Performance Evaluation and Microbial Shift of Sequen-cing Batch Biofilm Reactor Treating Synthetic Mariculture Wastewater Under Different Dissolved Oxygen at Aerobic Phase
2023-12-21HUOSiyueLIUWenjieZHAOChangkunLUShuailingWANGQianzhiSHEZonglianZHAOYangguoZHANGZhimingGUOliangJIJunyuanJINChunjiandGAOMengchun
HUO Siyue, LIU Wenjie, ZHAO Changkun, LU Shuailing, WANGQianzhi, 3), SHE Zonglian, ZHAO Yangguo, ZHANG Zhiming, * , GUO liang, JI Junyuan, 3), JIN Chunji, 3), and GAO Mengchun, 3), *
Performance Evaluation and Microbial Shift of Sequen-cing Batch Biofilm Reactor Treating Synthetic Mariculture Wastewater Under Different Dissolved Oxygen at Aerobic Phase
HUO Siyue1), 2), LIU Wenjie1), 2), ZHAO Changkun1), LU Shuailing1), 2), WANGQianzhi1), 3), SHE Zonglian1), ZHAO Yangguo1), 2), ZHANG Zhiming1), *, GUO liang1), JI Junyuan1), 3), JIN Chunji1), 3), and GAO Mengchun1), 3), *
The impact of dissolved oxygen (DO) at aerobic phase on the nitrogen removal, extracellular polymeric substances (EPS), microbial activity and microbial community of sequencing batch biofilm reactor (SBBR) have been evaluated in treating mariculture wastewater. The oxygen uptake rate and nitrification rate declined with DO concentration from 3–4 to 1–1.5mgL−1, whereas the denitrification rate had an increment. The activities of nitrifying enzymes reduced with the decrease of DO concentration at aerobic phase, but those of denitrifying enzymes illustrated opposite results. The nitrification and denitrification rates displayed the similar variation tendency with the relevant enzymatic activities as DO concentration decreased. The protein (PN) and polysaccharide (PS) content in EPS decreased as DO concentration declined, whereas the PN/PS ratio increased. The microbial community showed obvious difference as DO concentration decreased from 3–4 to 1–1.5mgL−1. The microbial co-occurrence, keystone taxa and significant difference illustrated some variations at different DO concentrations.
SBBR; mariculture wastewater; DO concentration; microbial activity; microbial community
1 Introduction
With the increasing seafood consumption and the continuous reduction of wild fishery resources, mariculture is developing rapidly in coastal countries and regions in recent years (FAO, 2020). At present, China has become the largest producer and consumer of mariculture pro- ducts in the world. During the mariculture process, feed residues and aquaculture excreta as wastes are ultimately dissolved or suspended in mariculture water (Grab., 2007; Huang., 2021). A quantity of mariculture wastewater is discharged from intensive mariculture systems along the coast, which can produce adverse impact on coastal waters. To protect offshore marine environment, it is essential to reasonably treat mariculture waste- water prior to discharge.
Mariculture wastewater usually has higher salinityand lower carbon/nitrogen (C/N) ratio (Sun., 2020). Compared with physical and chemical methods, biological processes are considered as good choices for treating mariculture wastewater due to low operating cost, low energy consumption and environmental friendliness. Many biological treatment systems have been proved to be effective for treating mariculture wastewater, including biofilter (Davidson., 2008), trickling filter (Eding, 2006), sequencing batch reactor (SBR) (Fontenot., 2007), constructed wetland (Liang., 2017), microbial fuel cell (Pugazhendi., 2021) and bio-elec- trochemical reactor (Sun., 2020).
Recently, sequencing batch biofilm reactor (SBBR) be- comes a promising method for treating mariculture waste- water owing to the characteristics of SBR and biofilm (Gao., 2018). An alternate aerobic and anoxic condition of SBBR can effectively remove nitrogen com- pound under a harmonious and balance interaction between nitrifying and denitrifying processes. Dissolved oxygen (DO) concentration at aerobic phase is a key factor to impact the balance between nitrification and denitrification of SBBR. Too much DO concentration at aerobic phase occurs good nitrification and organic matter degradation and inhibits the denitrification process due to high DO concentration and the insufficiency of carbon source. Too little DO concentration can restrain the nitrification process and decrease the nitrogen removal performance. Some reports have proved that the variation of DO concentration cause obvious impacts on the biological nitrogen removal performance (Gao., 2017; Wang., 2018). Due to low C/N ratio in mariculture wastewater, the variation of DO concentration at aerobic phase might impact the nitrogen removal of SBBR. Ne- vertheless, no research has reported the impact of DO at aerobic phase on the SBBR performance during treating mariculture wastewater.
The objects of this research consisted of a) investigating the impact of DO at aerobic phase on the SBBR performance during treating mariculture wastewater, b) assessing the nitrogen removal rate and enzymatic activity with DO from 3–4 to 1–1.5mgL−1at aerobic phase, c) exploring the variation of extracellular polymeric substances (EPS) under different DO concentrations at aerobic phase, and d) analyzing the effect of DO concentration at aerobic phase on the microbial community, co- occurrence and keystone taxa.
2 Materials and Methods
2.1 Experimental Setup
A lab-scale SBBR with fibrous carrier was used to treat synthetic mariculutre wastewater in the present research. The SBBR with 7.7L effect volume had 14cm inner diameter and 50cm effective height. Synthetic wastewater was pumped into the SBBR through a peristaltic pump, and treated water was discharged by a solenoid valve, which had a 50% volume exchange rate of each operation cycle. The hydraulic retention time of SBBR was 12h. An operation cycle was successively comprised of filling (5min), aerobic phase (120min), anoxic phase (210min), precipitation (20min) and drainage (5min). Air was supplied at aerobic phase through an aerating pump, and mixed liquid of SBBR was stirred at anoxic phase with a stirrer. DO at anoxic phase was below 0.5mgL−1. The different DO at aerobic phase was obtain by through controlling a gas flowmeter. DO at aerobic phase was in the range of 3–4, 2–3, 1.5–2 and 1–1.5mgL−1on day 1–20, 21–40, 41–64, and 65–93, respectively.
Prior to treating synthetic mariculture wastewater, the fibrous carrier was seeded by activated sludge, and the formed biofilm was acclimatized at marine salinity. At the end of the biofilm acclimation process, synthetic mariculture wastewater were treated under different DO concentrations during aerobic phase. Synthetic mariculture wastewater included (mgL−1): CH3COONa (80.8), NH4Cl (11.5), NaNO3(42.4), NaNO2(2.5), NaHCO3(15.0), KH2PO4(10.0), and seawater crystal (3×104). The COD, NH4+-N, NO2−-N and NO3−-N in the influent were 63.10 ±1.07, 3.14±0.11, 0.51±0.01 and 7.08±0.11mgL−1, res- pectively.
2.2 Analytical Methods
COD, NH4+-N, NO2−-N, NO3−-N, mixed liquor suspended solids (MLSS) and mixed liquor volatile suspended solids (MLVSS) were determined in accordance with standard methods (APHA, 2005). DO concentration was measured by a DO meter. The specific oxygen uptake rate (SOUR), specific ammonia-oxidizing rate (SAOR), specific nitrite-oxidizing rate (SNOR), specific nitrite- reducing rate (SNIRR) and specific nitrate-reducing rate (SNRR) of biofilm were analyzed according to the report of Wang. (2016). The ammonia mono-oxygenase (AMO) activity, nitrite oxidoreductase (NOR) activity, nitrate reductase (NR) activity, nitrite reductase (NIR) activity and dehydrogenase activity (DHA) were determined according to the report of Wang. (2016). The microbial community, microbial significant differences, and microbial co-occurrence and keystone taxa of biofilm samples were analyzed according to previous reports (Huang, 2020; Gao, 2021; Song, 2021). The COD, NH4+-N, NO2−-N, NO3−-N concentrations of the influent and effluent were determined every day. The sludge samples for the analysis of MLSS, MLVSS, EPS, LB-EPS, TB-EPS, SOUR, SAOR, SNOR, SNIRR, SNRR, AMO activity, NOR activity, NR activity, NIR activity and microbial community were obtained at 3–4, 2–3, 1.5–2 and 1–1.5mgL−1DO were measured on day 20, 40, 64, and 93, respectively.
