Extraction of Natural Nanostructured Hydroxyapatite from Pacific Cod (Gadus macrocephalus) Bone with a Thermostable Collagenolytic Protease and Its ex vivo Intestinal Bioavailability
2023-12-21GUOWeiLIShiyangJINGZhehuaandWUHaohao
GUO Wei, LI Shiyang, JING Zhehua, and WU Haohao, *
Extraction of Natural Nanostructured Hydroxyapatite from Pacific Cod () Bone with a Thermostable Collagenolytic Protease and ItsIntestinal Bioavailability
GUO Wei1), LI Shiyang2), JING Zhehua2), and WU Haohao2), *
1),,264003,2),,266003,
Natural nano-hydroxyapatite (HA) was extracted from Pacific cod () bone with a thermostable col-lagenolytic proteasein the present study. Conditions for the enzymatic reaction were optimized to be 60℃ and pH 7.0, and a desir- able extraction efficiency was achieved by using the crude collagenolytic protease.Dynamic light scattering, transmission electron microscopy and energy-dispersive X-ray analysis revealed that nano-HA are anionic spherical (about 110nm) particles mainly com- prised of calcium and phosphorus at an approximate ratio of 5:3. As evaluated with the mouseintestinal segments, the extracted nano-HA displayed comparable level of intestinal bioavailability to the positive control CaCl2. By treating with inhibitors (NaN3, ami- loride) and low temperature (4℃), clathrin-mediated endocytosis was assumed to involve the intestinal absorption of nano-HA. Over- all, the application of thermostable collagenolytic protease is proved to be a promising alternative method for nano-HA extraction from natural resource with improved ecological and biological value.
nano-hydroxyapatite; thermostable collagenolytic protease; Pacific cod () bone; intestinal bio-availability
1 Introduction
Calcium plays multifarious physiological roles in human body, including bone/tooth formation, cell division, signal transmission and hormone secretion. However, according to a global map of average dietary calcium intake,many countries, in particular Asian and African countries, have an average dietary calcium intake of less than 600mgd−1(Balk., 2017), resulting in billions of people sufferingfrom calcium deficiency. Long-term calcium deficiency in- duces dental deterioration, cataracts, cognitive symptoms, and osteoporosis, which increases the possibility of osteo- porotic fractures (Bove-Fenderson and Mannstadt, 2018). Early stages of calcium deficiency usually donot show re- markable clinical features and thus are difficult to be diag- nosed. Unawareness and lack of treatment, however, could be life-threatening. Taking calcium supplements to comple- ment dietary calcium intake has been widely recommend- ed in common clinical practice. While various calcium sup-plements and fortified food are available on the market,the absorption rates are reported to be less than 20% for most of them (Khashayar., 2015). Recent development in nanotechnology provides a new resolution for such dilem- ma and the formulation of dietary supplements has been exa- mined in nanoscale. Nano-sized calcium demonstrates high-er intestinal absorption compared with conventional formu- la and is therefore believed to be an effective supplement for calcium metabolism(Jampilek., 2019).
Fish processing industry produces numerous amounts ofby-products. In 2016, over 12.32 million tons of fish by-pro- ducts were derived from processing around the world (Cop-pola., 2021; FAO, 2021). These by-products were tra- ditionally converted toanimal feed andsoil fertilizer with limited economical value.Fish bone accounts for 10%–15% of total fish weight and more than 50% of the by-pro- ducts. So far, there has not been a consistent conclusion about how to valorize fish bone with proper treatments. Fish bone is mainly composed of nano-hydroxyapatite (HA) cry- stals within collagen fibrils, and hydroxyapatite is consider- ed an excellent bioavailable form of calcium(Kim and Men-dis, 2006). Attentions have therefore been devoted to tak- ing fish bone as a source of nano-HA.
Enzyme-catalyzed hydrolysis is a promising alternative to calcination in the extraction of nano-HA. We have report- ed a thermostable collagen-degrading strain of() with optimized con- ditions for collagenolytic protease production in a previous study (Jing., 2017). Here, the effects of temperature, pH, divalent ions, and inhibitors on the enzyme activity ofthis thermostable collagenolytic protease were evaluated in the extraction of nano-HA from cod bone. The extracted na- no-HA was then characterized with dynamic light scatter- ing (DLS), transmission electron microscopy (TEM) and energy-dispersive X-ray (EDX) analyses. Finally, the ab- sorption property of the nano-HA particle was measured by theabsorption experiment.
2 Materials and Methods
2.1 Materials
Pacific cod () bone was provided by Qingdao Longharmony Food Co., Ltd. (Qingdao, Chi- na) and stored at −20℃. The fish bone was boiled for 5minto remove the residual tissues and impurities after thawing at room temperature. Then the bone was cut into approxi- mately 2cm pieces and washed 3 times by ultrapure water. The cleaned pieces were ground and freeze-dried to obtain the fish bone powder.
was isolated from the fermentative solid waste anddeposited in our laboratory.
The bone powder medium contains glucose monohydrate (2.77gL−1), K2HPO4(1.92gL−1), NaH2PO4·2H2O (0.5gL−1), NaCl (5gL−1) and cod bone powder (20gL−1).
All reagents were of analytical grade and bought from commercial suppliers.
2.2 Extraction of the Crude Collagenolytic Protease
Briefly,strain was fermented at op-timum conditions (pH=8.0 and=50℃) for 16h in Try-ptic Soy Broth (TSB) (Hopebio-Technology Co., Ltd., Shan-dong, China) (Jing., 2017). Then the sample was cen- trifuged at 8000for 20min at 4℃ to remove the bacteria and debris. The cell-free supernatant was used as the crude enzyme solution and stored at −20℃.
2.3 Effect of Temperature, pH, Divalent Ions, and Inhibitors on Enzyme Activity
The enzyme activity was determined with Azocoll as a substrate. In brief, 0.01g Azocoll, 2.5mL Tris-HCl buffer (pH 7.0) and 1mL protease solution were mixed. The mix- ture was incubated for 24h at 60℃ with shaking (150rmin−1)and then the reaction was halted in ice bath for 10min. Af- ter centrifugation at 8000for 10min at 4℃, the absor- bance of the resulted supernatant was determined at 520nm with a microplate reader (Bio-tek, Vermont, USA). One unit of enzyme activity was defined as the amount of pro- tease that hydrolyzes 1μg Azocoll per minute. The relative enzyme activity was represented as the activity ratio between the specific and the optimum condition (60℃ and pH 7.0).
