Magnesium alloys in tumor treatment: Current research status,challenges and future prospects
2023-12-27YuchienHsuYupuLuSiyiWangYufengZhengDandanXiaYunsongLiu
Yuchien Hsu ,Yupu Lu ,Siyi Wang ,Yufeng Zheng ,Dandan Xia ,Yunsong Liu
a Department of Prosthodontics, Peking University School and Hospital of Stomatology, Beijing, 100081, China
bNational Center for Stomatology, National Clinical Research Center for Oral Diseases, National Engineering Research Center of Oral Biomaterials and Digital Medical Devices, Beijing Key Laboratory of Digital Stomatology, Research Center of Engineering and Technology for Computerized Dentistry Ministry of Health, NMPA Key Laboratory for Dental Materials, Beijing, 100081, China
cDepartment of Dental Materials, Peking University School and Hospital of Stomatology, Beijing, 100081, China
d Department of Prosthodontics, Shanghai Ninth People’s Hospital, Shanghai Jiao Tong University School of Medicine, College of Stomatology, Shanghai Jiao Tong University, National Center for Stomatology, National Clinical Research Center for Oral Diseases, Shanghai Key Laboratory of Stomatology,Shanghai Research Institute of Stomatology, China
e Department of Materials Science and Engineering, College of Engineering, Peking University, Beijing 100871, China
Abstract Cancer is a major threat to human life worldwide.Traditional cancer treatments,such as chemotherapy and surgery,have major limitations and can cause irreversible damage to normal tissues while killing the cancer cells.Magnesium (Mg) alloys are widely reported novel potential biomedical materials with acceptable mechanical properties and good osteogenic and angiogenic properties.In this review,we summarize the Mg alloys for antitumor applications,including pure Mg and Mg alloys (Mg-Ag,Mg-Gd,Mg-Li-Zn,Mg-Ca-Sr-Zn,et al.) fabricated by casting and extruding,selective laser melting methods.Mg alloys can exhibit antitumor effect on bone tumor,breast cancer,and liver tumor,etal.What’s more,after tumor tissue is eliminated,Mg alloys prevent tumor recurrence,fill tissue defects and promote tissue regeneration.The antitumor effects of Mg alloys are mainly due to their degradation products.Overall,Mg alloys show great potential in tumor treatments due to the dual function of antitumor and tissue regeneration.
Keywords: Antitumor;Cancer;Magnesium alloys;Mechanism;Tissue regeneration.
1.Introduction
Cancer is a major threat to human life worldwide.Currently,the main cancer treatments include chemotherapy,radiotherapy,and surgical excision of lesions.These traditional treatment methods have certain limitations,such as high surgical risk and trauma during treatment,severe adverse reactions,and a lack of specificity for radiotherapy,which can also damage healthy tissues [1,2].There is growing interest in exploring alternative therapies that are more targeted and less harmful to healthy tissues.Researchers have been striving to develop more effective therapies to fight cancer,given its high mortality rates.Many new cancer therapies are emerging,including (but not limited to) gene therapy [3,4],immunotherapy [5,6],and hyperthermia [7],all of which may improve treatment outcomes.
Nevertheless,the current methods of treating tumors are still relatively limited,and there is still a long way to go to achieve cures for all cancers.It is important to develop novel biomaterials that can simultaneously inhibit tumor cells without damaging healthy tissues.Much effort has been expended exploring next-generation biodegradable materials with clinical potential;one such approach is the use of magnesium(Mg) alloys in cancer treatment [8,9].
Mg-based biodegradable metals (BMs) are promising biomaterials for biomedical applications,due to their high biodegradability and good mechanical and biocompatibility properties [10].Mg has various biological functions in energy metabolism,macromolecule synthesis,and the expression of genetic information [11,12].Studies have confirmed the surgical success and tolerance of Mg-based absorbable metal stents in different coronary and pulmonary arteries and lower extremity vessels [13].Besides,Mg and its alloys are considered as potential materials for bone repair applications due to close elastic modulus to human bone,essential nutrient element,high mechanical properties,and good biocompatibility [10,14-16].Mg2+can promote osteogenic differentiation has also been well proved bothin vitroandin vivoexperiments [17].
In recent years,researchers are exploring new functions of Mg alloys.It was reported that the byproducts of Mg alloys,including H2,Mg2+,and Mg(OH)2,exhibited significant antitumor effects [18].Some studies have applied Mg alloys in osteosarcoma (OS) [19–31],breast [8,32-35],ovarian [36],prostate [37],colon [38,39],gallbladder [40],liver[41],and other cancers and confirmed their antitumor and anti-recurrence effects.Moreover,Mg alloys can promote tissue healing,which can fill the tissue defect after a tumor is eliminated,promoting healthy tissue regeneration,and improving patient prognosis.
This review focus on the Mg alloys developed for antitumor applications,summarizes the tumor in different sites of the body.Furthermore,the mechanisms of Mg alloys on tumor inhibition are discussed.Finally,we comments on the remaining challenges and perspectives of Mg alloys in cancer treatment.
2.Biological characteristics of tumors
A tumor is a collection of local cells with abnormal morphology and function under the action of various carcinogenic factors,which has the characteristics of heterogeneity,immortalization,invasion,and metastasis.The tumor microenvironment,which is composed of immune cells and inflammatory cells,tumor-associated fibroblasts,micro-vessels,cytokines,and chemokines and is closely related to tumor occurrence,growth,metastasis,and recurrence.The tumor microenvironment is typically weakly acidic,with low oxygen levels and high reactive oxygen species (ROS) levels,and immunosuppression (Fig.1).Enhanced aerobic glycolysis (i.e.,the Warburg effect)benefits tumor cell survival and invasion[42],and the large accumulation of lactic acid provides favorable conditions for the migration and immune escape of tumor cells[43,44].A hypoxic microenvironment activates the hypoxiainducible factor-1 (HIF-1) signaling pathway,which accelerates tumor growth,increases tumor invasiveness,promotes tumor metastasis,and leads to tumor drug resistance [45,46].Almost all tumor cells have an imbalance in the intracellular redox system and the ROS levels in tumor cells are abnormally increased.Prolonged high levels of oxidative stress for a long time can induce DNA damage,protein modification and genomic instability,leading to potential carcinogenic mutations [47].In addition,the immunosuppression of the microenvironment is closely related to tumor growth,invasion,and metastasis.The tumor-promoting M2 phenotype of tumor-associated macrophages assists cancer cell metastasis,angiogenesis,and proliferation via various anti-inflammatory mechanisms [48].Moreover,the tumor microenvironment actively recruits regulatory T lymphocytes (Tregs),whose expansion and activation seem to respond to signals generated by tumors,thereby leading to tumor escape [49].
3.Magnesium alloy degradation and byproducts
Mg and its alloys are prone to corrosion.In the degradation process,Mg loses electrons and undergoes anodic reaction (Eq.(1)) while a large number of electrons undergo a cathodic reaction with H2O (Eq.(2)).Subsequently,Mg2+generated by the anodic reaction reacts with OH-generated by the cathodic reaction to form the corrosion product Mg(OH)2(Eq.(3)).Mg(OH)2is unstable in aqueous solution,especially in the environment containing Cl-.A high Cl-concentration will accelerate the reaction of Mg(OH)2,thereby promoting the corrosion of Mg,which produces high concentrations of Mg2+and OH-(Eq.(4)).The corrosion of these biomaterials mainly involves galvanic pitting,filamentous,and total corrosion,stress corrosion cracking,and corrosion fatigue(Fig.2).
In galvanic corrosion,due to the low electrode potential of Mg,when Mg is in contact with other metals it generally serves as the anode for galvanic corrosion,while the cathode is a foreign metal directly in contact with the outside or a second phase or impurity phase inside the Mg alloy [50].Macroscopically,the galvanic corrosion of the Mg alloy matrix and internal second phase is total corrosion.Metals with a low hydrogen overpotential form corrosive micro-batteries with Mg,resulting in marked galvanic corrosion of Mg alloys.However,metals with a higher hydrogen overpotential have less corrosion effects on Mg alloys [51].The influence of the second phase on the corrosion of Mg alloys is well understood [52–54],but the phenomenon of cathode activation in the process of the corrosion of Mg alloys requires further exploration to determine whether it is the effect of impurities or a secondary phase,or a combination of both (Fig.2A).
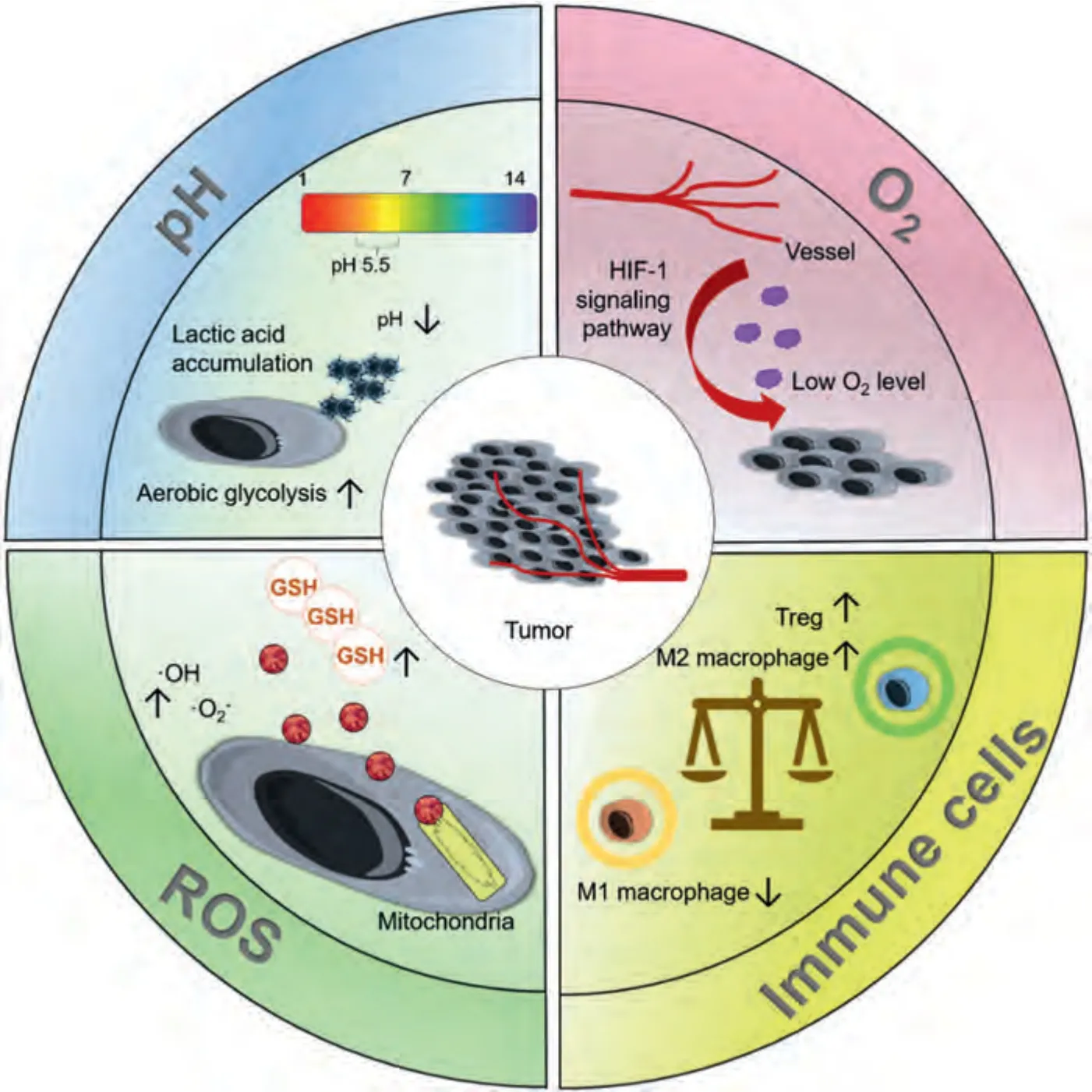
Fig.1.Biological characteristics of tumors.