3 Results and Discussion
3.1 SBBR Performance Under Different DO Concentration at Aerobic Phase
The COD and nitrogen removals of SBBR were investigated under different DO concentrations at aerobic phase (Fig.1). Due to lower influent COD concentration (63.10±1.07mgL−1), the COD removal efficiency stabilized at 94.20%±1.76% under different DO concentrations through the heterotrophic biodegradation of aerobic phase and anoxic phase (Fig.1a). Under the influent NH4+-N at 3.14mgL−1, the NH4+-N removal efficiencies were 91.09% ±1.98%, 92.53%±1.31% and 91.40%±1.22% at 3–4, 2–3 and 1.5–2mgL−1DO, respectively (Fig.1b). The present results demonstrated that the SBBR treating mariculure wastewater had a good ability of ammonia oxidization under the DO at over 1.5mgL−1in the present research. Nevertheless, the NH4+-N removal efficiency was only 78.64%±1.70% at 1–1.5mgL−1DO, which illustrated that the insufficiency of DO at the aerobic pha- se could inhibit the oxidation process of ammonia to nitrite and then further affected the nitrogen removal performance. The effluent NO2−-N was 0.46±0.32, 0.71±0.08, 0.63±0.10mgL−1at 3–4, 2–3 and 1.5–2mgL−1DO (Fig.1c), respectively. Compared with influent NO2−-N (0.51±0.01mgL−1), the effluent NO2−-N kept stable at 3–4, 2–3 and 1.5–2mgL−1DO. However, the effluent NO2−-N was zero at 1–1.5mgL−1DO except for day 63–67. The effluent NO2−-N was related to the influent NO2−-N, ammonia oxidization and nitrate reduction. Under the influent NO3−-N at 7.08mgL−1, the effluent NO3−-N stabilized at 0.21±0.18mgL−1under different DO concentrations, suggesting that almost all nitrate from the influent, ammonia oxidation and nitrite oxidation was reduced as nitrite or nitrogen gas.
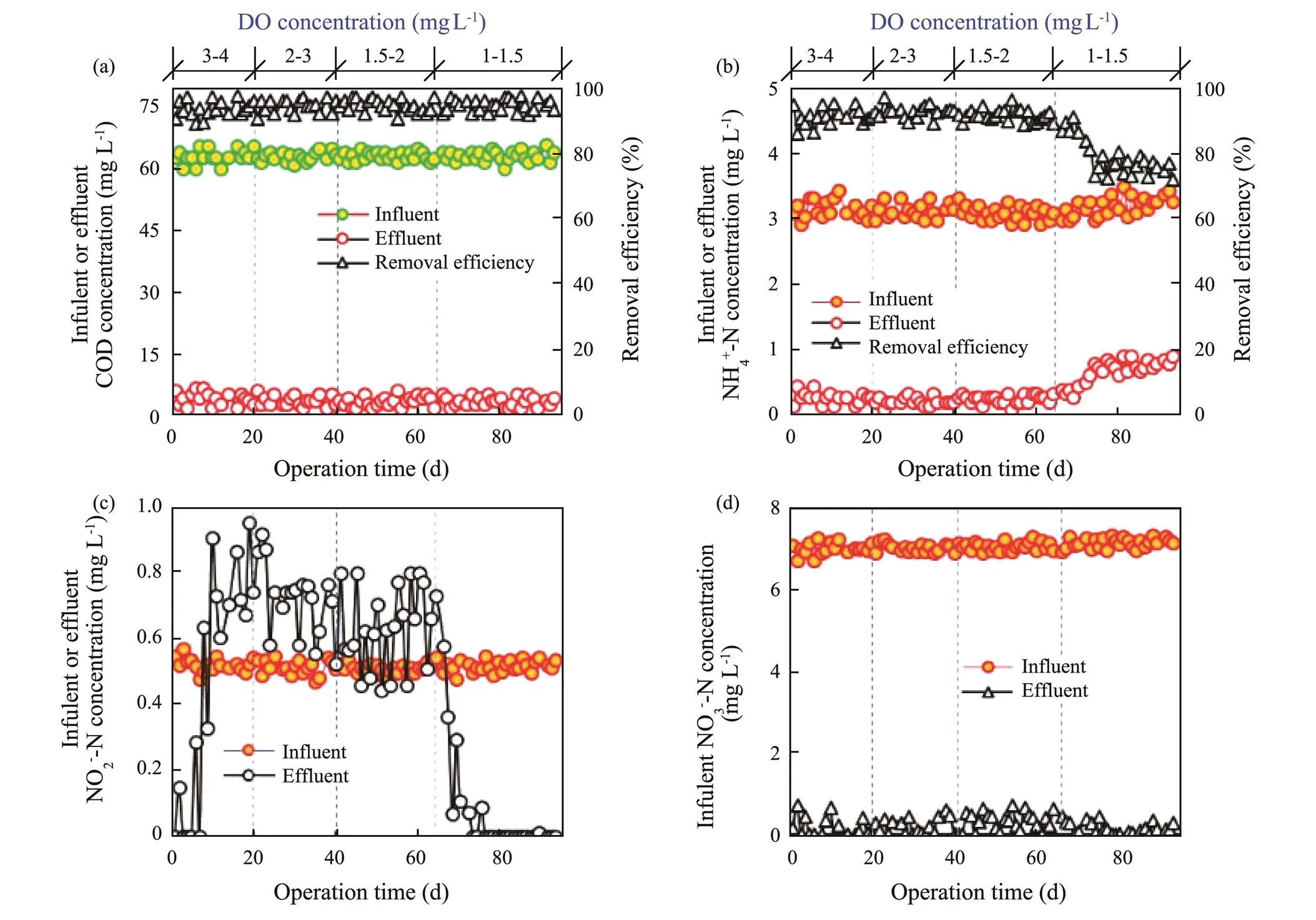
Fig.1 Variations of COD and nitrogen compound concentration in the influent and effluent under different DO concentrations of aerobic phase. (a), COD; (b) NH4+-N; (c), NO2−-N; (d), NO3−-N.
3.2 Effect of DO Concentration at Aerobic Phase on Oxygen Uptake Rate and Nitrogen Removal Rate
As depicted in Fig.2a, the SOUR had a declining trend with the DO concentration from 3–4 to 1–1.5mgL−1. In contrast with 3–4mgL−1DO, the SOUR declined by 10.81%, 26.58% and 44.12% at 2–3, 1.5–2 and 1–1.5 mgL−1DO, respectively. SOUR is bound up with the organic matter degradation and nitrification processes. Low DO concentration at aerobic phase restrained the organic matter biodegradation and the nitrification, which could result in a detrimental impact on the nitrogen removal. Both SAOR and SNOR declined with DO concentration from 3–4 to 1–1.5mgL−1(Fig.2b), which further proved that low DO concentration was unfavorable to the ammonia and nitrite oxidation. Compared with 3–4mgL−1DO, the SAOR and SNOR decreased by 24.07% and 16.70%, 47.09% and 35.54%, and 70.15% and 63.59% at 2–3, 1.5–2, and 1–1.5mgL−1DO, respectively. The decreasing degree of SAOR at each DO concentration was more than that of SNOR compared with 3–4mgL−1DO. The present results suggested that low DO concentration had higher inhibition on the ammonia oxidization than the nitrite oxidization. However, both the SNIRR and SNRR heightened as the DO concentration decreased from 3–4 to 1–1.5mgL−1, which demonstrated that low DO concentration was advantageous to denitrification process of SBBR. Compared with 3–4mgL−1DO, the SNIRR and SNRR heighted by 5.93% and 6.87%, 17.02% and 13.57%, and 42.09% and 41.88% at 2–3, 1.5–2 and 1–1.5 mgL−1DO, respectively, which demonstrated that low DO concentration had almost similar promoting effect on the SNIRR and SNRR. The present results illustrated that the variation of DO could obviously change the nitrifying and denitrifying rates and then further affect the nitrogen removal performance of SBBR treating mariculture wastewater.