The effect of temperature on the protease activity was de- termined at temperatures ranging from 50℃ to 70℃. The mixture would be kept in 60℃ water bath for 4h before measuring if the reaction was not conducted at 60℃. The effect of pH on the enzyme activity was investigated at pH=5.0–9.0 with different buffersunder the standard condi- tions. The stability of the protease was also examined by pre- incubating crude enzyme in the presence of ethylenediami- netetraacetic acid (EDTA), phenylmethanesulfonyl fluoride (PMSF) and bivalent ions (10mmolL−1). Enzyme activity assays were then performed under standard conditions.
2.4 Extraction of the HA
The HA was acquired by fermenting the bone powder withstrain or crude enzyme. When fer- mented with the strain, a 5mL suspension of(1×1010cfumL−1) was added to 95mL of bone powder solution and the reaction was conducted at pH 7 and 60℃ with shaking (150rmin−1). When fermented withthe crude enzyme, 100mL crude enzyme solution and 1.9g cod bone powder were mixed, and the mixture was ferment- ed at standard conditions with shaking. Fermentation brothwas sampled every day in a week. The samples were filter- ed with 0.22µm membrane to characterize the natural nano- HA.
To calculate the nano-HA concentration, the total and free calcium of the filtered broth were measured. The total cal- cium was determined by atomic absorption spectrophoto- meter after digestion, while the free calcium was determin- ed by the same method after ultrafiltration with a 3000Da cutoff membrane. The difference of the value was taken as the nano-calcium concentration (which was used to repre- sent the content of nano-HA).
2.5 Characterization of the HA
The filtered nano-HA solution was ultrafiltered with a 3000Da cutoff membrane and then diluted with ultrapure water. The ultrafiltration and dilution were repeated until no phosphorus was detected in the filtrate. The resulted na- no-HA was used for subsequent analyses.
DLS and TEM were utilized to characterize the nano-HA. DLS was performed with Zetasizer Nano ZS 90 (Malvern Instruments, Herrenberg, Germany). For TEM observations, sample solutions were dropped on the carbon-coated cop- per grids and air-dried at room temperature, followed by ob- serving under a JEM-2100Plus (JEOL, Tokyo, Japan) elec- tron microscope at 100kV. The EDXanalysis was carried out on an FEI-Tecnai G2TF20 to gain the elemental compo- sition of the HA.
2.6 Ex vivo Intestinal Bioavailability of Nano-HA
C57BL/6 mice were purchased from Jinan Pengyue Ex-perimental Animal Breeding Co., Ltd. (Jinan, China). Ani- mals were individually housed in an air-conditioned room maintained at 20–24℃ with a 55%–65% relative humidi- ty. All mice were fed with NIH-41 standard diet (Trophic Animal Feed High-Tech Co., Ltd., Nantong, China). All ex- periments were performed in accordance with the principlesof the National Institutes of Health (NIH)and were approved by the Committee on the Ethics of Animal Experiments of Ocean University of China (No. SPXY20210315).
Food was removed from the cages 12h before mice were euthanizedwith CO2suffocation followed by cervical dis- location. The abdomen was opened and the intestinal be- tween the stomach and the top of the cecum was removed. The ileum was separated and washed 3 times with sterile HBSS and then the contents were flushed.absorp- tion assay was carried out as follows: 0.4mL HBSS solution was instilled to the empty ileum. The ileum was ligatured at both ends and was then incubated with 10mL HBSS me- dia at 37℃. HBSS solution contained concentrated nano- HA or CaCl2(positive control) (both containing 5mmolL−1total calcium). For inhibition assays, 2mmolL−1amiloride (AMR) or 10mmolL−1NaN3was used. The temperature- dependence trial was performed by incubation at 4℃. The assays were terminated after 60min. The media was collect- ed, and calcium contents were measured by flame atomic absorption spectrometry (AA-6800, Shimadzu, Japan).
2.7 Statistical Analysis
Statistical analysis was conducted with SPSS 19.0 soft- ware. Results were displayed as means±SD and assessed by one-way ANOVA followed by Duncan’s new multiple range tests. Significant differences were defined at-va- lues<0.05.
3 Results
3.1 Optimization of Conditions for the Enzymatic Activity
The collagenolytic protease produced bywas active at all tested temperatures with an op- timum at 55℃, and there was no significant difference be-tween 55℃ (100%±3.70%) and 60℃ (97.32%±5.71%) (Fig.1a). The relative enzyme activity was dramatically re- duced to less than 70% when the temperature was increasedto 65℃. Since collagen degrades in temperatures above 60 ℃, 60℃ was selected for further experiments(Matteini., 2012).
The pH profiles of the protease were determined in five buffers with pH 5.0, pH 6.0, pH 7.0, pH 8.0 and pH 9.0. The enzyme demonstrated activity at all pH conditions with an optimum at pH 7.0 (Fig.1b), which was selected for the ex-traction of nano-HA. The relative enzyme activity was morethan 80% in the basic range, which is much higher than that in the acidic range (less than 40%). Thus, this protease ex-hibited activity for collagen hydrolysis mainly under the neu- tral or basic conditions.