In pitting and filamentous corrosion,as Mg is a selfpassivating metal,when Mg and Mg alloys are exposed to a non-oxidizing medium such as Cl-,the unstable oxide film on its surface is destroyed,forming pitting corrosion[55];corrosion pits are seen on the surface after gradually deepening.Active corrosion batteries generally have a protective coating or anodized oxide layer,which causes filamentous corrosion when it moves through the metal surface [56] (Fig.2B).
In stress corrosion cracking,Mg alloys crack when stress applied in an environment with corrosion does not reach half the yield strength [57].This corrosion is usually attributed to one of two mechanisms: continuous crack propagation caused by anodic dissolution of the crack tip or discontinuous crack propagation caused by a series of mechanical fractures of the crack tip[58];that is,stress corrosion cracking has dissolution and brittle fracture models (Fig.2C).
In corrosion fatigue,the fatigue strength increases with decreasing grain size,while the opposite is true for resistance to fatigue crack propagation [59].Corrosion fatigue cracks propagate in a trans-granular/inter-granular composite manner,and the same environment that accelerates stress corrosion crack propagation also accelerates corrosion fatigue crack propagation (Fig.2D).For example,the fatigue strength or fatigue life is significantly reduced in NaCl solution [60].In general,the corrosion mechanism of Mg and Mg alloys is roughly expressed by the above equation.
4.Application of magnesium alloys in tumor treatment
Pure Mg is regarded as a relatively safe material.However,the mechanical properties of pure Mg are not sufficiently strong and the degradation rate of Mg with impurities (iron,nickel,and copper) is particularly rapid,hindering its further application[10].Regardless of proper processing,the strength of pure Mg is low;specifically,the yield tensile strengths are 21 MPa for as-cast Mg,90–105 MPa for as-extruded Mg,and 115–140 MPa for as-rolled Mg [61].To improve the mechanical properties and degradation properties of Mg-based alloys for antitumor treatment,various approaches including alloying and surface modification have been adopted,as shown in Table 1.
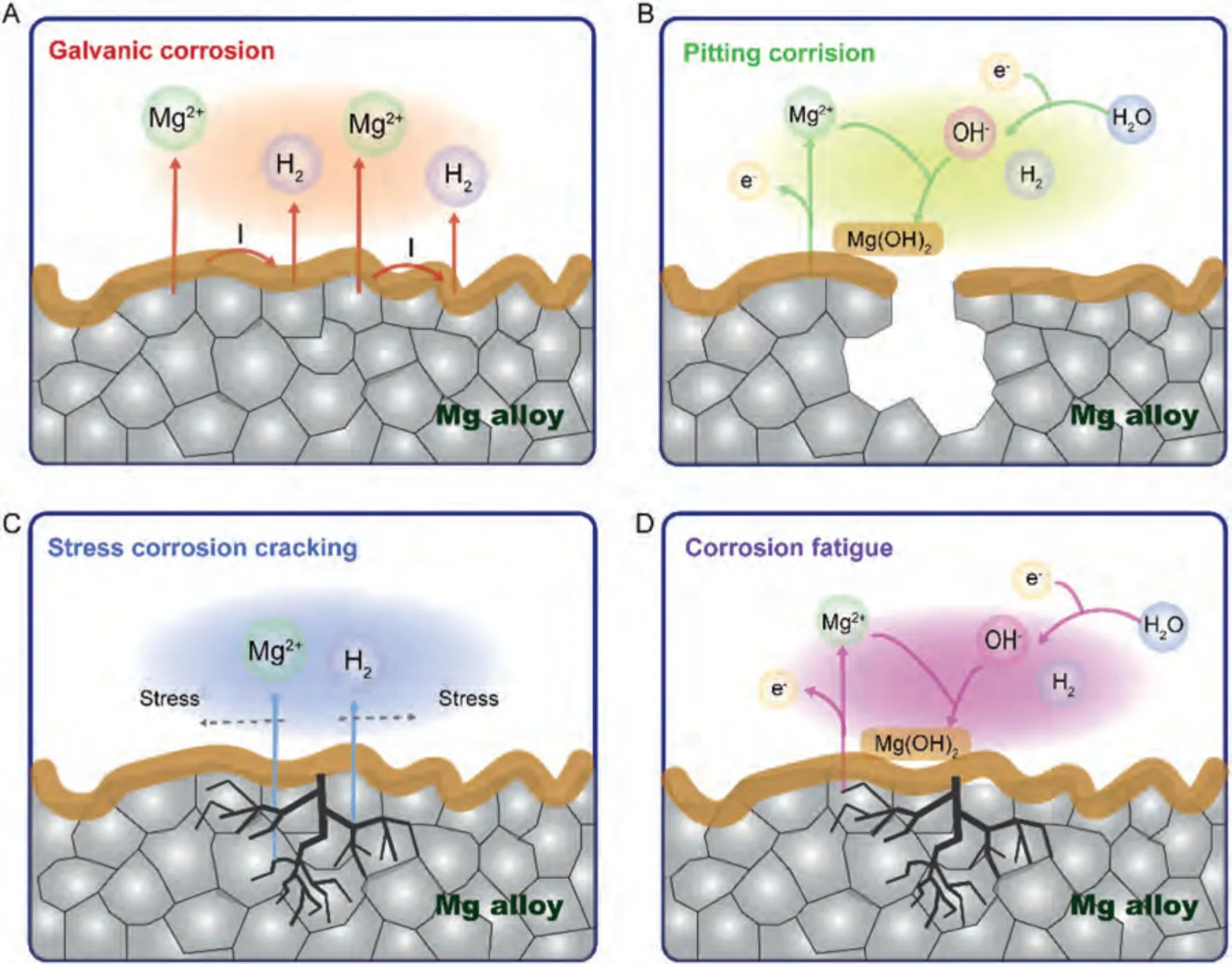
Fig.2.The corrosion schematic diagrams of Mg alloys.
Alloying is one of the most effective ways to improve the corrosion resistance and mechanical strength of pure Mg.The alloying element Ca refines the microstructure of Mg through the formation of thermally stable intermetallic phases,improving strength and creep properties under high temperatures[10].Wan et al.reported that the addition of an appropriate amount of Ca can effectively improve the flexural and compressive strength of pure Mg [62].To further optimize the mechanical properties and degradation rate of Mg-Ca binary alloys,the essential elements Zn and Sr have also been introduced for improved alloy strength and ductility.Berglund et al.[63] reported that the slowest degradation of the Mg-1Ca-0.5Sr alloy was 1.5 mm/year in Hank’s solution,but it remained able to maintain appropriate compressive strength(274 ± 4 MPa).On this basis,the addition of Zn can contribute to the formation of the eutectic phase and further reduce the alloy degradation rate.Wu et al.[22,64] reported the corrosion performance of a series of Mg-1Ca-0.5Sr-xZn(x=0,2,4,6%)alloys in Hank’s solution,where the addition of Zn significantly reduced the degradation rate of Mg-1Ca-0.5Sr in the initial stage.Additionally,Zn2+and Mg2+released from Mg-1Ca-0.5Sr-xZn significantly inhibited OS cell proliferation by altering the cell cycle and inducing cell apoptosis.Birblis et al.[65] found that the mechanical and degradation properties of Mg alloys can be effectively improved by adjusting the ratio of REEs with different solubilities and heat treatments.Shuai et al.[21] reported that the compressive strength of Mg-6Zn-0.5Zr-xLa (x=0.5,1.0,1.5 and 2.0 wt%) increased according to La content;the degradation rate decreased to 1.23 mm/year with an La content of 1.0 wt.%.Upon the addition of La,the mitochondrial membrane potential decreased,whereas the ROS increased,revealing a high inhibition rate for OS cells.
Surface modification is another effective way to decrease the degradation rate and improve the biocompatibility of Mg alloys.Kannan et al.[20]electrophoretically deposited samarium on anodized AZ31 Mg alloy.Compared with the bare alloy,the corrosion potential of the samarium coating shifted in the positive direction,and the corrosion current density was significantly reduced.The Bode phase angle diagram showed that the phase angle value in the mid-frequency region was higher than the value of the bare alloy;this finding indicated that the coating prevented the solution from penetrating the alloy,suggesting that the Mg alloy with samarium coating had greater corrosion resistance.Additionally,samariumcoated Mg alloys showed excellent performance with respect to anti-bone tumor relapse and metastasis effects.
Through a series of modification,Mg alloys gain better mechanical properties and degradation rates,which laid a good foundation for its anti-tumor application,including but not limited to osteosarcoma (OS) [19–31],breast [8,32-35],ovarian [36],prostate [37],colon [38,39],gallbladder [40],liver [41],and other cancers (Fig.3).
