science, 2011, 28(5): 770-775. (in Chinese)
[7] 贺普超. 葡萄学. 北京: 中国农业出版社, 1999: 244-247, 255.
HE P C. Viticulture. Beijing: China Agriculture Press, 1999: 244-247, 255. (in Chinese)
[8] GUSTAFSON F G. The cause of natural parthenocarpy. American Journal of Botany, 1939, 26(3): 135-138.
[9] DO AMARAL A L, DE OLIVEIRA P R D, CZERAINSKI A B C, CAMARGO U A. Embryo growth stages on plant obtention from crosses between seedless grape parents. Revista Brasileira de Fruticultura, 2001, 23: 647-651.
[10] CHEN W, LU L X. Endogenous hormones in relation to embryo development in litchi. Acta Horticulturae, 2001, 558: 247-250.
[11] QUITTENDEN L J, DAVIES N W, SMITH J A, MOLESWORTH P P, TIVENDALE N D, ROSS J J. Auxin biosynthesis in pea: Characterization of the tryptamine pathway. Plant Physiology, 2009, 151(3): 1130-1138.
[12] CHEN D, DENG Y T, ZHAO J. Distribution and change patterns of free IAA, ABP 1 and PM H+-ATPase during ovary and ovule development ofL. Journal of Plant Physiology, 2012, 169(2): 127-136.
[13] HOSSAIN A B M S, ALENAZI M M, TAHA R M. Seedless okra production by indole 3-acetic acid micro syringe injection on flower bud, ovary and shoot xylem and its vitamin and mineral content development: An innovation. Scientia Horticulturae, 2021, 283: 110010.
[14] BEVERIDGE C A, KYOZUKA J. New genes in the strigolactone- related shoot branching pathway. Current Opinion in Plant Biology, 2010, 13(1): 34-39.
[15] FRÉBORT I, KOWALSKA M, HLUSKA T, FRÉBORTOVÁ J, GALUSZKA P. Evolution of cytokinin biosynthesis and degradation. Journal of Experimental Botany, 2011, 62(8): 2431-2452.
[16] NOOKARAJU A, BARRETO A S, KARIBASAPPA G S, AGRAWAL D C. Synergistic effect of CPPU and benzyladenine on embryo rescue in six stenospermocarpic cultivars of grapevine. Vitis Journal of Grapevine Research, 2007, 46(4): 188-191.
[17] KHOSHANDAM L, DOULATI B H, JALILI M R, DARWISHZADEH R. Effect of BA and ovule developmental stages on embryo rescue in Perlette grape (L.) cultivar. European Online Journal of Natural and Social Sciences, 2017, 6: 1-9.
[18] ZHU P P, ZHANG J X. Effects of pre-bloom spraying thidiazuron and different embryo development media on seedless grape embryo rescue. New Zealand Journal of Crop and Horticultural Science, 2022: 1-28.
[19] SWAIN S M, REID J B, KAMIYA Y. Gibberellins are required for embryo growth and seed development in pea. The Plant Journal, 1997, 12(6): 1329-1338.
[20] CHENG C X, XU X Z, SINGER S D, LI J, ZHANG H J, GAO M, WANG L, SONG J Y, WANG X P. Effect of GA3treatment on seed development and seed-related gene expression in grape. PLoS One, 2013, 8(11): e80044.
[21] KHAVARI-NEJAD R A, NAJAFI F, RANJBARI M. The interactive effects of cadmium and GA3on tomato (Mill. cv. CH) plants photosynthesis, anthocyanin, proline and total phenolic contents. Romanian Journal of Biology, 2016, 2: 43-52.
[22] 崔梦杰, 王晨, 张文颖, 汤崴, 朱旭东, 李晓鹏, 房经贵. 无核葡萄研究进展. 植物生理学报, 2017, 53(3): 317-330.
CUI M J, WANG C, ZHANG W Y, TANG W, ZHU X D, LI X P, FANG J G. Research progress of seedless grape. Plant Physiology Journal, 2017, 53(3): 317-330. (in Chinese)
[23] MATILLA A J, MATILLA-VÁZQUEZ M A. Involvement of ethylene in seed physiology. Plant Science, 2008, 175(1/2): 87-97.
[24] TSAI W C, HSIAO Y Y, PAN Z J, KUOH C S, CHEN W H, CHEN H H. The role of ethylene in orchid ovule development. Plant Science, 2008, 175(1/2): 98-105.
[25] MOU W S, KAO Y T, MICHARD E, SIMON A A, LI D D, WUDICK M M, LIZZIO M A, FEIJÓ J A, CHANG C R. Ethylene-independent signaling by the ethylene precursor ACC inovular pollen tube attraction. Nature Communications, 2020, 11(1): 4082.