3.3 Effect of DO Concentration at Aerobic Phase on Key Enzymatic Activity
As the pollutant removal rates are closely related to their corresponding microbial enzymatic activity, the dehydrogenase and nitrogen removal enzyme activities of SBBR have been evaluated under different DO concentrations at aerobic phase (Fig.3). The microbial DHA decreased with the DO concentration from 3–4 to 1–1.5mgL−1(Fig.3a), which suggested that low DO concentration could lead to an inhibitory effect on the DHA. Compared with 3–4mgL−1DO, the DHA decreased by 12.59%, 26.16% and 41.35% at 2–3, 1.5–2, and 1–1.5mgL−1DO, respectively. The inhibitory effect of low DO concentration on the DHA could further impact the organic matter biodegradation. Both the AMO and NOR activities reduced as DO concentration decreased (Fig.3b), which illustrated that low DO concentration at aerobic phase inhibited the enzymatic activity of nitrification bacteria. Compared with 3–4mgL−1DO, the AMO and NOR activities declined by 26.90% and 15.52%, 50.63% and 37.93%, and 73.48% and 60.34% at 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively. The decline of AMO and NOR activities at low DO concentration inhibited the SAOR and SNOR. The AMO and NOR activities had similar varying trends with the SAOR and SNOR under different DO concentrations, suggesting that the nitrifying enzymatic activities determined the nitrification rate. However, both the NIR and NR activities heightened as the DO concentration decreased (Fig.3c), which illustrated that low DO concentration was beneficial to the enzymatic activities of denitrifying bacteria. Compared with 3–4mgL−1DO, the NIR and NR activity heightened by 7.47% and 13.39%, 19.42% and 19.64%, and 42.70% and 46.43% at 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively. The increment of NIR and NR activity at low DO concentration heightened the SNI RR and SNRR, which was advantageous to the denitrifying process of SBBR. The NIR and NR activities had similar changing tendencies with SNIRR and SNRR under different DO concentrations, suggesting the denitrifying enzymatic activities determined the denitrifying rate. The present results illustrated the decrease of DO concentration at aerobic phase inhibited nitrification enzymatic activities and promoted the denitrification enzymatic activities.
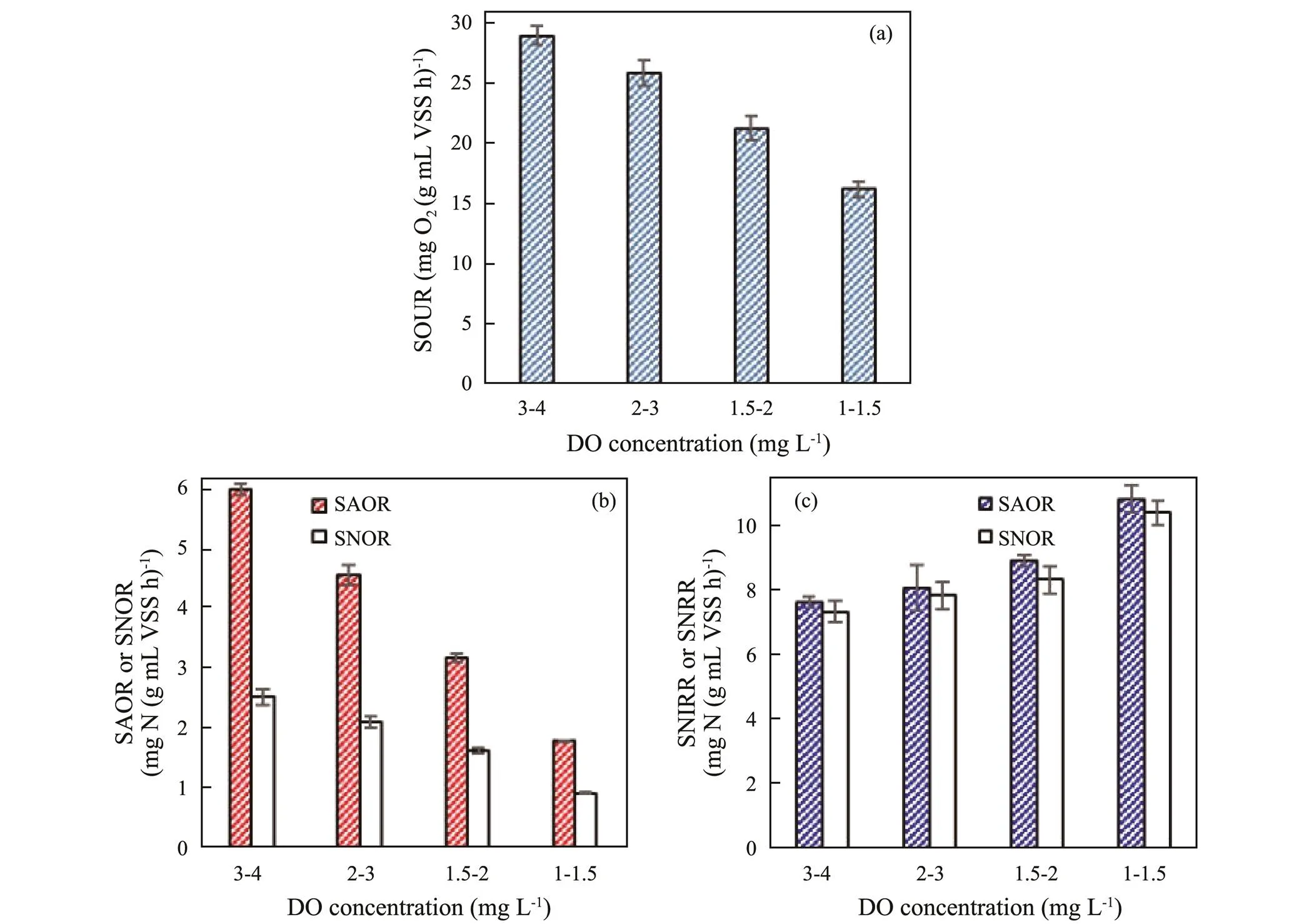
Fig.2 Effect of DO concentration at aerobic phase on the oxygen uptake rate and nitrogen removal rates of SBBR. (a) SOUR, (b) SAOR and SNOR, and (c) SNRR and SNIRR. The SOUR and nitrogen removal rates at 3–4, 2–3, 1.5–2, and 1–1.5mgL−1 DO were measured on day 20, 40, 64 and 93, respectively. Error bars represent standard deviations determined from measurements performed in triplicate.