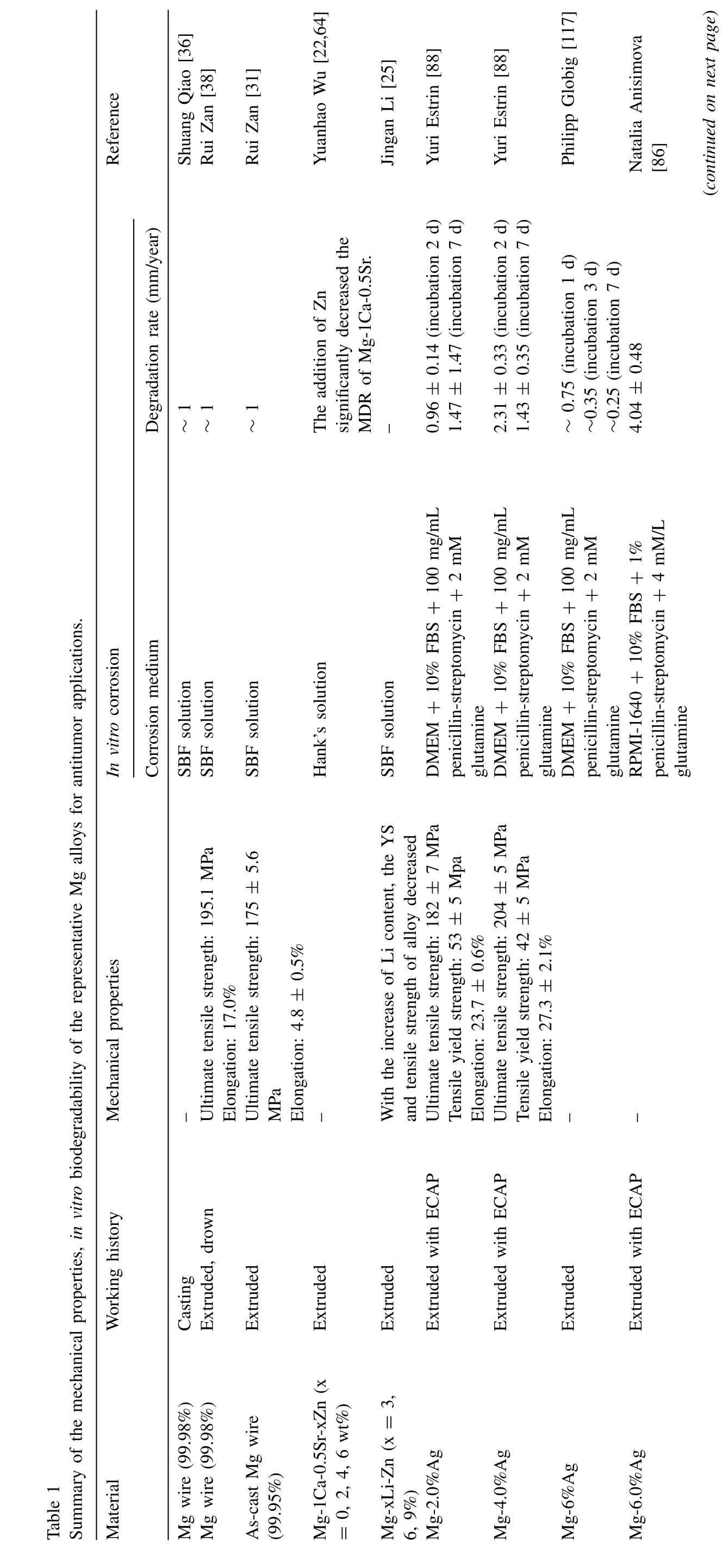

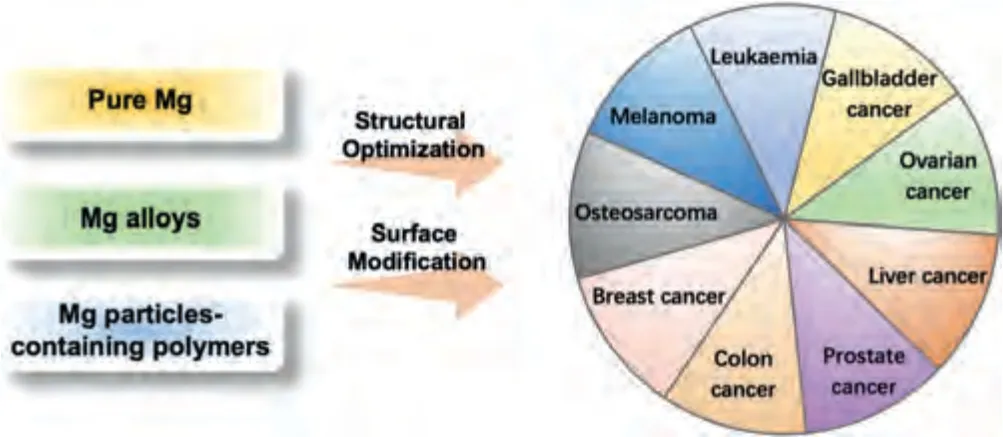
Fig.3.Magnesium alloys in antitumor applications.
4.1.Bone tumors
As a primary malignant bone tumor originating from bone mesenchymal cells,OS is common in children and adolescents,with high malignancy and rapid progression [66].The current treatment of OS is radical surgery combined with chemotherapy,and the extensive bone tissue loss and unavoidable residual tumor cells caused by surgery have a great impact on the patients’ prognosis and quality of life [67].Hence,it is important to develop novel biomaterials with dual functionalities,including the efficient elimination of residual cancer cells to prevent tumor recurrence and the ability to promote bone regeneration to repair large surgical defects.
Mg alloys can not only inhibit tumor cells growth but also promote new bone formation in the bone defect after tumor resection,showing the great potential of the multifunctional effect of Mg alloys in anti-bone tumor applications[19,28,38].Table 2 summarize the anti-bone cancer effects of Mg alloys in the literature,and Fig.4 displays some representative experimental results.
Pure Mg wires activate the transport of zinc finger protein Snail1 from the cytoplasm to the nucleus by releasing Mg2+,thereby inducing OS cell apoptosis and inhibiting OS cell proliferation.Furthermore,the hydroxide gas produced by Mg wires eliminates excessive intracellular ROS,inhibiting bone tumor cell growth [31].Milenin et al.[29] utilized MgCa0.7 alloy for the treatment of OS cells,they found that it could inhibit SaOS-2 cells.Additionally,the antitumor effect and degradation of Mg alloys can be improved by modifying the surface of the Mg alloy and loading active ingredients.Li et al.[26] developed a novel approach using a bisphosphonate (BP)-loaded microarc oxidation coated Mg-strontium alloy pellet to inhibit OS.The BP-coated Mg pellets destroyed tumors and prevented neoplasm recurrence by inducing apoptosis,necrosis,and synergistic effects of Mg degradation.Shao et al.[68] and Zhang et al.[30] fabricated a layered double hydroxide on Mg alloys surface,which enhanced its antitumor effects.
The porous structure of Mg alloys allows for cell infiltration and promotes the attachment and growth of bone-forming cells [30].As the alloy degrades,it releases Mg ions,which have antitumor effects on OS cells.This dual action of providing structural support while exerting anticancer properties makes Mg alloys a promising option for bone cancer therapy.
Besides the direct effect on tumor cells,Mg particle solutions absorb light throughout the entire ultraviolet–visible–near infrared region(300–900 nm),and have potential as photothermal agents (PTAs) [28].Photothermal therapy (PTT) is an established therapeutic strategy for cancer that harvests light energy and converts it into heat to raise the temperature of the surrounding environment and trigger tumor cell death [69,70].Compared with traditional therapies,PTT has the advantages of high accuracy,noninvasiveness,and precise spatiotemporal selectivity [71–74].Mg alloys have been explored as PTAs [28,41].Conventional PTAs mainly use metal elements with slow degradation rates such as copper (Cu)and gold.To achieve better photothermal conductivity,a high PTA concentration may produce side effects,such as high concentrations of Cu2+that significantly inhibit cell proliferation [75].Since Mg particles can be used directly as PTA without loading particles,the above drawback can be overcome,and good photothermal conversion properties and positive effects on surrounding healthy tissues can be achieved simultaneously.Long et al.[28] reported the synthesis of dualfunctional poly(lactide-co-glycolide)/Mg scaffolds with excellent photothermal conversion ability.Their study suggests that Mg alloys combined with PTT are potential biomaterials for OS applications.Table 3 summarizes the PTT conditions of Mg alloys used for antitumor applications from representative publications.
Additionally,the implanted bioactive scaffolds can stimulate the osteogenic differentiation capacity of bone marrow stromal cells (BMSCs) via Mg2+,thereby promoting the production of new bone in areas with bone deficiencies [28,76].Typically,bioactive scaffolds have porous structures,which facilitate nutrient transport and ion exchange,enabling the gradual release of Mg2+during degradation,thereby realizing truly dual-functional bioactive scaffolds [77].In addition to antitumor effects,Mg alloys can promote bone regenerationin vivoandin vitro[12,28,30,68,78-80].In vitroosteogenic development studies have examined rat BMSCs [12],rabbit BMSCs [78,79],MC3T3-E1 cells [28,80],human umbilical vein endothelial cells (HUVECs) [80],and C3H10T1/2 cells[30,68].The test groups performed significantly better based on alkaline phosphatase activity,alizarin red S staining,and the expression levels of osteogenic-related genes including runt-related transcription factor 2 (Runx2),bone sialoprotein(BSP),osteopontin (OPN),and osteocalcin (OCN).