[26] WASTERNACK C, FORNER S, STRNAD M, HAUSE B. Jasmonates in flower and seed development. Biochimie, 2013, 95(1): 79-85.
[27] FILGUEIRAS C C, MARTINS A D, PEREIRA R V, WILLETT D S. The ecology of salicylic acid signaling: primary, secondary and tertiary effects with applications in agriculture. International Journal of Molecular Sciences, 2019, 20(23): 5851.
[28] LI Z Q, JIAO Y T, ZHANG C, DOU M R, WENG K, WANG Y J, XU Y.positively regulate salicylic acid biosynthesis during seed abortion in Thompson Seedless. Plant Biotechnology Journal, 2021, 19(9): 1824-1838.
[29] FINKELSTEIN R R, TENBARGE K M, SHUMWAY J E, CROUCH M L. Role of ABA in maturation of rapeseed embryos. Plant Physiology, 1985, 78(3): 630-636.
[30] PONCE M, GUIÑAZÚ M, TIZIO R. Effect of putrescine on embryo development in the stenospermocarpic grape cvs Emperatriz and Fantasy. Vitis, 2002, 41: 53-54.
[31] MALIK A U, SINGH Z. Endogenous free polyamines of mangos in relation to development and ripening. Journal of the American Society for Horticultural Science, 2004, 129(3): 280-286.
[32] 郭印山, 郭修武, 张海娥. 葡萄胚胎发育与败育过程中胚珠的多胺含量变化. 植物生理学通讯, 2007, 43(1): 53-56.
GUO Y S, GUO X W, ZHANG H E. Changes in polyamine contents of ovules during grape (L.) embryo development and abortion. Plant Physiology Communications, 2007, 43(1): 53-56. (in Chinese)
[33] 贺军虎, 陈业渊, 赵小青, 陈华蕊. ‘金煌’杧胚正常与胚败育果实内源多胺的变化. 热带作物学报, 2013, 34(10): 1972-1976.
HE J H, CHEN Y Y, ZHAO X Q, CHEN H R. The change of endogenous polyamines in ‘Jinhuang’ mango fruit with normal and aborted embryo. Chinese Journal of Tropical Crops, 2013, 34(10): 1972-1976. (in Chinese)
[34] SHIOZAKI S, OGATA T, HORIUCHI S. Endogenous polyamines in the pericarp and seed of the grape berry during development and ripening. Scientia Horticulturae, 2000, 83: 33-41.
[35] ALBURQUERQUE N, EGEA J, BURGOS L, MARTÍNEZ- ROMERO D, VALERO D, SERRANO M. The influence of polyamines on apricot ovary development and fruit set. Annals of Applied Biology, 2006, 149: 27-33.
[36] CAO D D, HU J, ZHU S J, HU W M, KNAPP A. Relationship between changes in endogenous polyamines and seed quality during development ofshsweet corn (L.) seed. Scientia Horticulturae, 2010, 123(3): 301-307.
[37] RAMMING D W. The use of embryo culture in fruit breeding. HortScience, 1990, 25(4): 393-398.
[38] 朱佩佩, 罗燚佳, 向雯, 张明磊, 张剑侠. 抗寒无核葡萄杂种胚挽救及分子标记辅助选择. 中国农业科学, 2021, 54(6): 1218-1228.doi: 10.3864/j.issn.0578-1752.2021.06.012.
ZHU P P, LUO Y J, XIANG W, ZHANG M L, ZHANG J X. Rescue and molecular marker assisted-selection of the cold- resistant seedless grape hybrid embryo. Scientia Agricultura Sinica, 2021, 54(6): 1218-1228. doi: 10.3864/j.issn.0578-1752. 2021.06.012. (in Chinese)
[39] XU T F, GUO Y R, WANG W Y, YUAN X J, CHU Y N, WANG X W, HAN Y L, WANG Y J, SONG R, FANG Y L, WANG L J, XU Y. Effects of exogenous paclobutrazol and sampling time on the efficiency ofembryo rescue in the breeding of new seedless grape varieties. Journal of Integrative Agriculture, 2022, 21(6): 1633-1644.
[40] GARCIA-PAPI M A, GARCIA-MARTINEZ J L. Endogenous plant growth substances content in young fruits of seeded and seedless Clementine mandarin as related to fruit set and development. Scientia Horticulturae, 1984, 22(3): 265-274.
[41] 祁业凤, 刘孟军. 两个胚败育率不同的枣品种果实生育期内源激素的变化. 园艺学报, 2004, 31(6): 800-802.
QI Y F, LIU M J. Change of endogenous hormone in cultivars of Chinese jujube with different type of embryo abortion. Acta Horticulturae Sinica, 2004, 31(6): 800-802. (in Chinese)
[42] LULSDORF M M, YUAN H Y, SLATER S M H, VANDENBERG A, HAN X M, ZAHARIA L I, ABRAMS S R. Endogenous hormone profiles during early seed development ofand. Plant Growth Regulation, 2013, 71(2): 191-198.