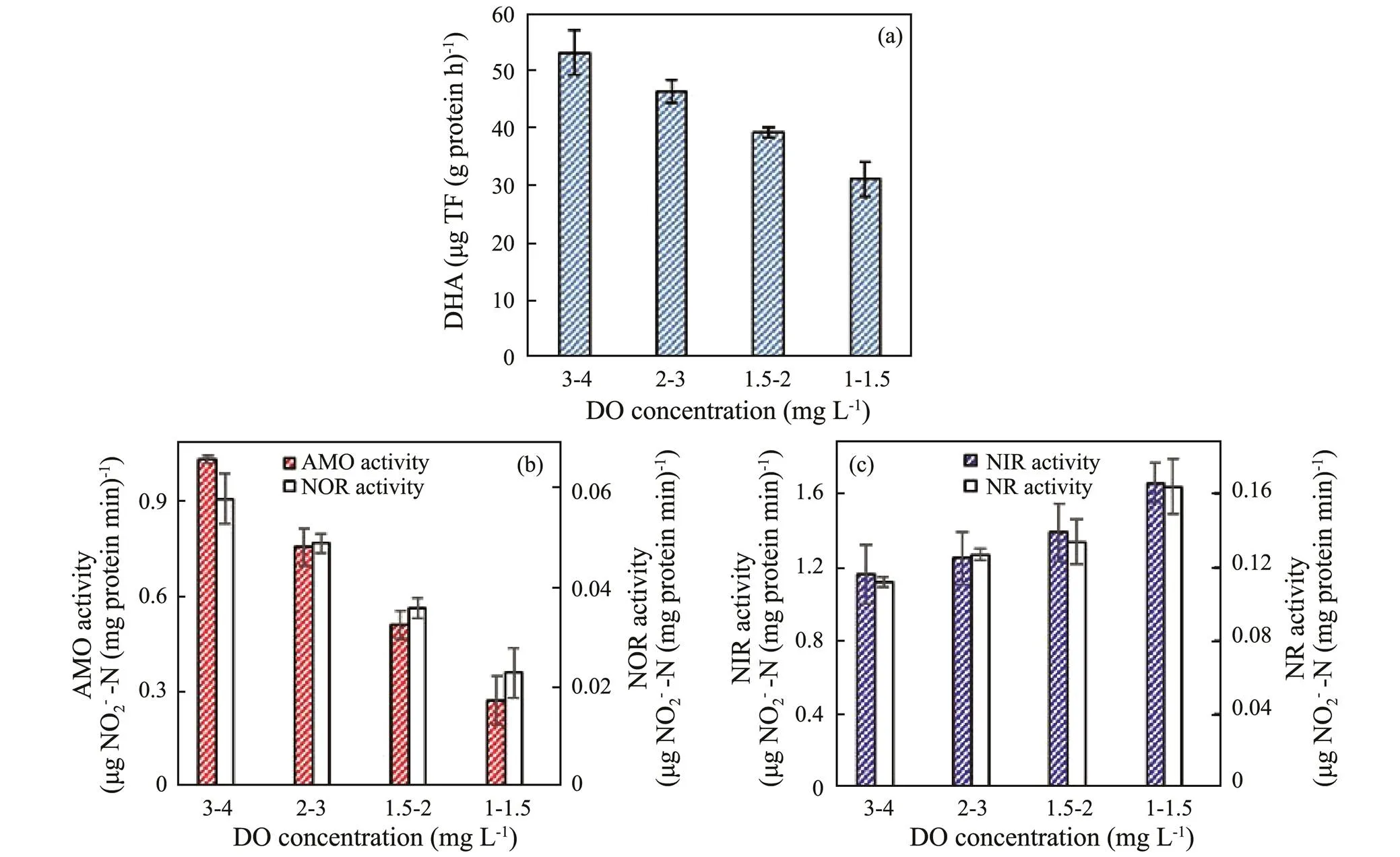
Fig.3 Effect of DO concentration at aerobic phase on the key enzymatic activities of SBBR. (a) DHA, (b) AMO and NOR activities, and (c) NR and NIR activities. The enzymatic activities at 3–4, 2–3, 1.5–2, and 1–1.5mgL−1 DO were measured on day 20, 40, 64, and 93, respectively. Error bars represent standard deviations determined from measurements performed in triplicate.
3.4 Effect of DO Concentration at Aerobic Phase on the EPS
EPS consisting of secreted by microbes acts a key role in microbial adhesion on the fibrous carrier, which has EPS has a dynamic double-layered structure of LB- and TB-EPS. Different operational parameters can affect the EPS production and composition (Sheng., 2010). The production and component of EPS from the biofilm were investigated at 3–4, 2–3, 1.5–2 and 1–1.5mgL−1DO on day 20, 40, 64, and 93, respectively. The EPS production gradually declined as DO decreased at aerobic phase (Fig.4a), suggesting that low DO concentration was detrimental to microbial EPS secretion. The production of TB-EPS was more than that of LB-EPS under different DO concentrations. Compared with 3–4mgL−1DO, the TB-EPS and LB-EPS production decreased by 15.52% and 33.03%, 30.54% and 50.34%, and 41.71% and 58.27% at 3–4, 2–3, 1.5–2 and 1–1.5mgL−1DO, respe- ctively. PN and PS have been regarded as major component in EPS. Both PN and PS content in LB-EPS or TB-EPS decreased as DO concentration declined from 3–4 to 1–1.5mgL−1(Figs.4b and 4c). The PN content in LB- or TB-EPS was obviously more than the PS content at each DO concentration. Compared with 3–4mgL−1DO, the PN and PS content in LB-EPS decreased by 32.00% and 39.13%, 47.14% and 65.74%, and 54.68% and 76.55% at 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively. Additionally, the PN and PS content in TB-EPS declined by 12.51% and 15.46%, 23.25% and 35.28%, and 30.45% and 54.27% at 2–3, 1.5–2 and 1–1.5 mgL−1DO, respectively. The decreasing degree of PS in EPS was more than that of PN content with the decrease of DO concentration. Generally, PN is positively charged due to amino group hydrolysis, whereas the PS has negative charges relating to of hydroxyl group hydrolysis (Yan., 2015). The PN/PS ratio in LB- or TB-EPS heightened as the DO concentration decreased. Higher PN/PS ratio demonstrated that EPS had a higher positive charge. Low PN/PS ratio was beneficial to biofilm stability (Miao., 2018). The variation of PN/PS ratio illustrated that low DO concentration at aerobic phase led to a negative effect the biofilm stability, which could affect the nitrogen removal performance of SBBR treating mariculture wastewater.
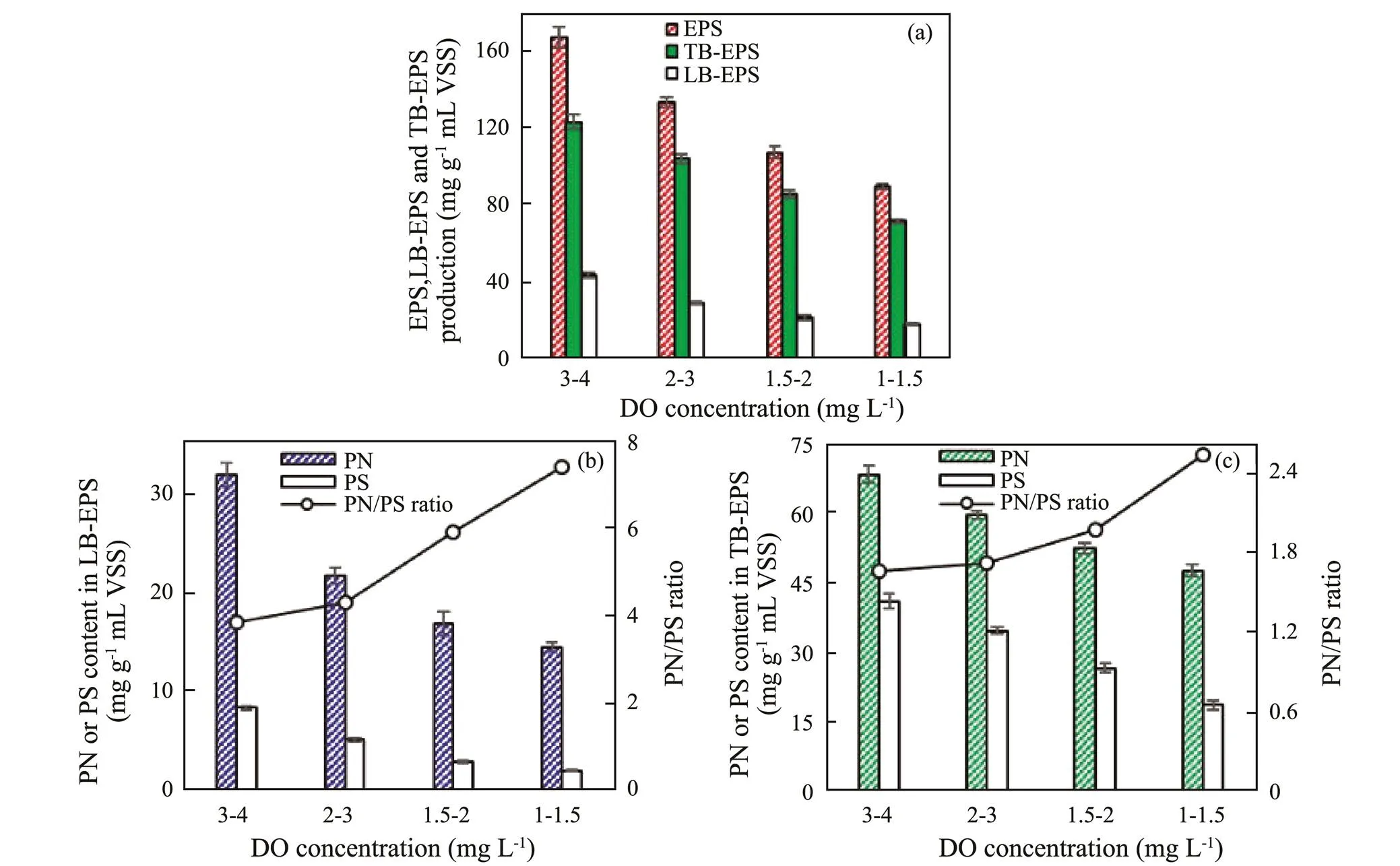
Fig.4 Effect of DO concentration at aerobic phase on the EPS production and composition from biofilm. (a) EPS, LB-EPS and TB-EPS contents, (b) PN content, PS content and PN/PS ratio in LB-EPS, and (c) PN content, PS content and PN/PS ratio in TB-EPS. The EPS production and composition at 3–4, 2–3, 1.5–2, and 1–1.5mgL−1 DO were measured on day 20, 40, 64, and 93, respectively. Error bars represent standard deviations determined from measurements performed in triplicate.