4.2.Breast cancer

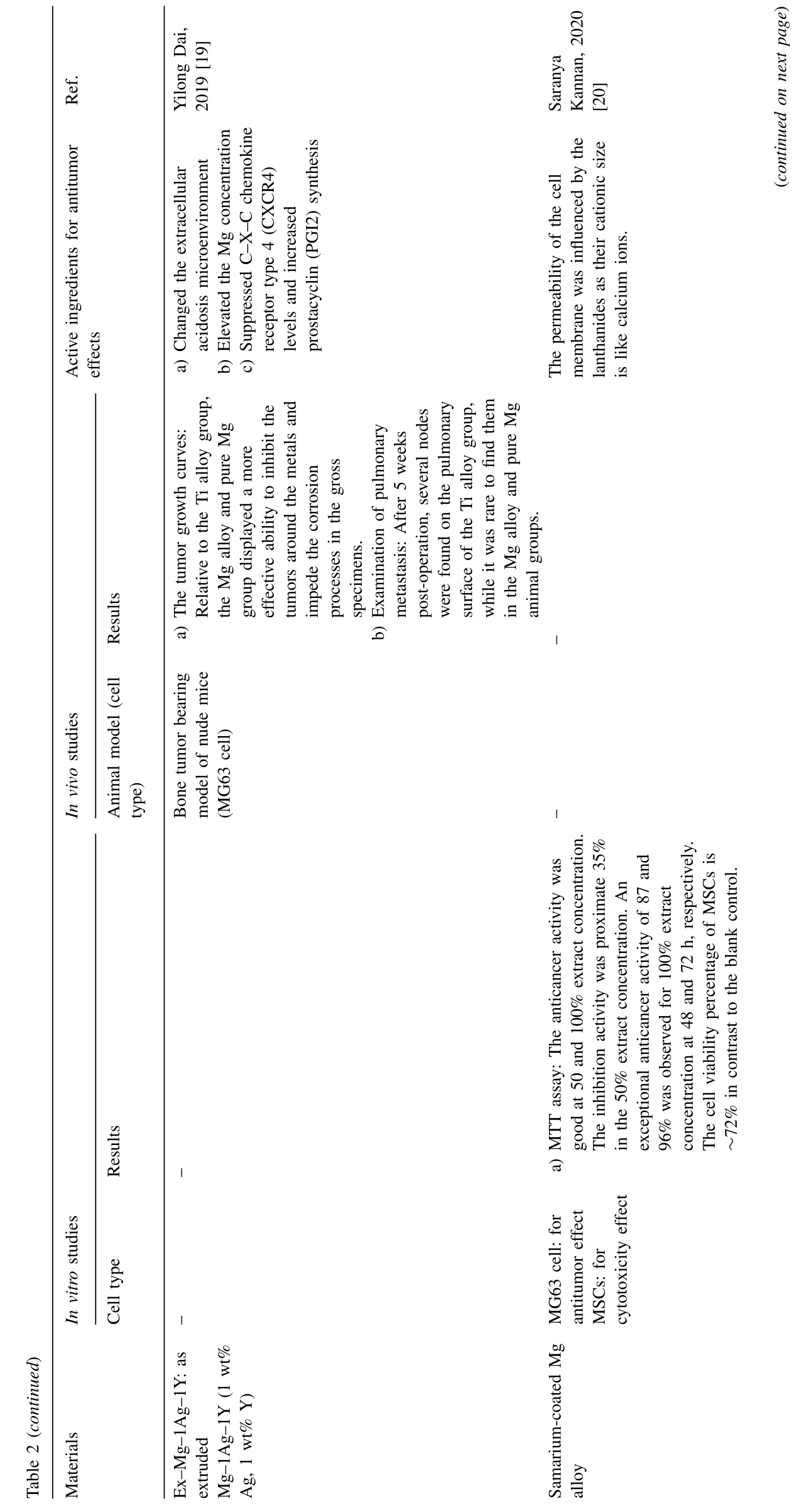
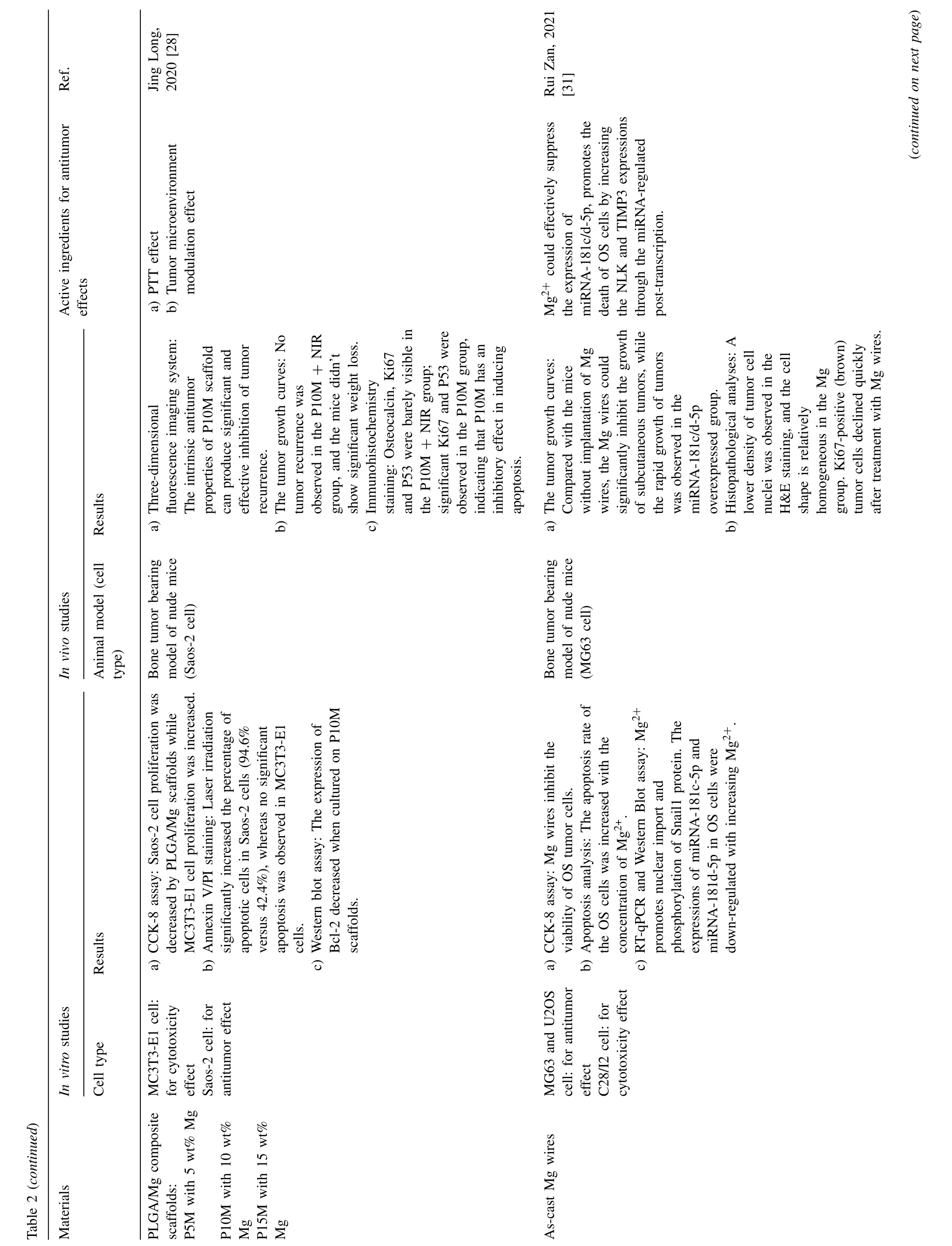
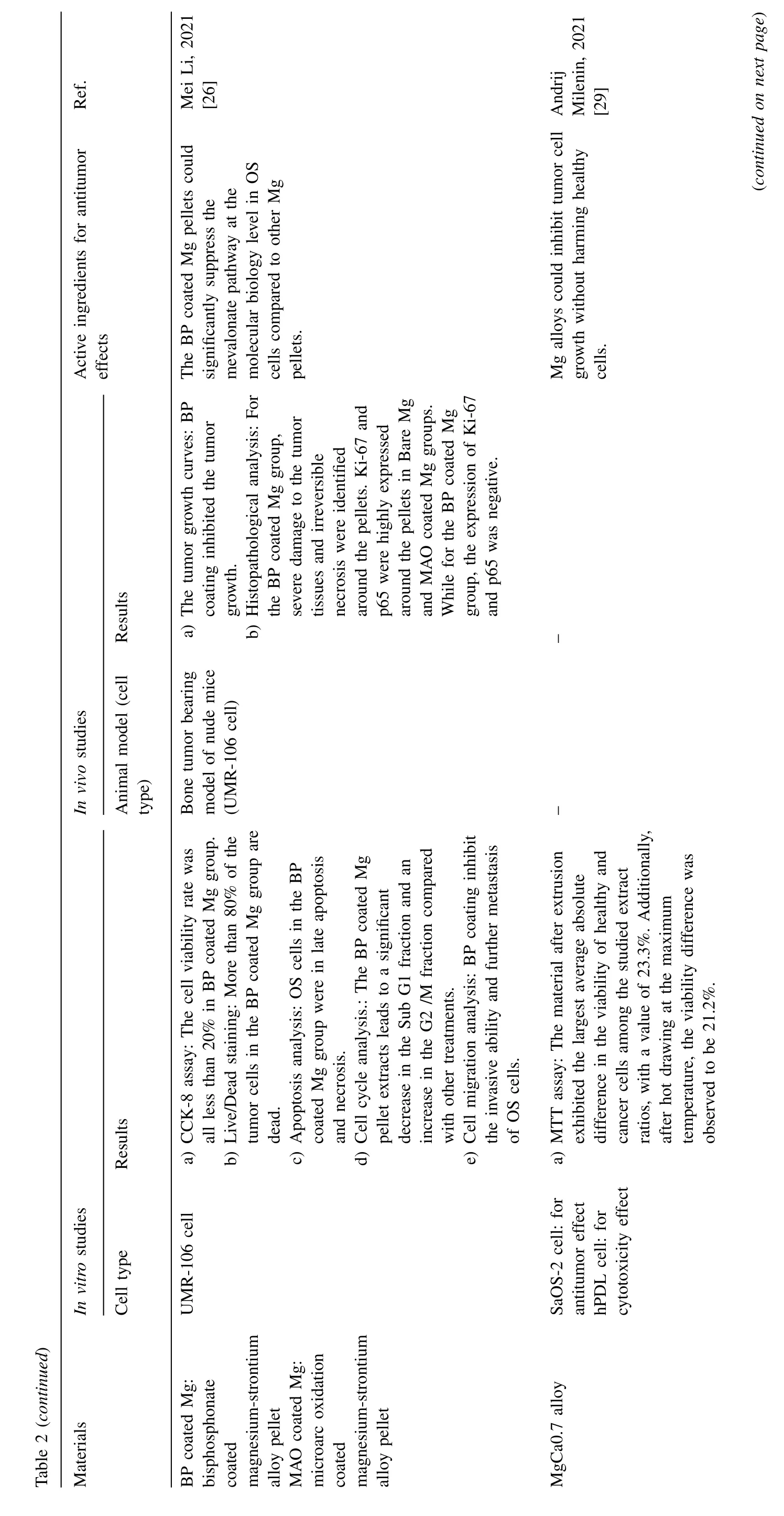
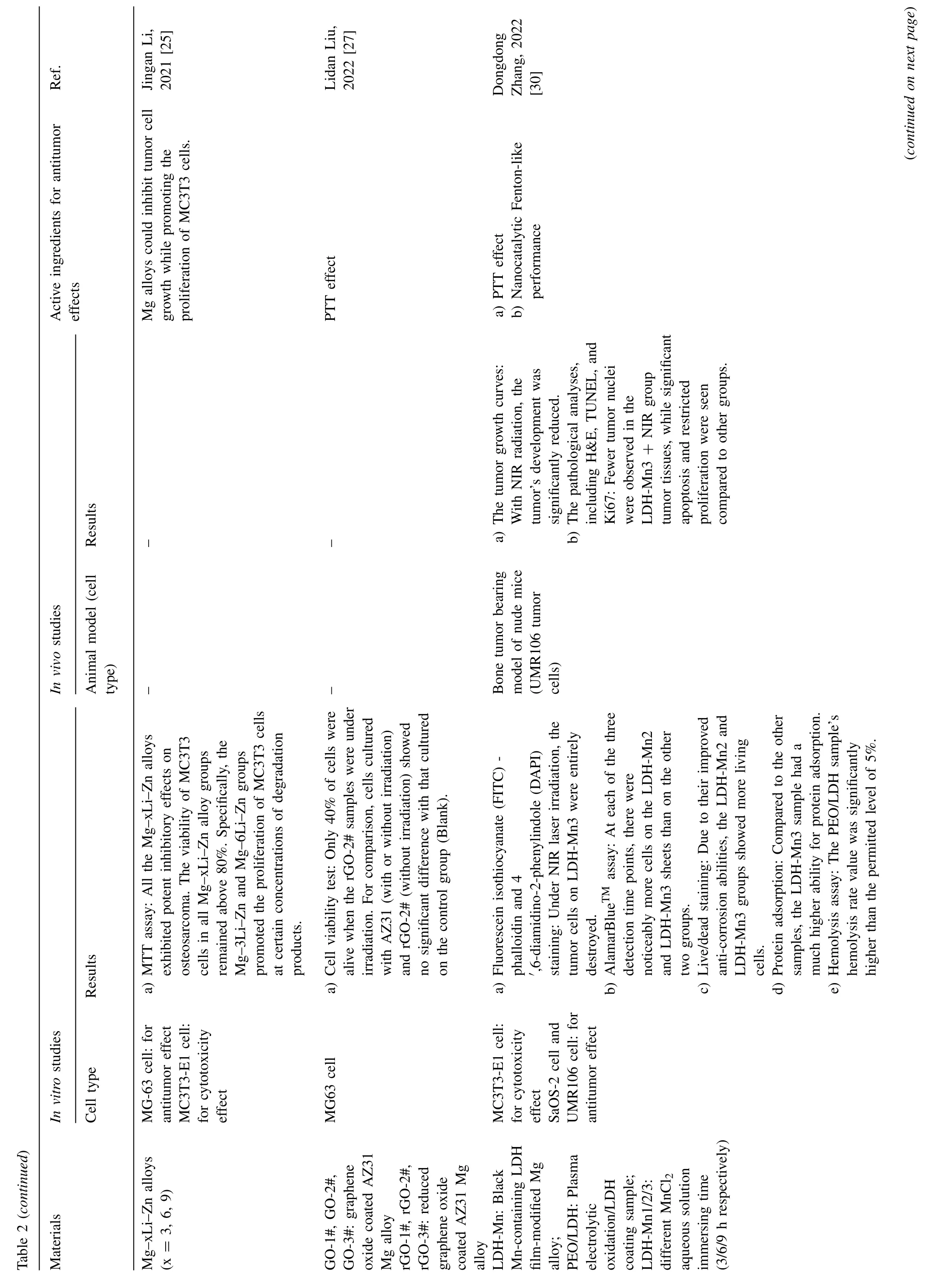
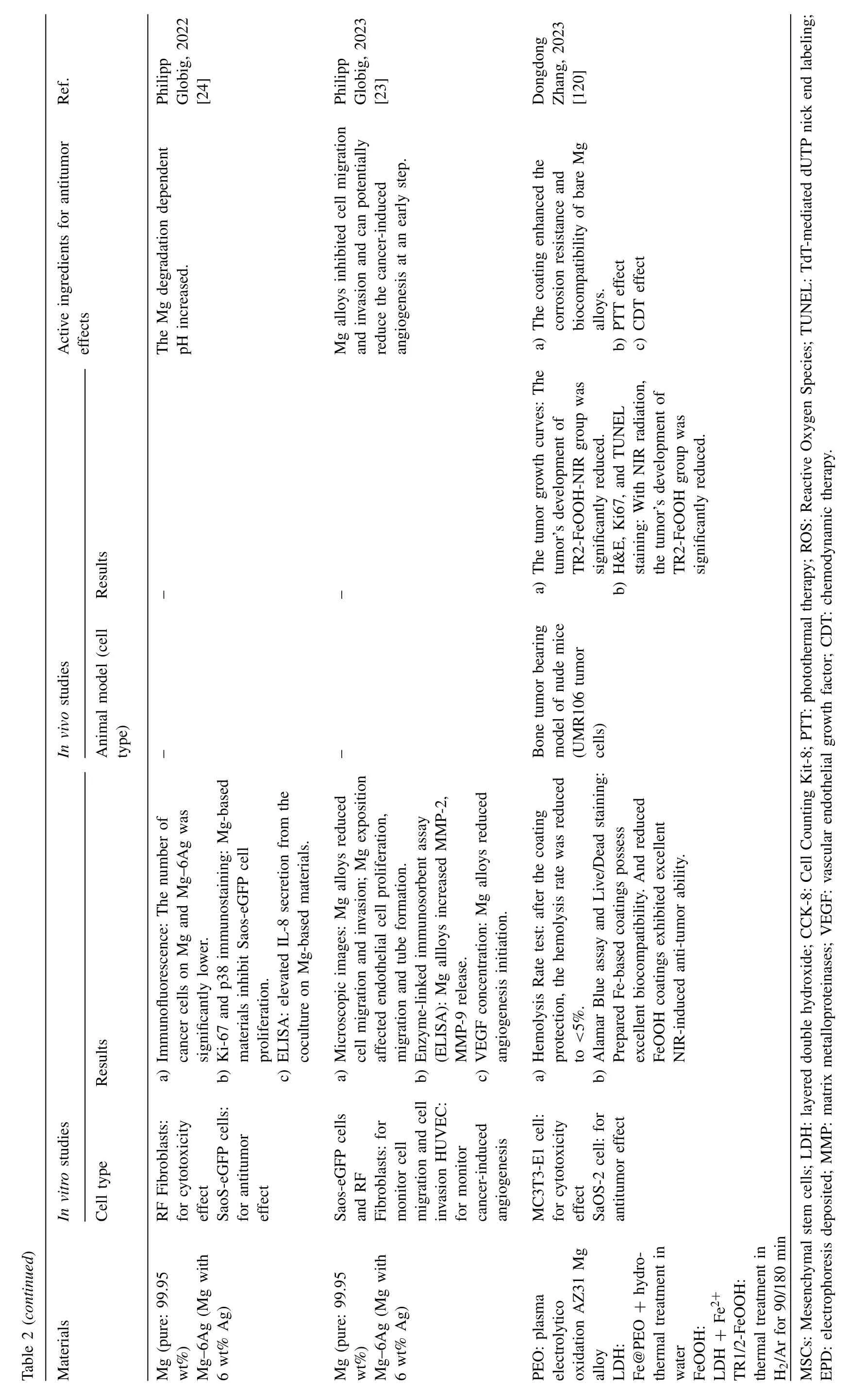
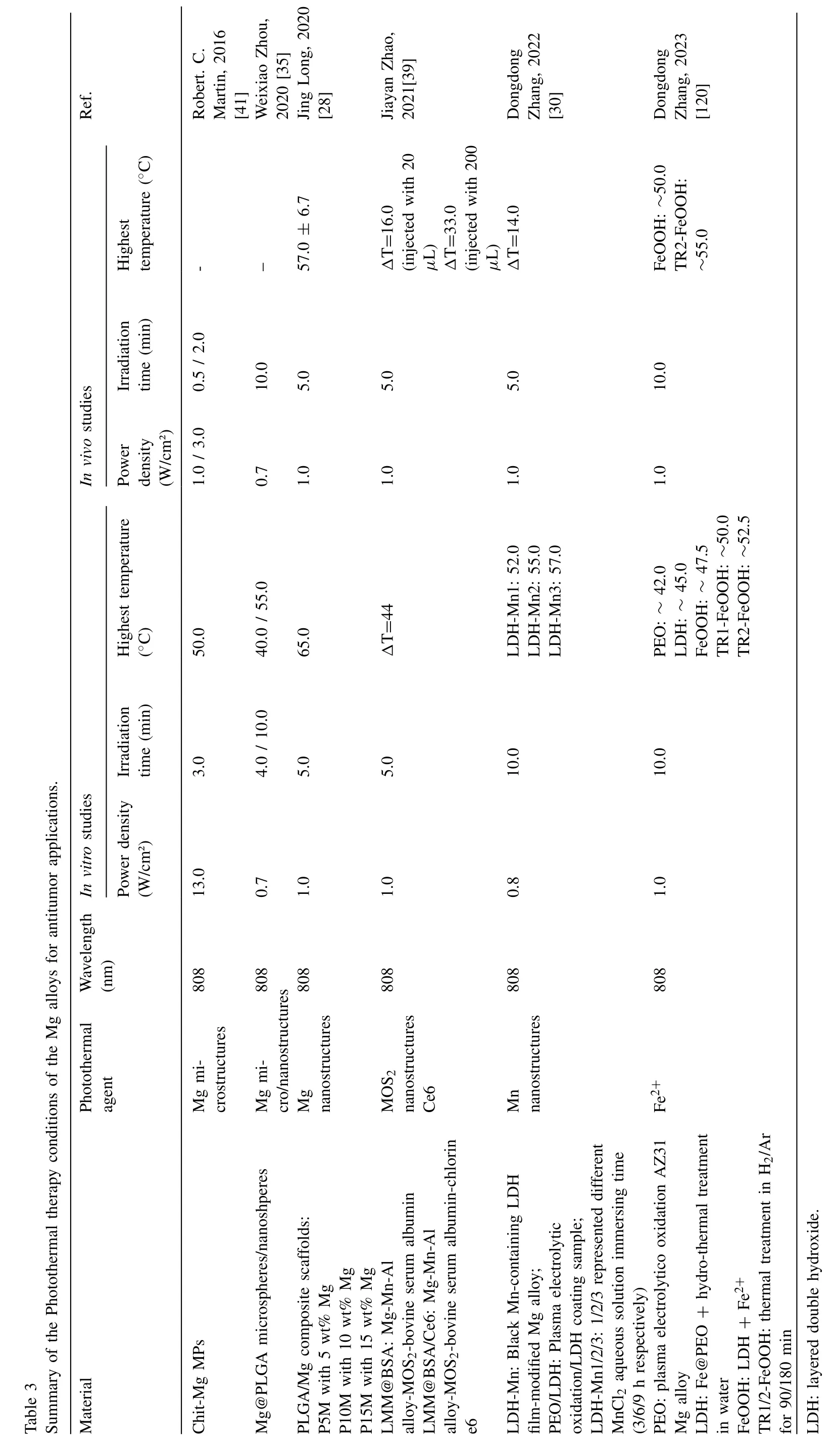
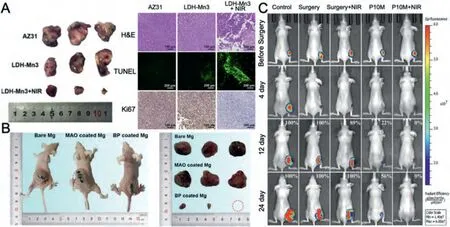
Fig.4.Representative antitumor effect of Mg alloys for bone cancer: A.Photographs,H&E,TUNEL,and Ki67 staining of tumor tissue after nude mice treated with AZ31,LDH-Mn3,LDH-Mn3+NIR,respectively.(Reproduced with permissions from Ref.[30]) B.Representative photos of tumor-bearing nude mice and tumor tissues collected from mice treated with different Mg pellets at day 28.(Reproduced with permissions from Ref.[26]) C. In vivo images of tumor-bearing mice right before surgical removal of the solid tumors and after accepting different treatments for 4,12 and 24 days.(Control: mice without implantation;Surgery: mice with PLGA scaffolds implantation;P10M: PLGA/Mg composite scaffolds with 10 wt% Mg).Reproduced with permissions from Ref.[28]) (LDH: layered double hydroxide;NIR: near-infrared light;MAO: microarc oxidation;BP: bisphosphonate).