[43] QIU Z L, WEN Z, YANG K, TIAN T, QIAO G, HONG Y, WEN X P. Comparative proteomics profiling illuminates the fruitlet abscission mechanism of sweet cherry as induced by embryo abortion. International Journal of Molecular Sciences, 2020, 21(4): 1200.
[44] ZHU P P, TIAN Y C, LIU Q Y, GE Q Y, ZHANG J X. Optimisation of embryo rescue for cold-resistant seedless grapevine. New Zealand Journal of Crop and Horticultural Science, 2022: 1-14.
[45] ZHU P P, GU B, LI P Y, SHU X, ZHANG X, ZHANG J X. New cold-resistant, seedless grapes developed using embryo rescue and marker-assisted selection. Plant Cell, Tissue and Organ Culture, 2020, 140(3): 551-562.
[46] EBADI A, MOGHADAM J E, FATAHI R. Evaluation of 22 populations achieved from controlled crossing between some seeded × seedless grapevine cultivars. Scientia Horticulturae, 2009, 119(4): 371-376.
[47] XUE W Y, LIU N, ZHANG T T, LI J, CHEN P P, YANG Y T, CHEN S X. Substance metabolism, IAA and CTK signaling pathways regulating the origin of embryogenic callus during dedifferentiation and redifferentiation of cucumber cotyledon nodes. Scientia Horticulturae, 2022, 293: 110680.
[48] 范建新, 邓仁菊, 王永清, 罗立娜, 韩树全, 刘荣. 火龙果茎段及花药愈伤组织内源激素含量的测定. 分子植物育种, 2017, 15(12): 5093-5102.
FAN J X, DENG R J, WANG Y Q, LUO L N, HAN S Q, LIU R. Determination of endogenous hormones in callus originated from stem and anther culture of pitaya. Molecular Plant Breeding, 2017, 15(12): 5093-5102. (in Chinese)
[49] MENCARELLI F, AGOSTINI R, BOTONDI R, MASSANTINI R. Ethylene production, ACC content, PAL and POD activities in excised sections of straight and bent gerbera scapes. Journal of Horticultural Science, 1995, 70(3): 409-416.
[50] WANG Y, MOPPER S, HASENSTEIN K H. Effects of salinity on endogenous ABA, IAA, JA, and SA in iris hexagona. Journal of Chemical Ecology, 2001, 27(2): 327-342.
[51] YALPANI N, SILVERMAN P, WILSON T M, KLEIER D A, RASKIN I. Salicylic acid is a systemic signal and an inducer of pathogenesis-related proteins in virus-infected tobacco. The Plant Cell, 1991, 3(8): 809-818.
[52] FLORES H E, GALSTON A W. Analysis of polyamines in higher plants by high performance liquid chromatography. Plant Physiology, 1982, 69(3): 701-706.
[53] MAHANIL S, GARRIS A J, OWENS C M, RAMMING D W, CADLE-DAVIDSON L. Development of molecular markers for powdery mildew resistance in grapevines. Acta Horticulturae, 2014, 1046: 91-99.
[54] 王飞, 王跃进, 周会玲, 万怡震, 杨进孝. 无核葡萄与中国野生葡萄杂种胚发育和败育的细胞学研究. 西北农林科技大学学报(自然科学版), 2005, 33(3): 61-65.
WANG F, WANG Y J, ZHOU H L, WAN Y Z, YANG J X. Cytological study of embryo development and abortion in hybrid progeny of seedless grape and Chinese wild grapes. Journal of Northwest A & F University (Natural Science Edition), 2005, 33(3): 61-65. (in Chinese)
[55] ROYO C, TORRES-PÉREZ R, MAURI N, DIESTRO N, CABEZAS J A, MARCHAL C, LACOMBE T, IBÁÑEZ J, TORNEL M, CARREÑO J, MARTÍNEZ-ZAPATER J M, CARBONELL- BEJERANO P. The major origin of seedless grapes is associated with a missense mutation in the MADS-box gene VviAGL11. Plant Physiology, 2018, 177(3): 1234-1253.
[56] SHAO F X, WANG S, ZHANG S Y, CHEN J, FENG C. Observation of embryo abortion characteristics ofmill. ‘Zhongqiusucui’. HortScience, 2021, 56(5): 595-602.
[57] 陈庭巧, 袁涛, 乔红雍, 徐珂. 大花黄牡丹二次枝对结实率的影响及胚珠败育生理机制研究. 西北农林科技大学学报(自然科学版), 2022, 50(9): 39-52.