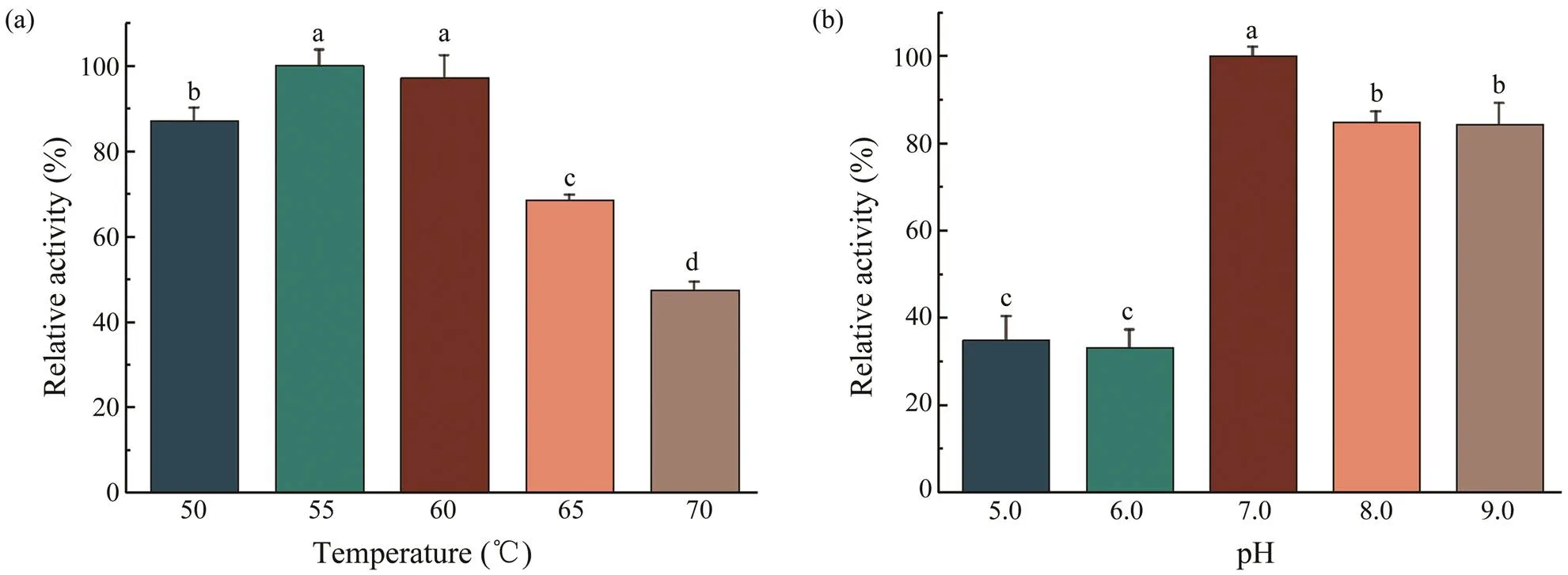
Fig.1 Effects of temperature and pH on the activity of the crude collagenolytic protease. (a) Effect of temperature on the activity of the collagenolytic protease. (b) Effect of pH on the activity of the collagenolytic protease. Data are expressed as means±SD (n=3) and assessed by one-way ANOVA followed by Duncan’s new multiple range tests. Different super- script letters (a–d) denote statistically significant differences (P<0.05).
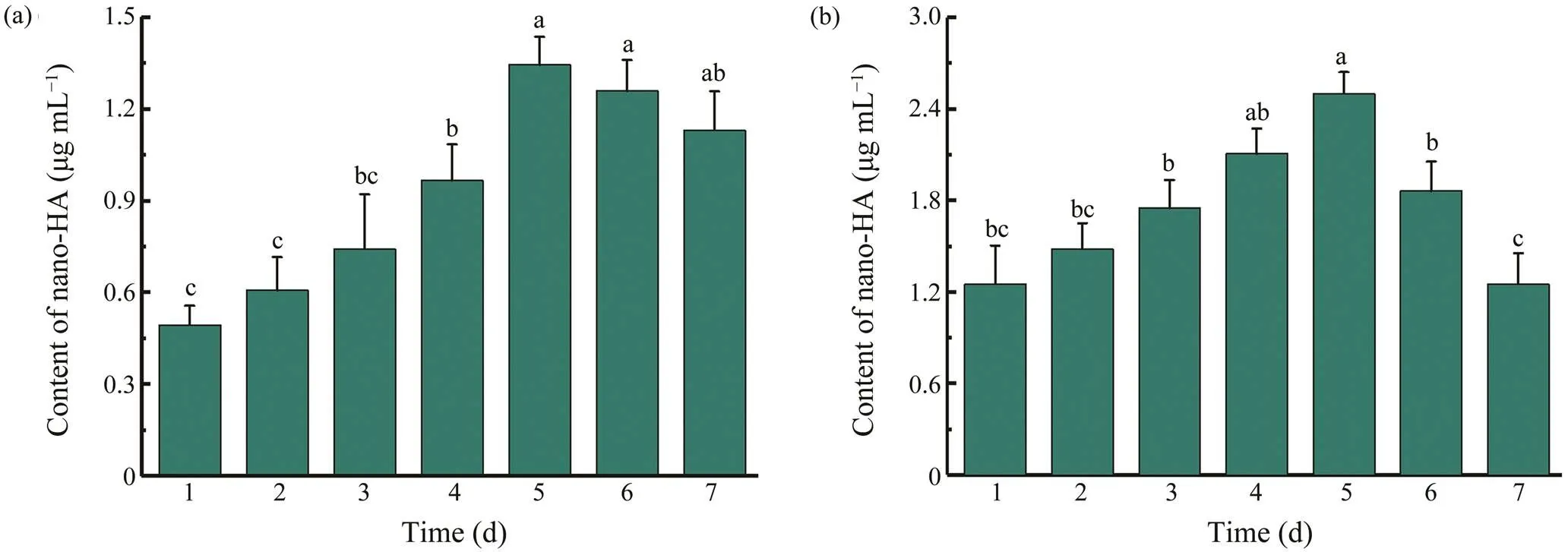
Fig.2 Extraction efficiencies of nano-HA from cod bone by (a) fermentation with B. glycinifermentans and (b) enzymolysis with the crude collagenolytic protease. Data are as means±SD (n=3) and assessed by one-way ANOVA followed by Duncan’s new multiple range tests. Different superscript letters (a–c) denote statistically significant differences (P<0.05).