3.5 Effect of DO Concentration at Aerobic Phase on Microbial Richness and Diversity
Table 1 shows that the effective sequence was catalogized as 585, 566, 587 and 612 operational taxonomic units (OTUs) at 3–4, 2–3, 1.5–2 and 1–1.5mgL−1DO during aerobic phase on day 20, 40, 64, and 93, respectively. The Shannon and Simpson indexes displayed an increment trend with DO concentration from 3–4 to 1–1.5mgL−1at aerobic phase, respectively. However, the Chao 1 and ACE indexes firstly decreased from 3–4 to 2–3mgL−1DO and subsequently increased from 1.5–2 to 1–1.5mgL−1DO. As shown in Fig.5, the common OTUs were 514 among the obtained samples, which illustrated that many microorganisms always existed in SBBR. The common OTUs were catalogized to be Proteobacteria (39.5%), Bacteroidetes (17.9%), Chloroflexi (13.4%), Planctomycetes (11.7%), Actinobacteria (3.1%), Spirochaetes (2.1%), Firmicutes (1.2%), Latescibacteria (1.2%), Acidobacteria (1.2%), Verrucomicrobia (1.2%) and others (7.6%). The unique OTU number relating special microorganisms was 74, 54, 56 and 92 at 3–4, 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively. Additionally, the common OTUs also appeared between two DO concentrations or among three DO concentrations.
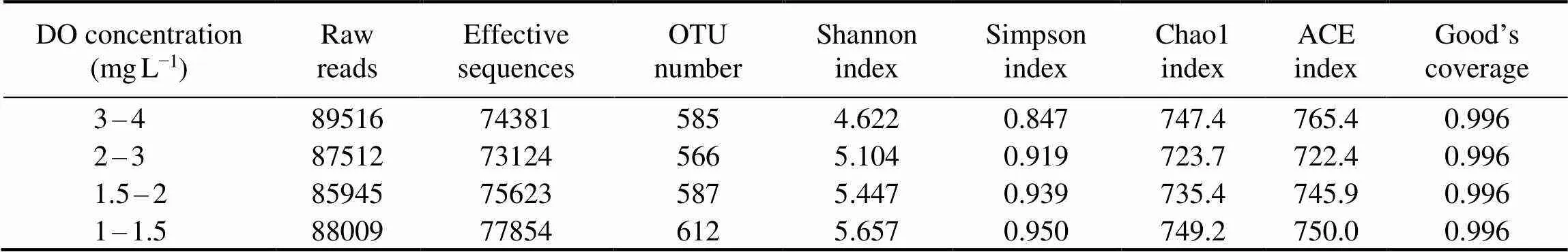
Table 1 Microbial richness and diversity of SBBR under different DO concentrations
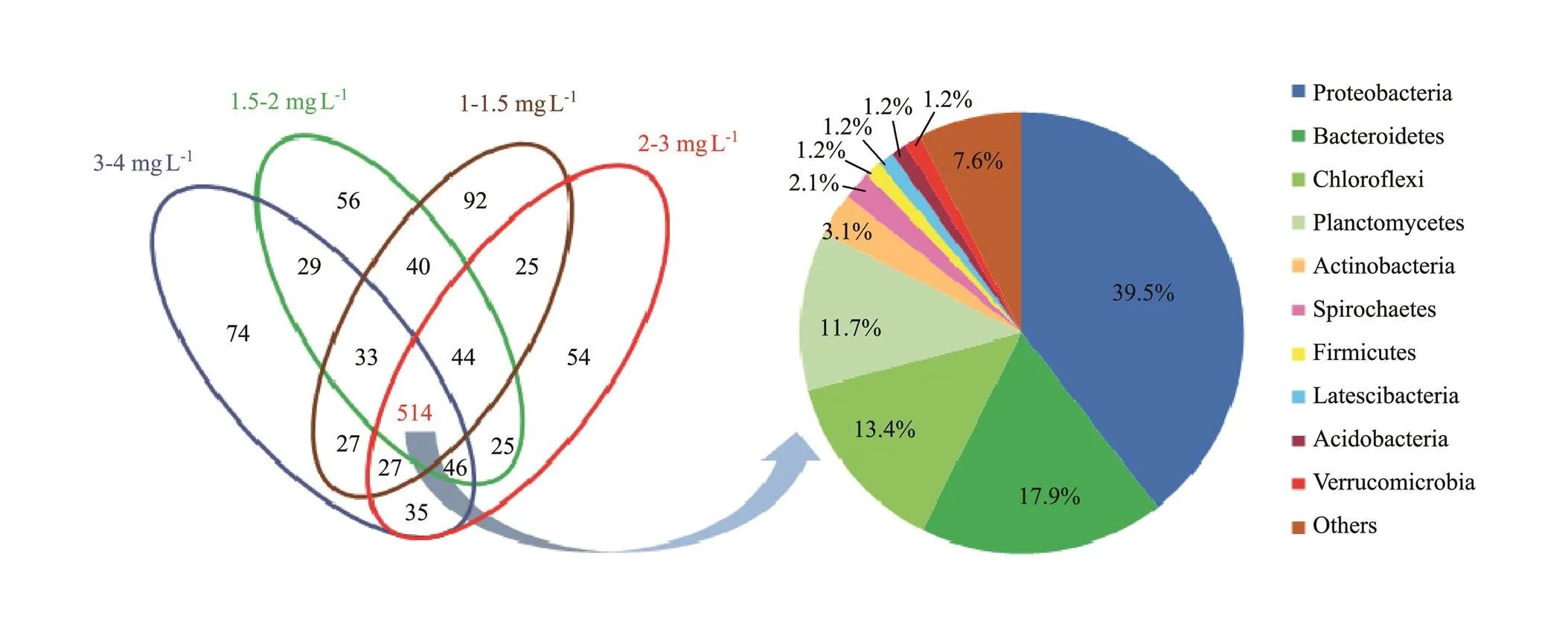
Fig.5 Venn diagram of microbial community at different DO concentrations. The shared OTUs were analyzed at the phylum level.