Breast cancer is the most diagnosed cancer among women worldwide,and one of the leading causes of cancer-related death in women [81].Current treatment for breast cancer typically involves a combination of surgery,chemotherapy,radiation therapy,and targeted therapy.These treatments are tailored to the specific characteristics of the tumor and aim to remove or shrink the tumor,eliminate cancer cells,and prevent recurrence [82].Advances in personalized medicine and targeted therapies have improved treatment outcomes and patient survival.Nevertheless,ongoing research and clinical trials are focused on developing novel treatment approaches to enhance the effectiveness and minimize side effects of breast cancer therapy.In particular,the treatment of triple-negative breast cancer remains a major challenge due to its greater degree of malignancy and the lack of genetic targets [83].The chemotherapeutic agents currently used are mainly injected via artificial blood vessels,and have bioavailability issues;thus,local drug precision delivery systems and local tumor ablation have been introduced for breast cancer treatment.Porous drug-loadable Mg alloys and the tumor-suppressive effect of the Mg alloy degradation product H2have increased interest in Mg alloys for breast cancer treatment.Fig.5 and Table 4 summarize the literature on the anti-breast cancer effects of Mg alloys.
Mg alloys with porous structures can be engineered,allowing for the controlled release of therapeutic agents.Liu et al.[32] invented MgNF@PEG/PMMVP [pH-sensitive polyethylene glycol-b-(poly(methyl methacrylate)-co-poly(4-vinylpyridine) (PEG-bPMMVP) coated with Mg nanoflowers],which can be implanted near a tumor and release Mg2+.As the Mg degrades over time,Mg2+directly targets the cancer cells while minimizing damage to healthy tissues.Mg alloy implants can be placed strategically near a tumor,providing the sustained controlled release of therapeutic agents directly to the affected area.This targeted drug delivery approach holds promise for enhancing the effectiveness of chemotherapy,while minimizing systemic side effects.
Recent reports have highlighted the therapeutic potential of H2in free radical-related breast cancer.Given that Mg alloys release H2during their biodegradation,it is reasonable to consider Mg alloys a promising material with therapeutic properties for inhibiting breast cancer [8,84].Recently,Liu et al.[32] reported pH-sensitive polymer-coated Mg nanoflowers for breast cancer treatment.In the tumor microenvironment,the continuous generation of H2was facilitated by the disassembly of the polymeric shell triggered by the acidic conditions.This disassembly allowed Mg to react with water,resulting in the production of H2bubbles,which induce transient cavitation and mechanical rupture of lysosomes.They also disrupt cellular energy metabolism,while simultaneously inducing high levels of oxidative stress.The combined effects of these processes ultimately lead to cancer cell death.
4.3.Other tumor types
While the applications mentioned above highlight specific tumor types,the potential use of Mg alloys in cancer treatment is not limited to these cases.Further research is exploring the efficacy of Mg alloys in other malignancies,including but not limited to prostate [37,86],colorectal [38,39,85],liver [41],ovarian [36],esophageal [16],and gallbladder[40] cancers,as well as melanoma [37,87],leukemia [88],and cholangiocarcinoma[89].Fig.6 provides representativeinvivoexperimental results of Mg alloys for other tumor types.Table 5 summarizes the specificin vivoandin vitroexperimental results,and related active ingredients of the reported Mg alloys.
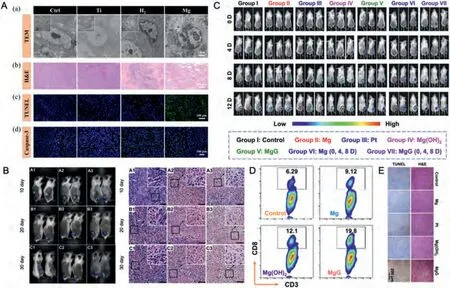
Fig.5.Representative antitumor effect of Mg alloys for breast cancer: A.(a) TEM photos of the tumor tissues at various scales;(b) H&E images of tumor tissues dissected from each group after different treatments on day 24;(c) Apoptosis was analyzed by TUNEL staining in tumor tissue after 24 days of treatment;(d) Confocal laser scanning microscopy of caspase-3 staining in tumor tissues after different treatments.(Reproduced with permissions from Ref.[34]) B. In vivo and H&E images of the tumors in animals at 10,20,and 30 days post operation.(Control:A1,A2,A3;P-Mg: B1,B2 and B3;AO-HT-Mg: C1,C2 and C3).(Reproduced with permissions from Ref.[8]) C.Bioluminescence images of mice bearing subcutaneous 4T1 tumors expressing firefly luciferase(Luc-4T1) to display the therapeutic efficacy of mice after various treatments.D.The flow cytometric analysis results of CD8+T cells (CD3+CD8 +)within the tumors after different treatments.E.Microscopy images of H&E and TUNEL stained tumor slices collected from mice post different treatment groups.(C,D,and E reproduced with permissions from Ref.[85]) (P-Mg: Pure Mg metal;AO-HT-Mg: P-Mg coated with MgO;MgG: Mg-based galvanic cell.).
Zan et al.[38] invented Mg staples for wound closure after the surgical resection of colorectal tumors to inhibit tumor cells.In vivo,the intestinal wounds of rabbits with Mg staples healed gradually with no adverse effects,such as leakage or inflammation;simultaneously,the colorectal tumor cells were inhibited because of the increased concentration of Mg ions and released hydrogen.Peng et al.[40] reported a Mg biliary stent that enables both bile drainage and the treatment of gallbladder cancer.Both the abovementioned Mg biliary stent and staples are degraded directly in the body without removing them at a secondary surgery,which reduces the patient’s pain and is expected to be an ideal material for biodegradable implants.Wang et al.[87] combined Mg with galliumindium alloy which produced a new biomedical material that can adapt to any irregular skin surface.Mg-GaIn plays the antitumor effect of Mg and the plasticity characteristics of liquid metal simultaneously which shows a good application prospect in melanoma.
5.The antitumor mechanism of magnesium alloys
The antitumor effects of Mg alloys are mainly due to their degradation products,including Mg2+,H2,OH-,and Mg(OH)2.These products mainly inhibit tumor progression by regulating the acidic tumor microenvironment,resisting oxidative stress,resolving tumor inflammation,inhibiting tumor cell proliferation,promoting apoptosis,and reducing migration and invasion directly (Fig.7).The tumor microenvironment tends to be weakly acidic,with high ROS levels and immunosuppression.The degradation of Mg and Mg alloys directly neutralizes acid,increasing the tumor microenvironment pH,thereby inhibiting tumor progression [90].In response to the high oxidative stress of tumors,H2downregulates the excessive ROS and improves endogenous antioxidant enzyme levels to inhibit tumor development [91].Regarding the immune suppression of tumors,the degradation products Mg2+and H2reduce inflammation,although the specific mechanism needs to be explored [92,93].Mg2+and H2also directly inhibit tumor cell proliferation and promote apoptosis [94,95].Therefore,Mg alloys may be potential biomaterials for antitumor treatment.Here,we summarize the antitumor mechanisms of the active components of Mg,including Mg2+,H2,OH-,and Mg(OH)2.

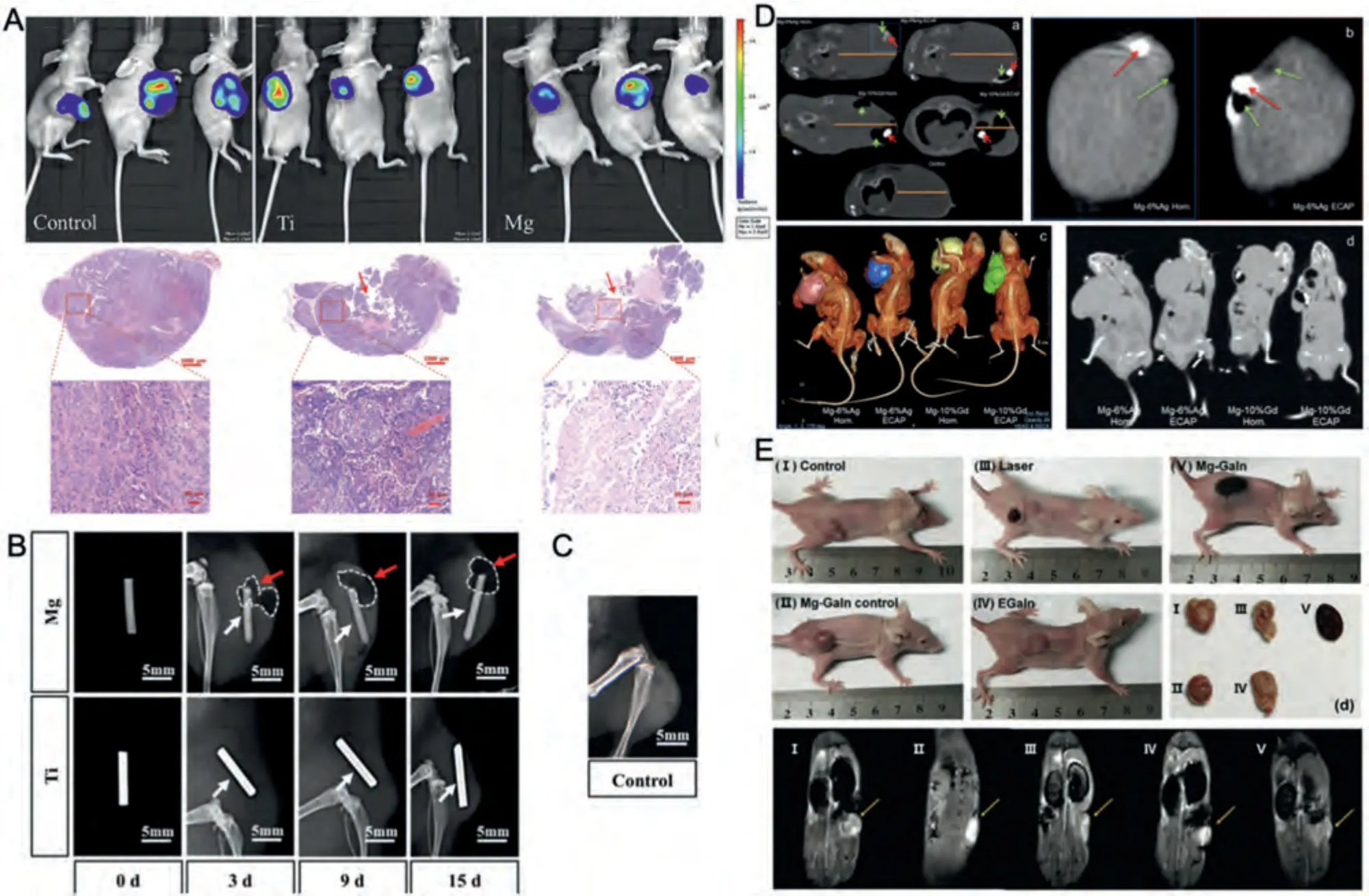
Fig.6.Representative other types of tumors: A.Bioluminescence and H&E stained images of ovarian tumor-bearing mice implanted with and without metallic wires on the 25th day.Red arrow indicated the position of Mg or Ti implantation.(Reproduced with permissions from Ref.[36]) B.Soft X-ray photography of Hepatoma tumor-bearing mice with Mg and Ti wires on the 3rd,9th,and 15th days.The white arrows show Mg and Ti wires,and the red arrows show the gas (white-dashed) around Mg wires.C.Soft X-ray photography of the blank control group on the 15th day.(B and C reproduced with permissions from Ref.[89]) D.Longitudinal and transversal CT scans of tumoral nodes in melanoma tumor-bearing mice with inoculated melanoma after 10 days of intratumorally implantation of alloy pins.An orange line marks the diameter of a tumoral node,green arrows indicate gas bubbles,and a red arrow points to the alloy pin.(а) A transversal section of the body of a mouse with an inoculated tumor;(b) a longitudinal section of the tumor for Mg-6%Ag Hom.and Mg-6%Ag ECAP;(c) CT images of tumoral nodes containing gas accumulated in cavities in mice with implanted Mg-6%Ag and Mg-10%Gd pins.The images represent 3D rendering of the reconstructed tumoral nodes of the groups of mice;(d) longitudinal section of tumor-bearing mice with different alloy implants.(Reproduced with permissions from Ref.[86]) E.The 6th day photographs of melanoma tumor-bearing mice after three times treatments.d) The resected tumor tissues from tumor-bearing mice after three times treatments.e) The 6th day MR images.(Reproduced with permissions from Ref.[87]) (HOM.: homogenized;ECAP:Equal-channel angular pressing).