Proteases could be classified according to their sensiti-vities to diverse inhibitors (Rao., 1998). In the pre-sence of EDTA, the collagenolytic protease activity de-creased to 54.67%±2.45%, while in the presence of PMSF, the relative enzyme activity decreased to 41.96%±1.69%(Table 1). Since EDTA and PMSF were inhibitors of me-talloproteinase and serine protease, respectively (Jaouadi., 2010), the results indicate that this enzyme requires both metallic and serine cofactors to catalyze collagen hy- drolysis. Hydrolysis of metalloprotease and serine proteasetakes place at various peptide bonds (Sorapukdee.,2020), thus the collagenolytic protease in the present study would show a broad range of specificity.
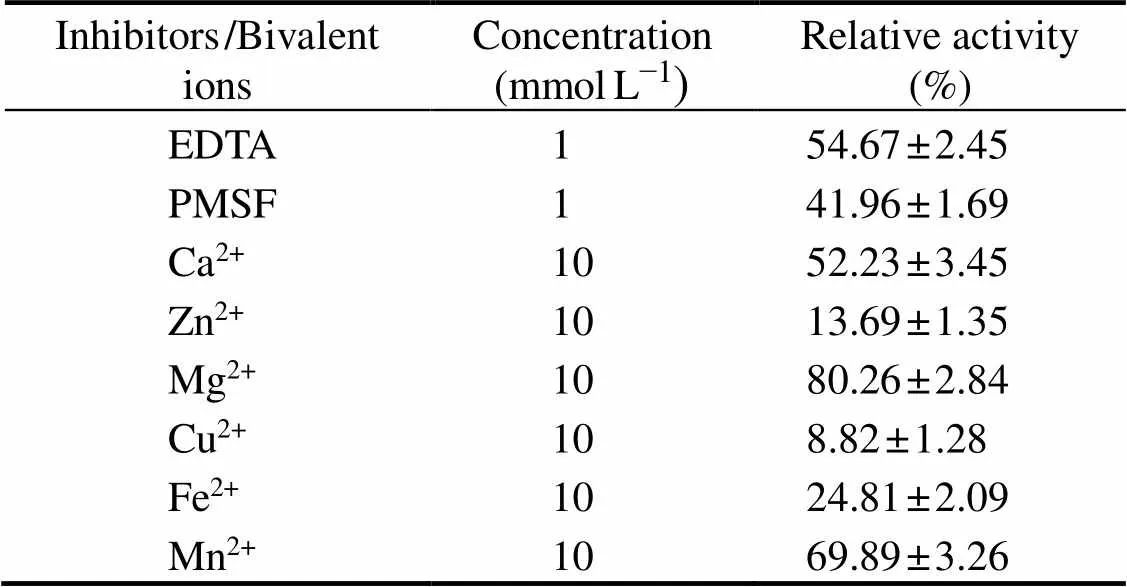
Table 1 Effects of protease inhibitors and bivalent ions on the activity of collagenolytic protease
Notes: Data are expressed as means±SD (=3) and assessed by one-way ANOVA followed by Duncan’s new multiple range tests.
Metal ions influence the protease activities by altering active binding sites and destabilizing enzyme structure, and the effects varied among proteases (Jaouadi., 2010;Aissaoui., 2017). As shown in Table 1, the relative en- zyme activity was sharply reduced to 8.82%±1.28% in the presence of Cu2+, while it was only decreased to 80.26%±2.84% in the presence of Mg2+. When the reactions were performed with divalent metal ions Ca2+, Zn2+, Fe2+, andMn2+, the relative enzyme activities were measured to be52.23%±3.45%, 13.69%±1.35%, 24.81%±2.09%, and 69.89%±3.26%, respectively. Therefore, the impact of me- tal ions on the activity of the protease is highly metal ion- dependent. The fish bone contained abundant Ca and tracemetals such as Fe, Zn and Cu (Luo., 2020), which needs to be considered in the fermentation.
3.2 Extraction Yield of Nano-HA
The extraction yield of nano-HA in the reaction mixtures was monitored during the 7-day fermentation of cod bone powder. As Figs.2a and 2b demonstrated,the contents of nano-HA continued to increase until day 5 and then de- creased in both the strain and crude enzyme broths. The aggregation of nano-HA at the later stage of fermentation might result in the decreased contents. Nanoparticles aggre-gation has been observed in many reported nanocomposites(Zare, 2016) and there is few efficient technologies to so- lve this issue. Compared withstrain sample (Fig.2a), broth with the rude enzyme (Fig.2b) ex- hibited a higher maximal content of nano-HA (2.50μgmL−1. 1.34μgmL−1), which was likely due to a higher concen- tration of collagenolytic protease. A similar increased trend was observed for the protein concentration in the broth fer-mented with the rude enzyme (data not shown).
3.3 Size and Morphology of Nano-HA
The TEM images of nano-HA in broths fermented with either thestrain or the crude enzyme were shown in Figs.3a and 3b, respectively. Both nano-HA nanoparticles extracted from fermenting with strain and crude enzyme were spherical and exhibited comparable dia- meter range with moderate polydispersity. Diameters of theparticles extracted with the strain ranged from 65nm to 159nm and the average particle diameter was 115 to 23nm. Dia- meters of the nano-HA extracted with the crude enzyme ranged from 69nm to 157nm with an average diameter of 113nm±29nm. There was no significant difference between the two results.
The size distribution of nano-HA in suspension was fur- ther checked by DLS. As presented in Figs.3c and 3d, the nanoparticles are normally distributed in a narrow size with one single peak. The z-average sizes of the nano-HA were measured to be 173nm±38nm (extracted with the strain) and 189nm±42nm (with the crude enzyme). DLS mea- sures hydrodynamic radius while TEM provides the actual particle radius, which can contribute to the noted varia- tion in the average particle sizes (Lim., 2014).
Whole area EDX mapping analysis revealed that both nano-HAs were composed mainly of calcium and phospho-rus with an estimated ratio of 5:3 (Figs.3e and 3f). Trace amounts of sodium and magnesium were also detected, and both of them were proved to be able to improve the bioac- tivity of the nano-HA (Sossa., 2018). The high Cu sig- nal can be attributed to the carbon-coated copper grids for TEM (Zhang and Su, 2009).