CHEN T Q, YUAN T, QIAO H Y, XU K. Effect of secondary branches on seed setting rate and physiological mechanism of ovule abortion in. Journal of Northwest A & F University (Natural Science Edition), 2022, 50(9): 39-52. (in Chinese)
[58] TANG D M, WANG Y J, CAI J S, ZHAO R H. Effects of exogenous application of plant growth regulators on the development of ovule and subsequent embryo rescue of stenospermic grape (L.). Scientia Horticulturae, 2009, 120(1): 51-57.
[59] EBADI A, AALIFAR M, FARAJPOUR M, FATAHI MOGHADDAM M R. Investigating the most effective factors in the embryo rescue technique for use with ‘Flame Seedless’ grapevine (). The Journal of Horticultural Science and Biotechnology, 2016, 91(5): 441-447.
[60] JIAO Y T, LI Z Q, XU K Y, GUO Y R, ZHANG C, LI T M, JIANG Y X, LIU G T, XU Y. Study on improving plantlet development and embryo germination ratesembryo rescue of seedless grapevine. New Zealand Journal of Crop and Horticultural Science, 2018, 46(1): 39-53.
Changes of Endogenous Hormones and Polyamines During Ovule Development of Stenospermocarpic Seedless Grape
ZHU PeiPei, QIN HaoXiang, ZHANG JianXia
College of Horticulture, Northwest A&F University/State Key Laboratory of Crop Stress Biology in Arid Areas/Key Laboratory of Horticultural Plant Germplasm Resource Utilization in Northwest China, Ministry of Agriculture and Rural Affairs, Yangling 712100, Shaanxi
【Objective】By exploring the effects of endogenous hormones and polyamines on embryo development during the ovule development of seedless grape, this study provided a theoretical basis for promoting embryo development by spraying exogenous hormones before anthesis and adding exogenous hormones into the medium in vitro culture of ovule.【Method】In this study, the European grape (L.) variety Jingxiu and its F1generation stenospermocarpic seedless variety Qinxiu were used as test materials. The content of endogenous hormones and polyamines in ovule of fruit at different developmental stages were compared by high performance liquid chromatography mass spectrometry (HPLCMS) and ultra performance liquid chromatography (UPLC).【Result】The content of IAA, ZT, ZR, and iPAS reached their highest values at 39 DAF (days after flowering) for Jingxiu and 42 DAF for Qinxiu, and the highest values of the former were higher than those of the latter (more than 1.5 times). For the content of JA and SA, Jingxiu started to rise sharply at 36 DAF (2 000 ng∙g-1and 6 500 ng∙g-1, respectively), and reached the highest values at 39 DAF (6 500 ng∙g-1and 10 000 ng∙g-1, respectively), and then, which were declining and remaining at a high level at 42-45 DAF, while Qinxiu was declining and remaining at a low level from 36 DAF (3 500 ng∙g-1and 3 000 ng∙g-1, respectively). The ACC content of Jingxiu was almost 0 ng∙g-1at 39 DAF, then increased rapidly and reached its highest value (1 200 ng∙g-1) at 45 DAF, while Qinxiu reached its highest value (900 ng∙g-1) at 39 DAF, then declined to 0 ng∙g-1at 42 DAF, and remained at a very low level. The ABA content of Jingxiu was almost 0 ng∙g-1from 36 to 45 DAF, while that of Qinxiu rose sharply from 0 ng∙g-1at 39 DAF to the highest value (900 ng∙g-1) at 42 DAF. The trends of Put (putrescine), Spm (spermine), and Spd (spermidine) were similar for the two varieties, but Jingxiu was consistently higher than Qinxiu. The highest values of Put and Spd were 42 DAF for Jingxiu and 39 DAF for Qinxiu, except for the highest values of Spm, which were all found at 42 DAF. 【Conclusion】 The lower content of growth promoting substances (IAA, CTK, GA3, ACC, JA, SA, Put, Spd, and Spm) and the higher content of growth inhibiting substances (ABA), and lower ratios of (IAA+GA3)/ABA, (IAA+ZT+GA3)/ABA, Spm/PAs, (Spd+Spm)/Pas, and (Spd+Spm)/Put in the ovules of the seedless variety Qinxiu compared with the seeded variety Jingxiu might be one of the main reasons for seedless grape embryo abortion. Therefore, in the process of seedless grape embryo rescue, the embryo abortion could be inhibited by spraying before flowering or adding to media a certain concentration of growth promoting substances.
stenospermocarpic seedless grapes; ovules; embryo; endogenous hormones; polyamines
10.3864/j.issn.0578-1752.2023.23.018
2023-04-18;
2023-08-15
陕西省重点研发计划-乡村振兴科技专项(2022FP-31)
朱佩佩,E-mail:825965951@qq.com。通信作者张剑侠,E-mail:zhangjx666@126.com
(责任编辑 赵伶俐)