3.6 Effect of DO Concentration at Aerobic Phase on Microbial Significant Differences
To further investigate the microbial community under different DO concentrations at aerobic phase, the microbial significant differences were investigated by LEfSe analysis. The circle from inner to outer in cladogram was successively phylum, class, order, family and genus, respectively (Fig.6a). The nodes marked with different colors on the circle were considered to be significant biomarkers. As shown in Fig.6b, a total of 42 biomarkers for all the DO concentrations exhibited significant difference (< 0.05) with an LDA threshold of 3.5, which included 3 phylum, 4 class, 9 order, 9 family, and 17 genera. Among these, Bacteroidetes was significantly enriched at 3–4 mgL−1DO concentration, while Proteobacteria and Planctomycetes were more abundant at 1–1.5mgL−1DO concentration. Bacteroidetes, Proteobacteria, and Planctomycetes are common in bioreactor, which are reported to have the capabilities of nitrogen removal (Wang., 2019; Liu., 2020). Planctomycetacia and Alphaproteobacteria were markedly enriched at 1–1.5 and 1.5–2mgL−1DO, respectively. Bacteroidia and Gammaproteobacteria were two kinds of classes with obvious difference at 3–4mgL−1DO. Many genera identified as biomarkers displayed relatively high abundance (>0.1%), such as,,,,, and, and they were closely related to nitrogen removal (Hu., 2000; Kaye., 2004; Fahrbach., 2006; Trubitsyn, 2014; Sun., 2015; Miao., 2017). These findings suggested that the significant differences in the microbial distribu- tion under different DO concentrations reflect the variation of microbial community in the SBBR.
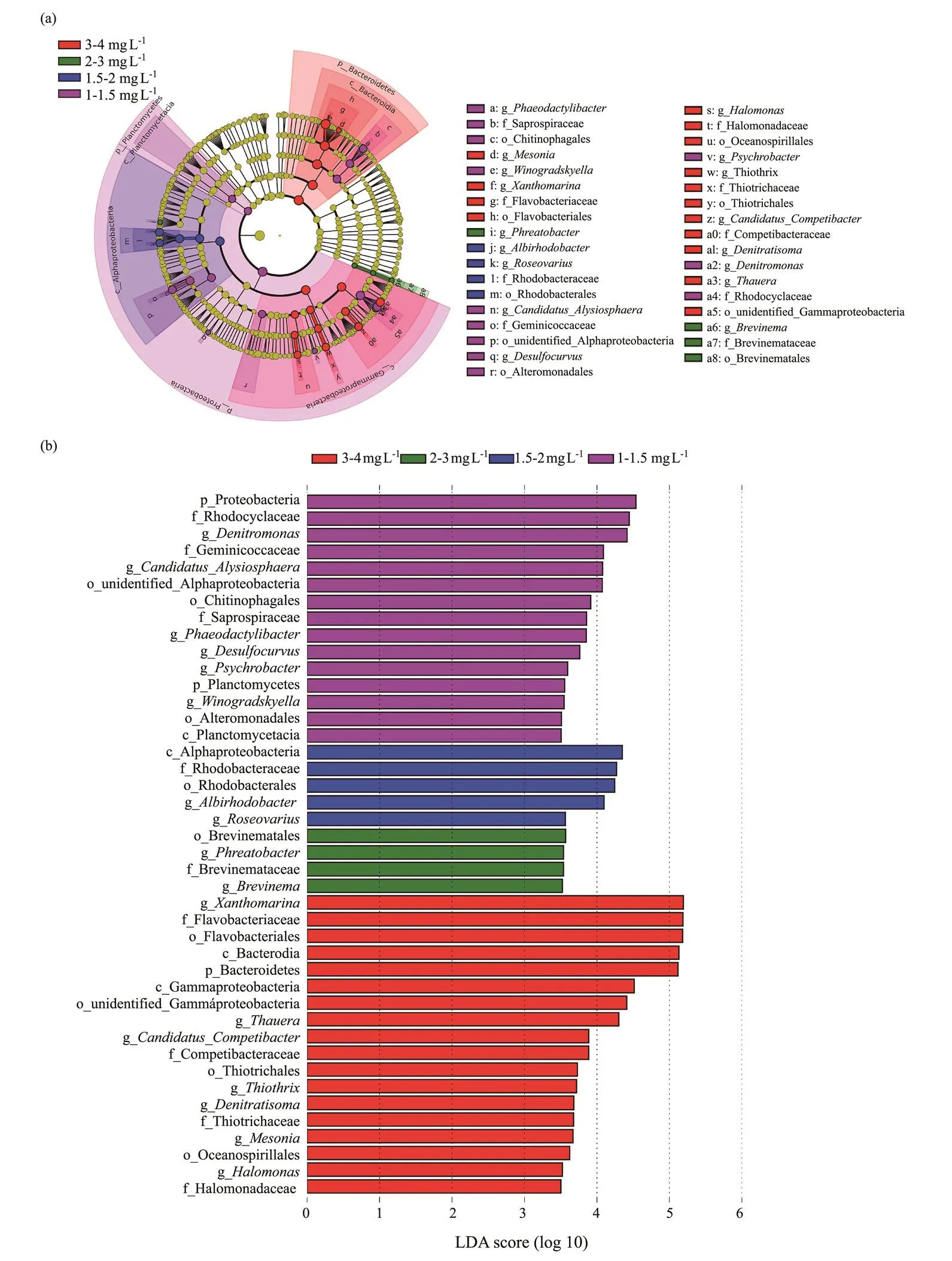
Fig.6 Linear discriminant analysis (LDA) coupled with effect size algorithm (LEfSe) analysis showing the different biomarkers at different DO concentrations. (a), Cladogram of all the samples; (b), LDA score of the biomarkers from all the samples.
3.7 Effect of DO Concentration at Aerobic Phase on Microbial Co-Occurrence and Keystone Taxa
The variation of environmental factors can lead to the difference of microbial network co-occurrence and biotic interaction (Ya., 2021; Yuan., 2021). When DO at 3–4mgL−1, the microbial networks based on the genus correlation of bacteria genus under different DO concentrations was established at 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively. As illustrated in Fig.7, the edge number was 115, 353 and 372 at 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively, demonstrating that the network complexity gradually increased with the decrease of DO concentration. Additionally, some topological parameters at different DO concentrations were investigated to analyze the network stability (Table 2). Compared with 2–3 and 1–1.5mgL−1DO, the microbial network at 1.5–2mgL−1DO had more average neighbor number (19.027), higher network density (0.529) and clustering coefficient (0.818), and shorter characteristic path length (1.634), suggesting that these genera at 1.5–2mgL−1had a closer connection. Additionally, most positive associations of microbial network are ascribed to the species cooperative behaviors of ecosystem, including parasitism, commensalism and mutualism, while the negative associations are responsible for predation, amenalism, and competition (Faust and Raes, 2012). The positive correlations accounted for 51.3%, 49.0%, and 54.3% of total microbial associations at 2–3, 1.5–2 and 1–1.5mgL−1DO, respectively. Hence, different DO concentrations at aerobic phase could lead to the difference of ecosystem structure in the SBBR.