3.4 Ex vivo Intestinal Bioavailability of Nano-HA
permeability assay using ‘intestinal sacs’ is a ra- pid and sensitive method for evaluating intestinal transportof a specific molecule with the additional advantage of site specificity (Mateer., 2016). Endocytosis and non-spe- cific macropinocytosis are important mechanisms for the transcellular transport of nanoparticles in mammal intestine (Oh and Park, 2014). Here, AMR and NaN3were added to inhibit the macropinocytosis and energy-dependent endo- cytosis pathways, respectively. The intestinal bioavailabi- lity of nano-HA was calculated in terms of the calcium ab- sorption, and the results were displayed in Fig.4. The per- centage of nano-HA transported to parenteral medium was 28.7%±3.2%, which is comparable to the intestinal bio- availability of CaCl2(32.7%±2.0%,>0.05). The extract- ed nano-HA thus was considered to be well absorbed in the gut lumen. When treated with inhibitors, the intestinal bio- availability of group HA+NaN3were significantly decreased(20.9%±2.7%), while the group HA+AMR revealed no no-table differences compared with group nano-HA in calcium absorptivity (26.6%±2.8%). The results indicated that the absorption of nano-HA was likely dominated by the ener- gy-consuming clathrin-mediated endocytosis, instead of ma- cropinocytosis. Furthermore, a clear decline of calcium ab- sorption was observed with low temperature (4℃) treatment (Fig.4). The reduction was probably due to the reduced fluidity of cell membrane at 4℃, which might be another evidence that endocytosis is involved in the intestinal ab- sorption of nano-HA.
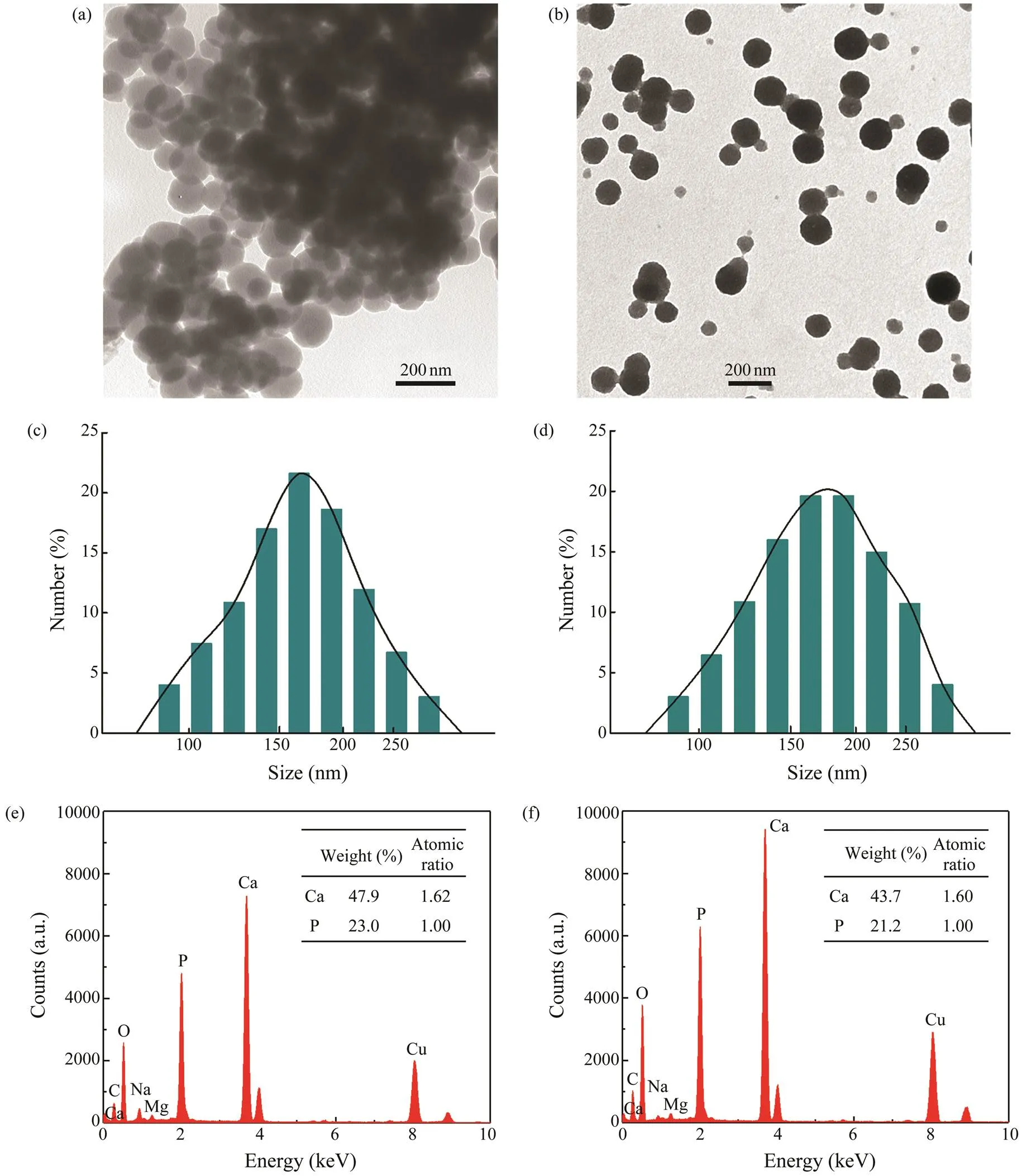
Fig.3 Transmission electron microscopy, dynamic light scattering and whole area energy-dispersive X-ray results for nano- HA extracted from the B.glycinifermentans strain (a, c, e), and nano-HA extracted with the crude collagenolytic protease (b, d, f).