5.1.Mechanism of Mg2+ on tumor inhibition
The corrosion product Mg2+inhibits tumor progression mainly by inhibiting tumor cell proliferation,promoting apoptosis,suppressing migration and invasion,and improving tumor inflammation.Specifically,Mg2+inhibits tumor proliferation through transient receptor potential melastatin7 (TRPM7) related signaling pathway and protein phosphatase 1D (PPM1D) [18,96,97],and promotes tumor apoptosis through AKT/mTOR and Bax signaling pathways [98].Mg2+may against tumor invasion and metastasis by inhibiting the tumor necrosis factor-α(TNF-α)/interleukin-1 (IL-1)/NF-κB signaling pathway [60,99].Moreover,Mg2+improves tumor inflammation by reducing the release of inflammatory factors such as IL-1α,IL-6,NO,and vascular cell adhesion molecule (VCAM) [100] (Fig.8).Additionally,Mg2+also regulates protein endocytosis and channel proteins to inhibit tumor angiogenesis and multi-drug resistance [18].
5.2.Mechanism of H2 on tumor inhibition
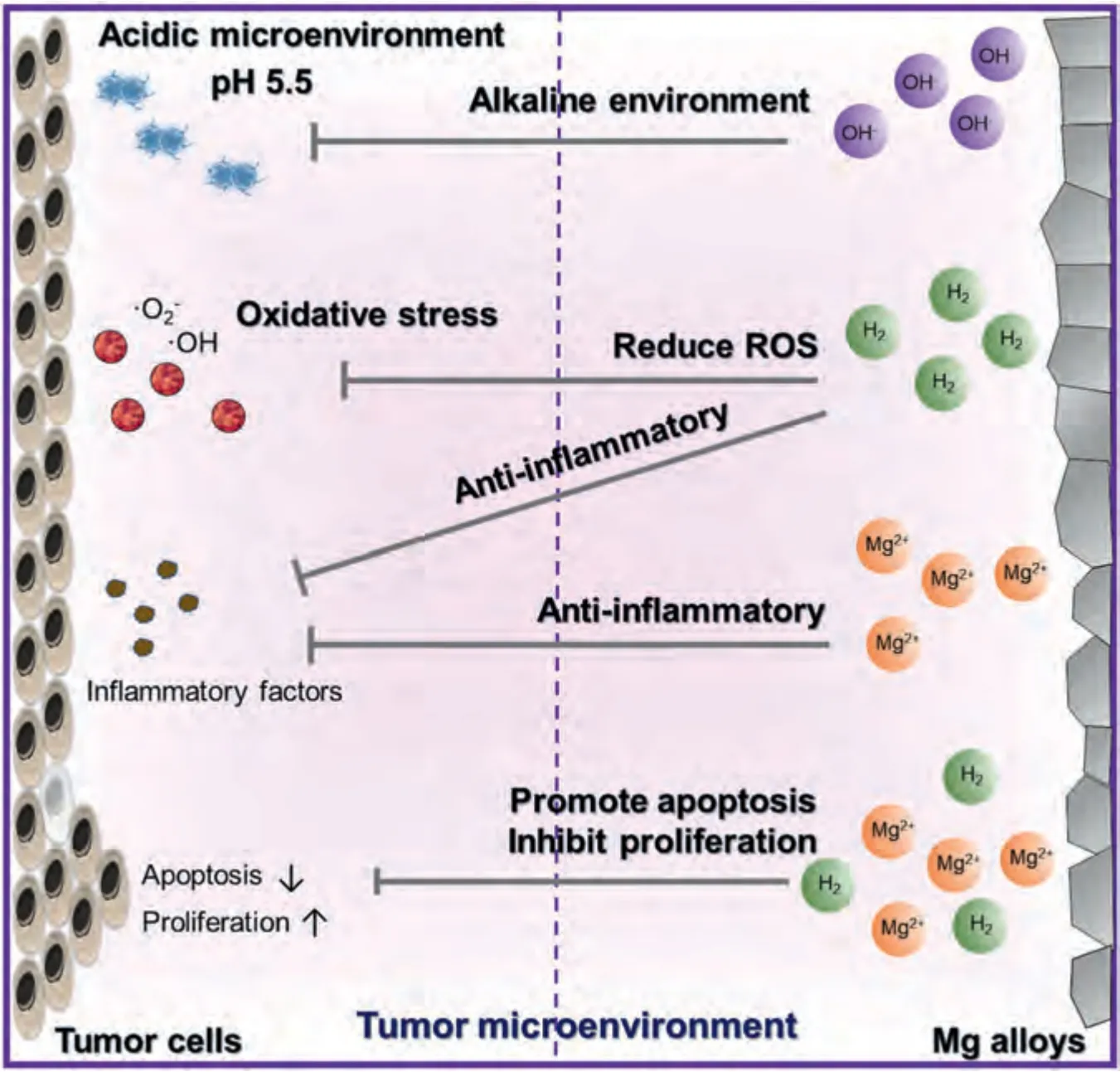
Fig.7.Tumors biological characteristics and Mg alloys material properties.
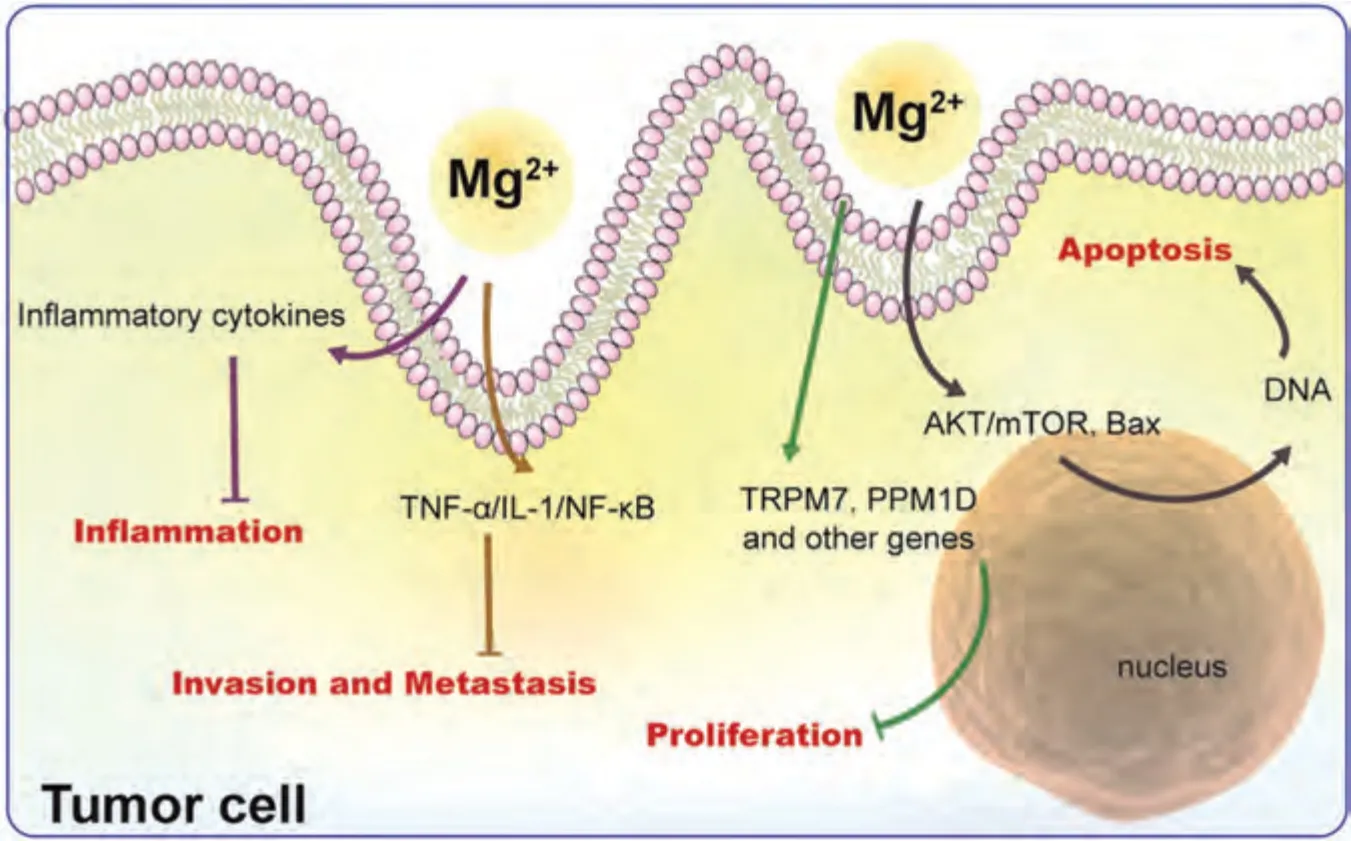
Fig.8.Antitumor mechanisms of Mg2+.