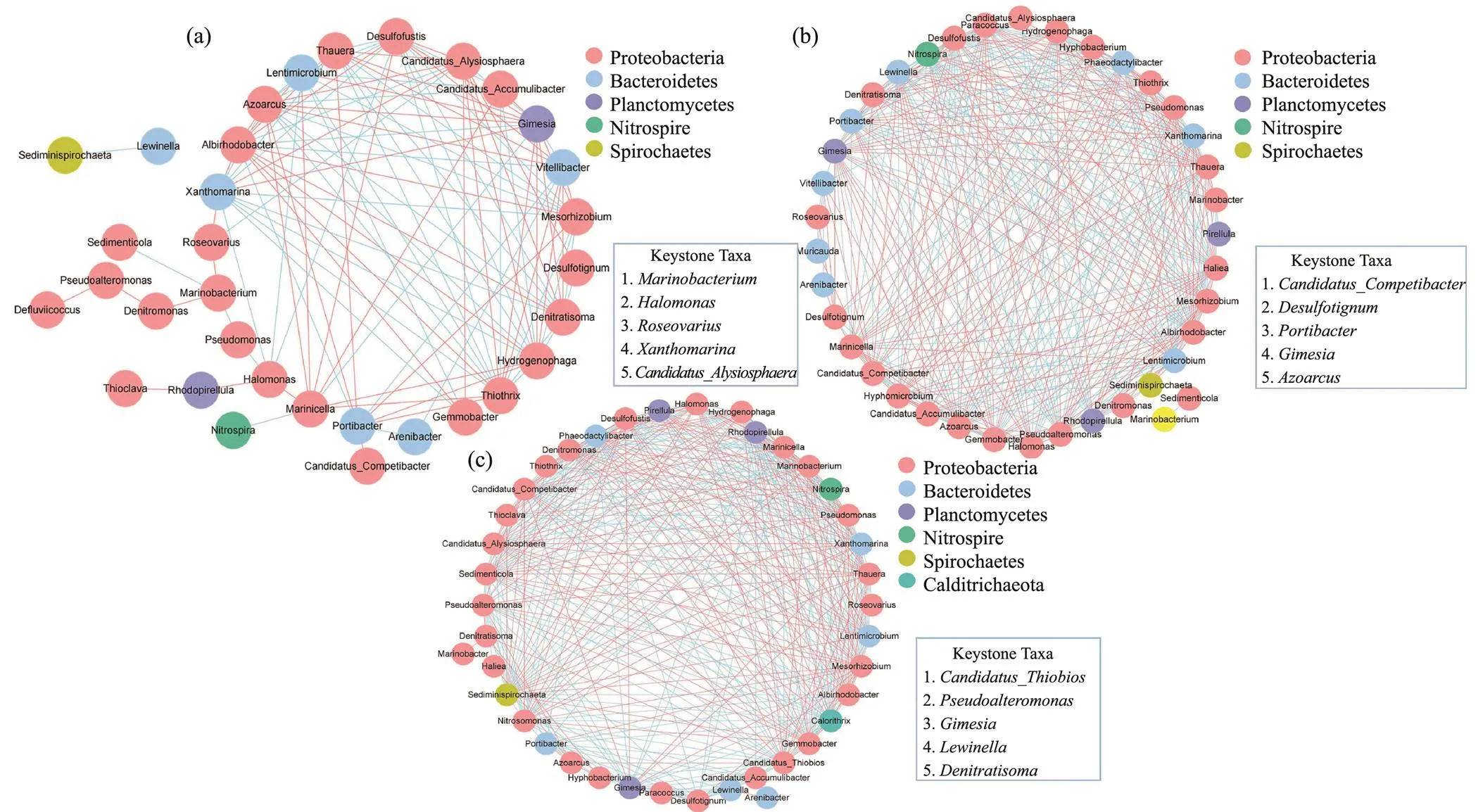
Fig.7 Network analysis showing bacterial co-occurrence and modular patterns under different DO concentrations. (a), DO concentration from 3–4mgL−1 to2–3mgL−1; (b), DO concentration from 3–4mgL−1 to 1.5–2mgL−1; (c), DO concentration from 3–4mgL−1 to 1–1.5mgL−1. The genera with relative abundances greater than 0.1% at least 1 sample were selected. Blue solid lines and red lines represent significantly strong positive (r>0.9) and negative (r<−0.9) linear relationships, respectively.
The network-based approach can clearly show the microbial integrated association and better understand the structure and function of microbial communities, especially keystone taxa (Berry and Widder, 2014; Banerjee., 2016). As shown in Table 3, the keystone taxa were obviously different under different DO concentrations due to high betweenness centrality. The keystone taxa includ- ed,,,andat 2–3mgL−1DO,,,,andat 1.5–2mgL−1DO, and,,,andat 1–1.5mgL−1DO. The network structure had strong linkages with ecosystem function, and the altered network could result in difference in the role of individual members (Yuan., 2021). Some denitrifying genera were considered as keystone taxa with the decrease of DO concentration, such as,, and. The relative abundance of aerobic denitrifying generaandde- clined as the decrease of DO concentration due to the in- sufficiency of DO.was the keystone taxa at 1.5–2 and 1–1.5mgL−1DO, and its relative abundance gra- dually increased with the decrease of DO concentration. It was the main reservoir ofthat are responsible for dissimilatory nitrite reduction, thereby resulting in the accumulation of effluent NH4+-N (Guo., 2021). Previous studies reported that low abundance genera disproportionately impacted on the various ecosystems (Zhao., 2021), which happened to similar results in this study.
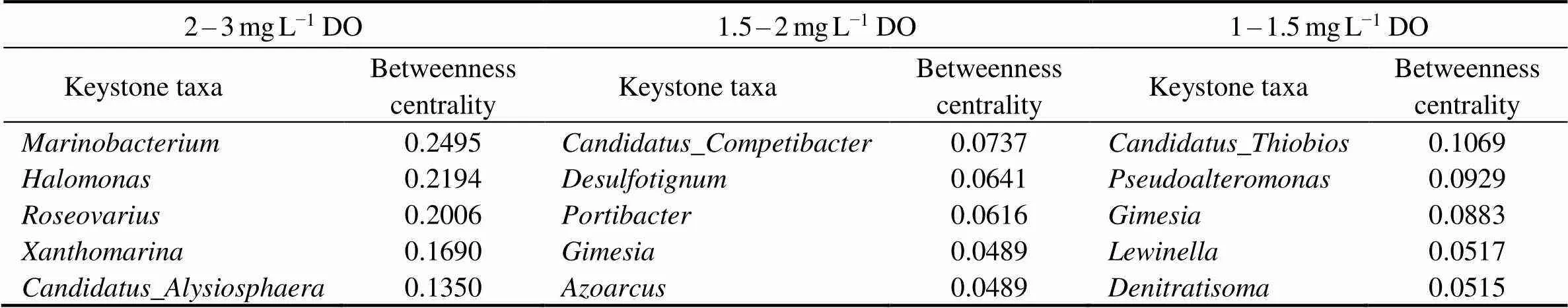
Table 3 Betweenness centrality of the keystone taxa under different DO concentrations
4 Conclusions
The impact of DO at aerobic phase on the performance, microbial activity and microbial community of SBBR were evaluated in treating mariculture wastewater. The SOUR, SAOR and SNOR decreased with the decrease of DO concentration, whereas the SNIRR and SNRR had opposite results, suggesting that lower DO concentration was unfavorable to the nitrification process. The activities of nitrification and denitrification enzymatic activities determined the nitrifying and denitrifying rates, respectively. The PN and PS content in EPS declined as DO concentration decreased, whereas the PN/PS ratio increased. The variation of DO concentration obviously varied the microbial abundance and microbial diversity and then further affected the SBBR performance. The microbial co-occurrence, keystone taxa and significant difference illustrated some variations under different DO concentrations.
Acknowledgements
The work was supported by the National Natural Science Foundation of China (No. 52070172), and the Fundamental Research Funds for the Central Universities (No. 201964003).
APHA, 2005.. 21st ed. American Public Health Association,Washington, D. C., USA, 1-22.
Banerjee, S., Kirkby, C. A., Schmutter, D., Bissett, A., Kirkegaard, J. A., and Richardson, A. E., 2016. Network analysis reveals functional redundancy and keystone taxa amongst bacterial and fungal communities during organic matter decomposition in an arable soil., 97: 188-198.
Berry, D., and Widder, S., 2014. Deciphering microbial interactions and detecting keystone species with co-occurrence networks., 5: 219.
Davidson, J., Helwig, N., and Summerfelt, S. T., 2008. Fluidized sand biofilters used to remove ammonia, biochemical oxygen demand, total coliform bacteria, and suspended solids from an intensive aquaculture effluent., 39: 6-15.
Eding, E. H., Kamstra, A., Verreth, J. A. J., Huisman, E. A., and Klapwijk, A., 2006. Design and operation of nitrifying trickling filters in recirculating aquaculture: A review., 34: 234-260.