4 Discussion
Conventional technologies to extract nano-HA from fish boneinclude calcination with or without acidic/basic treat- ment, hydrothermal synthesis, and laser ablation (Terzioğlu., 2018). The long-term heating involved in most of the methods above is accompaniedwith collagen degradation and energy consumption, and those acid-/base-assisted me- thods have posed problems to environmental protection and sustainability. Enzyme-catalyzed hydrolysis provides eco- friendly and mild processing conditions, resulting in protein recovery during the nano-HA extraction. Proteases utilized to degrade collagens usually are from mammals or bacte- ria(Zhang., 2015). Bacteria are ubiquitously distri- buted throughout the biosphere and some of them can sur- vive under extremely high temperature conditions. Colla- genolytic proteases fromthermophilic bacterium are ther- mally stable up to 50–60℃.Okamoto. (2001) puri- fied a collagenolytic protease from thermophilicsp. strain MO-1 at 60℃. The thermostable serine collage- nase separated by Petrova. (2006) was most active at 60–65℃. Zhang. (2022) reported the optimal tem- perature to be 70℃ for the enzyme mTSS against Azocoll. From the results of the present study, the optimum reactioncondition for collagenase is at 55–60℃ (Fig.1a), which will facilitate a more efficient separation of HA from collagen (Matteini., 2012).
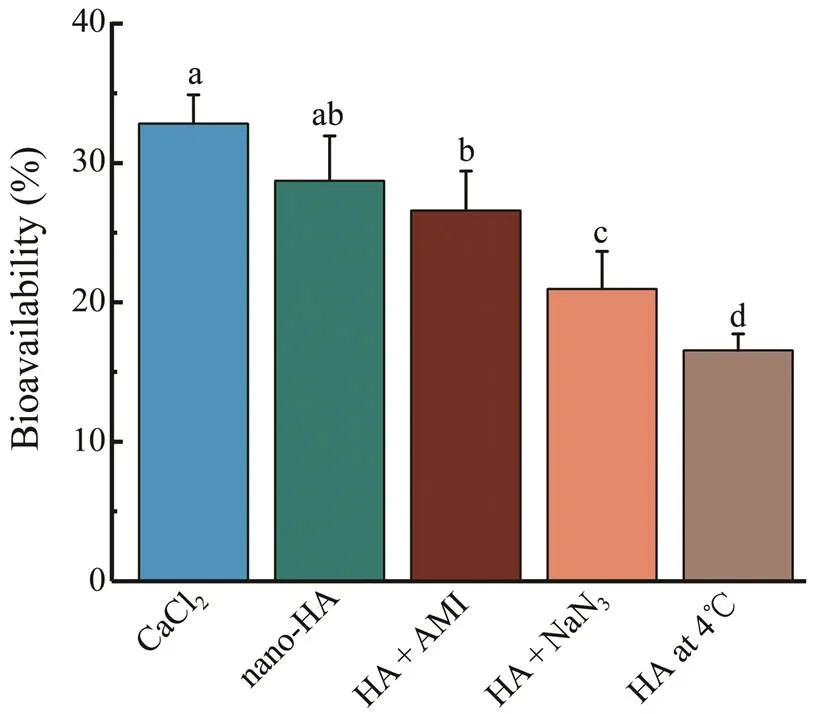
Fig.4 Effects of amiloride (AMI), NaN3 and low tempera- ture (4℃) treatments on intestinal bioavailability of nano- HA ex vivo. The neutral HBSS containing 5mmolL−1 CaCl2was taken as the positive control. Data are expressed as means±SD (n=3) and assessed by one-way ANOVA fol- lowed by Duncan’s new multiple range tests. Different su- perscript letters (a–d) denote statistically significant diffe- rences (P<0.05).
Electron microscopy analysis shows fish bone-based ma-terials of different shapes (Terzioğlu., 2018). In the pre- sent study, the Pacific cod bone was enzymatically disso- ciated, and the resulted HA particles manifested a sphericalmorphology (Figs.3c and 3d). Similar morphology was dis- played in the previous reports of Jin. (2011) and Yin. (2015), in which grinding, surfactant and thermal treat- ment have been experimented. Flake-like (Ivankovic.2010) and rod-like (Boutinguiza., 2012) were alsocommon morphology. It was reported that the geometry ofHA nano-particles influenced its bioactivity, cytotoxicity and biomaterial properties (Dubey and Tomar, 2009; Le- bre., 2017). Zhao. (2013) observed that needle- and plate-shaped nano-HA stimulated cell death and IL-6 expression in BEAS-2B cultures more notably than sphere-shaped nano-HA. Lebre. (2017) investigated the re- lation between the characteristics of HA particles and the innate immune response, and found that needle-shaped HA particles, but not spherical, significantly enhanced cytokinesecretion. Another study convinced that the sphere-like HAis less toxic than plate-like HA (Huang., 2019). There- fore, spherical nano-HA might have a certain advantage.
Particle size is another factor to affect the biological be- havior of the nano-HA. On one hand, nano-HA with small- er diameter exhibited higher bioactivity than the bigger ones (Shi., 2009; Dey., 2014). On the other hand, small- er HA particles generated a prolonged inflammatory re- sponse and moresignificant cytotoxicity (Lebre., 2017). The biological response of HA is such a complicated to- pic that more comprehensive study is required. Particle size is also thought to considerably affect the absorption path- ways employed by the nanoparticles. The small particles with diameters of nearly 60nm are most likely to be en- gulfed by caveolae (Kou., 2013). The size of clathrin- coated vesicles mediated endocytosis ranges from 100 to 200nm, while the size involved in macropinocytosis is morethan 500nm (Rennick., 2021). In this study, the mean diameter of nano-HA extracted from fish bone is approxi- mately 110nm (Figs.3a and 3b), implying little chance for calcium transport by caveolae or macropinocytosis. Clath- rin-mediated endocytosis was thus assumed to be the most possible absorption mechanism. Additionally, the reducedintestinal bioavailability in HA+NaN3group (Fig.4) indi- cated that the energy-dependent transport mediates the up- take of the HA nanoparticles (Zeng., 2012).
5 Conclusions
Nano-HA was extracted from Pacific cod bone in a greenway with a thermostable collagenolytic protease. Fig.5 is a schematic outline of the process. The resulted particles were characterized as anionic spheres with diameters of about 110nm and were mainly comprised of calcium and phos- phorus at an approximate ratio of 5:3. Theabsorp- tion experiment using mouse intestinal segments revealed a high level of intestinal bioavailability of the extracted na- no-HA. The clathrin-mediated endocytosis is probably in- volved in the intestinal absorption of nano-HA. The ther- mostable collagenolytic protease is proved to be a promis-ing alternative method for natural nano-HA extraction, and studies are underway to purify the protease and further im- prove the extraction efficiency of nano-HA.