H2has a surprising ability to inhibit tumor progression by inhibiting tumor proliferation,promoting apoptosis,improving inflammation,and regulating oxidative stress(Fig.9).Specifically,H2inhibits tumor cell proliferation via the PI3K/AKT signaling pathway [101] and promotes tumor cell apoptosis via NF-κB-related signaling pathway [95].decreasing the number of tumor cells sharply.H2also downregulates proinflammatory cytokines (e.g.,TNF-αand IL-6) to alleviate tumor inflammation [102] and also regulates micro-RNA (e.g.,miR-21 and miR-199) to control tumor inflammation [103].In terms of oxidative stress,H2inhibits vascular endothelial growth factor (VEGF) and ERK signaling downregulation by reducing excessive ROS [104] and activates the body’s own antioxidant system through the production of endogenous antioxidants,such as catalase(CAT)and superoxide dismutase (SOD) [105].H2also regulates oxidative stress and the Nrf2 signaling pathway to ameliorate genomic instability and mutation in tumor cells [106].Therefore,H2,the degradation product of Mg and Mg alloys,has a potential role in tumor treatment.
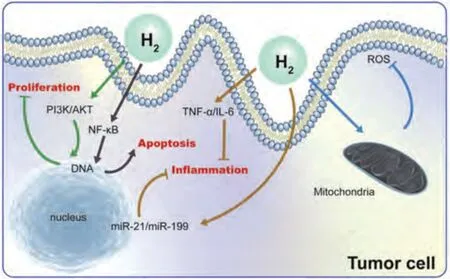
Fig.9.Antitumor mechanisms of H2.
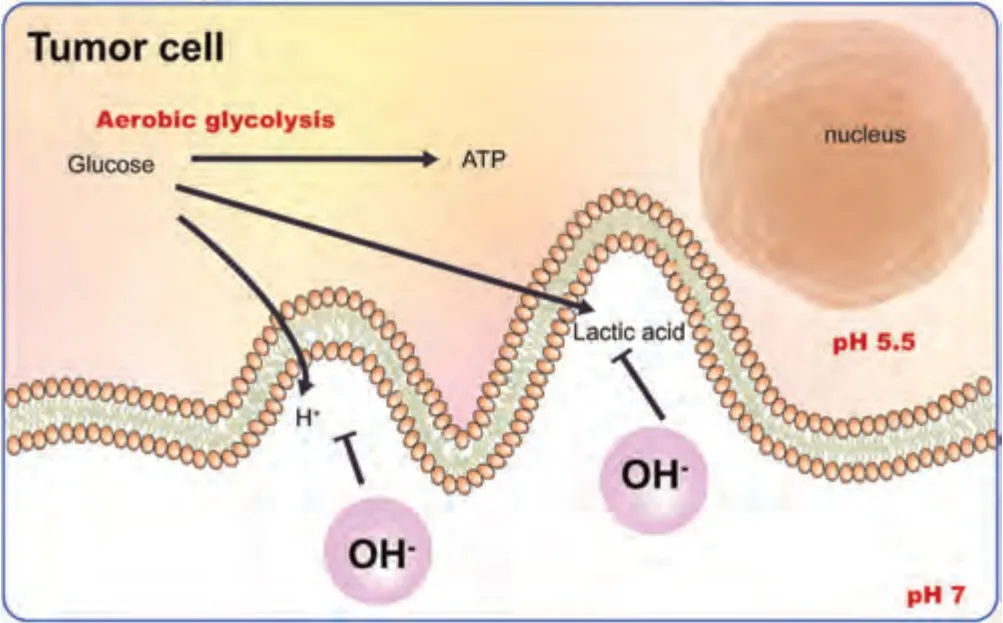
Fig.10.Antitumor mechanisms of OH-.
5.3.Mechanism of OH- on tumor inhibition
Aerobic glycolysis is the main method of energy production in tumor cells.One molecule of glucose is converted to two molecules of lactic acid,two molecules of H+and two molecules of ATP.The accumulated lactic acid and excess H+diffuse into the extracellular matrix forming an acidic microenvironment that promotes cell proliferation and invasion[107].Therefore,alkalizing the tumor microenvironment is an effective antitumor strategy.Notably,during the degradation process of Mg,the OH-generated by cathodic reactions and the decomposition of Mg(OH)2neutralizes acidic substances,increasing the pH value,and thereby inhibiting tumor progression (Fig.10).
5.4.Mechanism of Mg(OH)2 on tumor inhibition
The corrosion product Mg(OH)2also achieves tumorsuppressive effects through various pathways,which include inhibiting tumor cell proliferation,promoting tumor cell apoptosis,and regulating tumor-related inflammation (Fig.11).Mg(OH)2may inhibit the replicative immortality of tumor cells by inhibiting the PI3K/AKT signaling pathway[18,108] and activate mTORC1 to inhibit tumor cell proliferation [18,109].In terms of regulating tumor cell death,Mg(OH)2may also promote tumor cell apoptosis by regulating the BDNF/TrkB signaling pathway [18,110].Moreover,Mg(OH)2may inhibit ROS production [18] and suppress tumor-associated inflammation by inhibiting the inflammasome [18,111].Finally,in terms of genomic instability and mutation,Mg(OH)2may ameliorate DNA double-strand breaks to inhibit tumor progression [18,112].Therefore,Mg has excellent application prospects in inhibiting tumors and improving the tumor microenvironment.
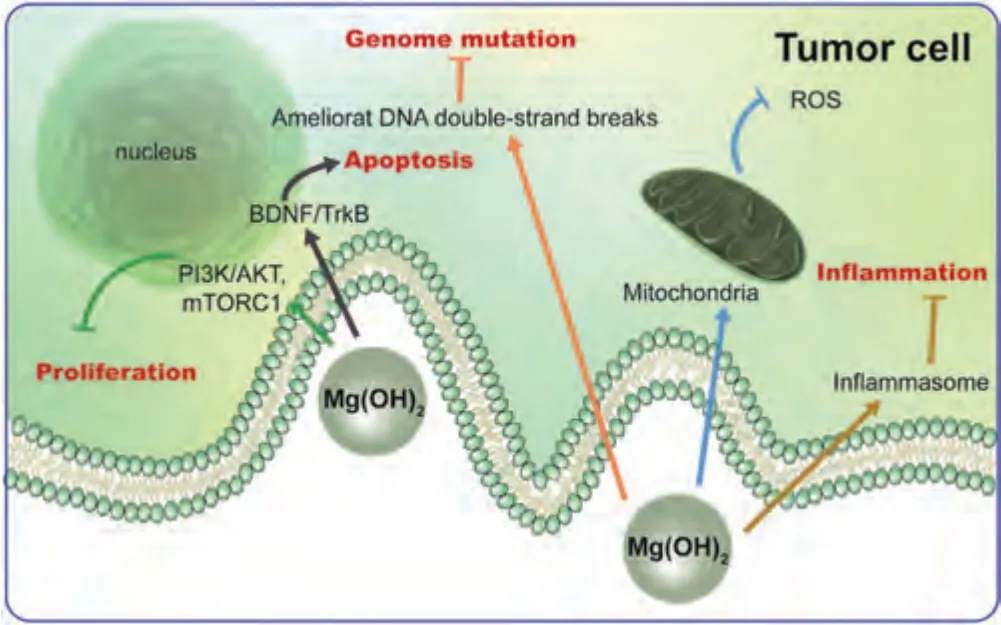
Fig.11.Antitumor mechanisms of Mg(OH)2.
6.Challenges and perspectives
In clinical practice,malignant tumors are traditionally treated via surgical resection followed by chemotherapy/radiation to kill the remaining tumor cells.Moreover,the tissue defects caused by resection of the primary focus,substantially decrease survival rates and have a devastating impact on patients’ lives.Therefore,there is an urgent need for highly selective,non-invasive treatments and advanced biomaterials that can simultaneously fight tumors and promote osteogenesis.
Studies confirm the positive contribution of appropriate external stimulation to tumor treatment,including thermal stimulation [7],electrical stimulation [113],magnetic stimulation[114],and light stimulation [69],and the pH response [115].The use of Mg alloys combined with near infrared light,pH modulation,and other external stimulation has achieved good efficacy for tumor treatment.However,because the external stimulation conditions are strictly limited,unreasonable use may produce irreversible damage to the surrounding healthy tissues.Therefore,the precise control of Mg alloys and stimulation is needed.When Mg alloys are combined with PTT,normal tissue can tolerate short periods of irradiation.If the duration and power of the irradiation are not controlled,the deep tissue cannot be heated with pinpoint precision,such that the heat may have harmful effects on normal cells and normal tissue surrounding the tumor [77,116].The irradiation power and duration should be adjusted carefully to achieve a suitable target temperature.As reported,the temperature of local thermotherapy needs to exceed 50 °C to achieve an antitumor effect,but hyperthermia exceeding 50 °C can cause irreversible DNA and protein denaturation in cancerous locations.While the temperature range suitable for osteogenic differentiation is 40–42 °C.Thus,precise temperature control is necessary during PTT in conjunction with Mg alloys.A temperature-sensitive Mg alloy with a large temperature conversion range combined with a gradient-controlled heating process will likely achieve the goals of two-stage antitumor treatment.
The biosafety of an implanted material is an important consideration.On the one hand,attention should be paid to regulate the release of degradation products to achieve better antitumor effects.On the other hand,Hepatic and renal clearance,the clearance cycle and complete degradation time,and the acute and chronic toxicity of degradation products of Mg alloys should be evaluated to ensure their biosafety before clinical application and further clinical translation.It is also necessary to consider how to modify the size,surface morphology,internal structure,and surface charge of Mg alloys to minimize nonspecific damage to normal cells.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence this paper.
Acknowledgement
This study was supported by the National Key R&D Program of China [grant number 2021YFC2400700],the National Natural Science Foundation of China [grant numbers 82170929,81970908] and the Beijing Natural Science Foundation [L222090,L222030].
杂志排行
Journal of Magnesium and Alloys的其它文章
- Magnesium and its alloys for better future
——The 10th anniversary of journal of magnesium and alloys - Twin-solute,twin-dislocation and twin-twin interactions in magnesium
- Recent advances on grain refinement of magnesium rare-earth alloys during the whole casting processes: A review
- Magnesium research in Canada: Highlights of the last two decades
- Structure-function integrated magnesium alloys and their composites
- Recent research progress on magnesium alloys in Korea: A review