Fahrbach, M., Kuever, J., Meinke, R., Kämpfer, P., and Hollender, J., 2006.gen. nov., sp. nov., a 17β-oestradiol-degrading, denitrifying betaproteobacterium.,56 (7): 1547-1552.
FAO, 2020.. Food and Agriculture Organization of the United Nations.
Faust, K., and Raes, J., 2012. Microbial interactions: from networks to models., 10 (8): 538- 550.
Fontenot, Q., Bonvillain, C., Kilgen, M., and Boopathy, R., 2007. Effects of temperature, salinity, and carbon: Nitrogen ratio on sequencing batch reactor treating shrimp aquaculture wastewater.,98: 1700-1703.
Gao, F., Li, Z. W., Chang, Q. B., Gao, M. C., She, Z. L., Wu, J.,., 2018. Effect of florfenicol on performance and microbial community of a sequencing batch biofilm reactor treating mariculture wastewater., 39 (3): 363-372.
Gao, Y., Li, Y., Ren, L., Sha, M. Q., Lv, D. Q., Wang, S.,., 2021. Bio-clogging mitigation in vertical subsur- face flow constructed wetlands using rhamnolipids-citric acid compound., 426: 131278.
Gao, Y. F., Zhang, C. S., Rong, H. W., Zheng, G. L., and Zhao, L. M., 2017. The effect of dissolved oxygen concentration (DO) on oxygen diffusion and bacterial community structure in moving bed sequencing batch reactor (MBSBR)., 108: 86-94.
Grab, R., Avnimelech, Y., Defoirdt, T., Bossier, P., and Verstraete, W., 2007. Nitrogen removal techniques in aquaculture for a sustainable production., 270: 1-14.
Guo, H. H., Gu, J., Wang, X. J., Song, Z. L., Qian, X., Sun, W.,., 2021. Negative effects of oxytetracycline and copper on nitrogen metabolism in an aerobic fermentation system: Characteristics and mechanisms., 403: 123890.
Hu, T. L., and Kung, K. T., 2000. Study of heterotrophic nitrifying bacteria from wastewater treatment systems treating acry- lonitrile, butadiene and styrene resin wastewater., 42 (3-4): 315-321.
Huang, F., Pan, L. Q., He, Z. Y., Zhang, M. Y., and Zhang, M. Z., 2021. Heterotrophic nitrification-aerobic denitrification characteristics and antibiotic resistance of two bacterial consortia fromandwith effective nitrogen removal in mariculture wastewater., 279: 111786.
Huang, Z. J., Kong, F. L., Li, Y., Xu, G. M., and Wang, S., 2020. Advanced treatment of effluent from municipal wastewater treatment plant by strengthened ecological floating bed., 309: 123358.
Kaye, J. Z., Marquez, M. C., Ventosa, A., and Baross, J. A., 2004.sp. nov.,sp. nov.,sp. nov. andsp. nov.: Halophilic bacteria isolated from deep- sea hydrothermal-vent environments., 54: 499-511.
Liang, Y. X., Zhu, H., Bañuelos, G., Yan, B. X., Zhou, Q. W., Yu, X. F.,., 2017. Constructed wetlands for saline wastewater treatment: A review., 98: 275-285.
Liu, S. L., Daigger, G. T., Liu, B. T., Zhao, W. Y., and Liu, J., 2020. Enhanced performance of simultaneous carbon, nitrogen and phosphorus removal from municipal wastewater in an anaerobic-aerobic-anoxic sequencing batch reactor (AOA- SBR) system by alternating the cycle times., 301: 122750.
Miao, L., Zhang, Q., Wang, S. Y., Li, B. K., Wang, Z., Zhang, S. J.,., 2018. Characterization of EPS compositions and microbial community in an Anammox SBBR system treating landfill leachate., 249: 108-116.
Miao, Y., Wang, Z., Liao, R. H., Shi, P., and Li, A. M., 2017. Assessment of phenol effect on microbial community structure and function in an anaerobic denitrifying process treating high concentration nitrate wastewater., 330: 757-763.
Pugazhendi, A., Alreeshi, G. G., Jamal, M. T., Karuppiah, T., and Jeyakumar, R. B., 2021. Bioenergy production and treatment of aquaculture wastewater using saline anode microbial fuel cell under saline condition., 21: 101331.
Sheng, G. P., Yu, H. Q., and Li, X. Y., 2010. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review., 28: 882-894.
Song, C. G., Zhao, C. K., Wang, Q. Z., Lu, S. L., She, Z. L., Zhao, Y. G.,., 2021. Impact of carbon/nitrogen ratio on the performance and microbial community of sequencing batch biofilm reactor treating synthetic mariculture wastewater., 298: 113528.
Sun, J. Q., Liu, L. F., and Yang, F. L., 2020. Successful bio- electrochemical treatment of nitrogenous mariculture wastewater by enhancing nitrogen removalsynergy of algae and cathodic photo-electro-catalysis., 743: 140738.
Sun, L., Zhao, X. X., Zhang, H. F., and Zhang, Y. Q., 2015. Biological characteristics of a denitrifying phosphorus-accu- mulating bacterium., 81: 82-88.
Trubitsyn, I. V., Belousova, E. V., Tutukina, M. N., Merkel, A. Y., Dubinina, G. A., and Grabovich, M. Y., 2014. Expansion of ability of denitrification within the filamentous colorless sulfur bacteria of the genus., 358 (1): 72-80.
Wang, J. Y., Rong, H. W., and Zhang, C. S., 2018. Evaluation of the impact of dissolved oxygen concentration on biofilm microbial community in sequencing batch biofilm reactor., 125 (5): 532-542.
Wang, S., Gao, M. C., She, Z. L., Zheng, D., Jin, C. J., Guo, L.,., 2016. Long-term effects of ZnO nanoparticles on nitrogen and phosphorus removal, microbial activity and microbial community of a sequencing batch reactor., 216: 428-436.
Wang, X. C., Shen, J. M., Kang, J., Zhao, X., and Chen, Z. L., 2019. Mechanism of oxytetracycline removal by aerobic gra- nular sludge in SBR.,161: 308-318.
Ya, T., Du, S., Li, Z. Y., Liu, S. D., Zhu, M. H., Liu, X. J.,., 2021. Successional dynamics of molecular ecological network of anammox microbial communities under elevated salinity., 188: 116540.
Yan, L. L., Liu, Y., Wen, Y., Ren, Y., Hao, G. X., and Zhang, Y., 2015. Role and significance of extracellular polymeric substances from granular sludge for simultaneous removal of organic matter and ammonia nitrogen., 179: 460-466.
Yuan, M. M., Guo, X., Wu, L. W., Zhang, Y., Xiao, N. J., Ning, D. L.,., 2021. Climate warming enhances microbial network complexity and stability., 11: 343-348.
Zhao, X., Yang, Y., Feng, K., Wang, X. H., Liu, B. F., Xie, G. J.,., 2021. Self-regulating microbiome networks ensure functional resilience of biofilms in sand biofilters during manganese load fluctuations., 188: 116473.
(May 31, 2022;
October 7, 2022;
December 14, 2022)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
E-mail: mengchungao@outlook.com
E-mail: zhangzhiming@ouc.edu.cn
(Edited by Ji Dechun)
杂志排行
Journal of Ocean University of China的其它文章
- Effects of 5-Azacytidine (AZA) on the Growth, Antioxidant Activities and Germination of Pellicle Cystsof Scrippsiella acuminata (Diophyceae)
- Using Rn-222 to Study Human-Regulated River Water-Sediment Input Event in the Estuary
- Parameterization Method of Wind Drift Factor Based on Deep Learning in the Oil Spill Model
- YOLOv5-Based Seabed Sediment Recognition Method for Side-Scan Sonar Imagery
- A Metadata Reconstruction Algorithm Based on Heterogeneous Sensor Data for Marine Observations
- Large Active Faults and the Wharton Basin Intraplate Earthquakes in the Eastern Indian Ocean