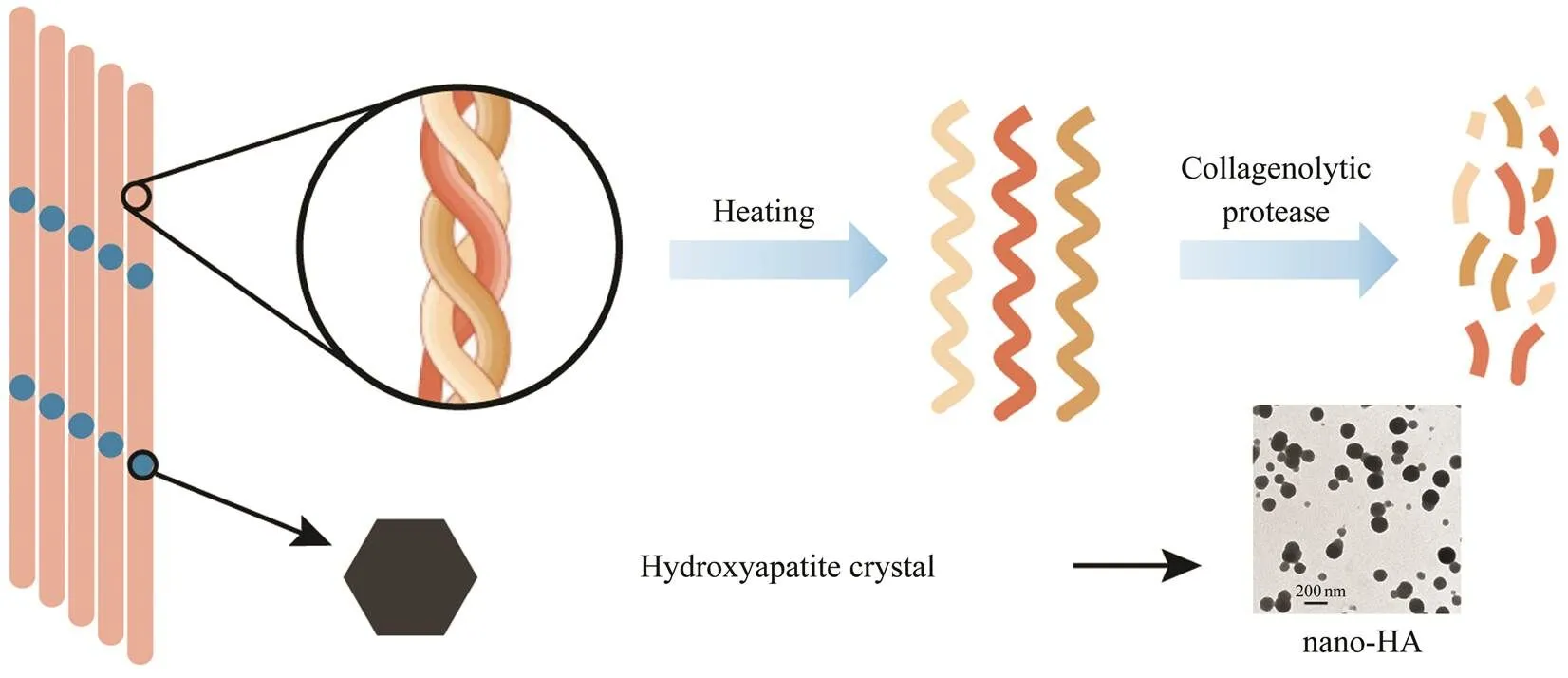
Fig.5 Process of nano-HA released from Pacific cod bone by the collagenolytic protease. Three strands of the collagen he- lix are unwound with heating beyond 60℃, and the peptides are cleaved by the thermostable collagenolytic protease. Si- multaneously, the nano-HA is released with the disruption of collagen architecture.
Acknowledgements
This work was supported by the Natural Science Foun- dation of Shandong Province (No. ZR202102270334), and the National Key Research and Development Program of China (No. 2020YFD0901004).
Aissaoui, N., Chobert, J., Haertle, T., Marzouki, M. N., and Abi- di, F., 2017. Purification and biochemical characterization of a neutral serine protease from. Use inantibacterial peptide production from a fish by-product hydro- lysate.,182(2): 831-845, DOI:10.1007/s12010-016-2365-4.
Balk, E. M., Adam, G. P., Langberg, V. N., Earley, A., Clark, P., and Ebeling, P. R., 2017. Global dietary calcium intake amongadults: A systematic review., 28 (12):3315-3324, DOI:10.1007/s00198-017-4230-x.
Boutinguiza, M., Pou, J., Comesaña, R., Lusquiños, F., De Carlos, A., and León, B., 2012. Biological hydroxyapatite obtained from fish bones., 32 (3):478-486, DOI:10.1016/j.msec.2011.11.021.
Bove-Fenderson, E., and Mannstadt, M., 2018. Hypocalcemic dis- orders., 32 (5): 639-656, DOI:10.1016/j.beem.2018.05.006.
Coppola, D., Lauritano, C., Palma Esposito, F., Riccio, G., Rizzo, C., and de Pascale, D., 2021. Fish waste: From problem to va- luable resource., 19 (2): 116, DOI: 10.3390/md19020116.
Dey, S., Das, M., and Balla, V. K., 2014. Effect of hydroxyapa- tite particle size, morphology and crystallinity on proliferation of colon cancer HCT116 cells., 39: 336-339, DOI: 10.1016/j.msec.2014.03.022.
Dubey, D. K., and Tomar, V., 2009. Role of hydroxyapatite crys- tal shape in nanoscale mechanical behavior of model tropo- collagen-hydroxyapatite hard biomaterials., 29 (7): 2133-2140, DOI: 10.1016/j.msec.2009.04.015.
FAO, 2021. Fishery and aquaculture statistics 2019. In:. Rome, 7-13.
Huang, L. H., Sun, X. Y., and Ouyang, J. M., 2019. Shape-depen-dent toxicity and mineralization of hydroxyapatite nanoparti- cles in A7R5 aortic smooth muscle cells., 9 (1): 18979, DOI: 10.1038/s41598-019-55428-9.
Ivankovic, H., Tkalcec, E., Orlic, S., Ferrer, G. G., and Schauperl, Z., 2010. Hydroxyapatite formation from cuttlefish bones: Kine-tics., 21 (10): 2711-2722, DOI: 10.1007/s10856-010-4115-4.
Jampilek, J., Kos, J., and Kralova, K., 2019. Potential of nanoma- terial applications in dietary supplements and foods for specialmedical purposes., 9 (2): 296, DOI: 10.3390/nano9020296.
Jaouadi, B., Abdelmalek, B., Fodil, D., Ferradji, F. Z., Rekik, H., Zaraî, N.,., 2010. Purification and characterization of a thermostable keratinolytic serine alkaline proteinase fromsp. strain AB1 with high stability in organic solvents., 101 (21): 8361-8369, DOI: 10.1016/j.biortech.2010.05.066.
Jin, Y. G., Fu, W. W., and Ma, M. H., 2011. Preparation and struc- ture characterization of soluble bone collagen peptide chelat- ing calcium., 10 (50): 10204-10211, DOI: 10.5897/AJB10.1923.
Jing, Z., Wu, H., Zhu, S., Gao, F., and Zeng, M., 2017. Screen- ing of thermostable collagenase strain and optimizing condi- tions for enhancing thermostable collagenase production., 42 (12): 7, DOI: 10.13684/j.cnki.spkj.2017.12.003 (in Chinese with English abstract).
Khashayar, P., Keshtkar, A., Ebrahimi, M., and Larijani, B. J., 2015. Nano calcium supplements: Friends or foes?, 1(1): 32-33, DOI: 10.18314/jbo.v1i2.46.
Kim, S. K., and Mendis, E., 2006. Bioactive compounds from ma-rine processing byproducts–A review., 39 (4): 383-393, DOI: 10.1016/j.foodres.2005.10.010.
Kou, L., Sun, J., Zhai, Y., and He, Z., 2013. The endocytosis and intracellular fate of nanomedicines: Implication for rational de- sign., 8 (1): 1-10, DOI: 10.1016/j.ajps.2013.07.001.
Lebre, F., Sridharan, R., Sawkins, M. J., Kelly, D. J., O’Brien, F. J., and Lavelle, E. C., 2017. The shape and size of hydroxyapa- tite particles dictate inflammatory responses following implan- tation., 7 (1): 2922, DOI: 10.1038/s41598-017-03086-0.
Lim, J., Yeap, S. P., Che, H. X., and Low, S. C., 2014. Charac-terization of magnetic nanoparticle by dynamic light scatter- ing., 8: 381, DOI: 10.1186/1556-276X-8-381.
Luo, J., Zhou Z., Yao, X., and Fu, Y., 2020. Mineral-chelating pep-tides derived from fish collagen: Preparation, bioactivity and bioavailability.–, 134: 110209, DOI: 10.1016/j.lwt.2020.110209.
Mateer, S. W., Cardona, J., Marks, E., Goggin, B. J., Hua, S., and Keely, S., 2016.intestinal sacs to assess mucosal per- meability in models of gastrointestinal disease., 2016(108): e53250, DOI: 10.3791/53250.
Matteini, P., Cicchi, R., Ratto, F., Kapsokalyvas, D., Rossi, F., and de Angelis., 2012. Thermal transitions of fibrillar collagen un- veiled by second-harmonic generation microscopy of corneal stroma., 103 (6): 1179-1187, DOI: 10.1016/j.bpj.2012.07.055.
Oh, N., and Park, J. H., 2014. Endocytosis and exocytosis of na- noparticles in mammalian cells., 9(S1): 51-63, DOI: 10.2147/IJN.S26592.
Okamoto, M., Yonejima, Y., Tsujimoto, Y., Suzuki, Y., and Wata- nabe, K., 2001. A thermostable collagenolytic protease with a very large molecular mass produced by thermophilicsp. strain MO-1., 57: 103-108, DOI: 10.1007/s002530100731.
Petrova, D. H., Shishkov, S. A., and Vlahov, S. S., 2006. Novel thermostable serine collagenase fromsp. 21E: Purification and some properties., 46(4): 275-285, DOI: 10.1002/jobm.200510063.
Rao, M. B., Tanksale, A. M., Ghatge, M. S., and Deshpande, V. V., 1998. Molecular and biotechnological aspects of microbial proteases., 62 (3): 597-635, DOI: 10.1128/mmbr.62.3.597-635.1998.
Rennick, J. J., Johnston, A. P. R., and Parton, R. G., 2021. Key principles and methods for studying the endocytosis of bio- logical and nanoparticle therapeutics., 16 (3): 266-276, DOI: 10.1038/s41565-021-00858-8.
Shi, Z., Huang, X., Cai, Y., Tang, R., and Yang, D., 2009. Size effect of hydroxyapatite nanoparticles on proliferation and apop- tosis of osteoblast-like cells., 5 (1): 338-345, DOI: 10.1016/j.actbio.2008.07.023.
Sorapukdee, S., Sumpavapol, P., Benjakul, S., and Tangwatcha- rin, P., 2020. Collagenolytic proteases fromB13 andS6 and their specificity toward collagen with low hydrolysis of myofibrils.–, 126: 109307, DOI: 10.1016/j.lwt.2020.109307.
Sossa, P. A. F., Giraldo, B. S., Garcia, B. C. G., Parra, E. R., and Arango, P. J., 2018. Comparative study between natural andsynthetic hydroxyapatite: Structural, morphological and bio-activity properties., 23 (4): e8, DOI: 10.1590/S1517-707620180004.0551.
Terzioğlu, P., Öğüt, H., and Kalemtas, A., 2018. Natural calcium phosphates from fish bones and their potential biomedical ap- plications., 91 (10): 899-911, DOI: 10.1016/j.msec.2018.06.010.
Yin, T., Park, J. W., and Xiong, S., 2015. Physicochemical pro- perties of nano fish bone prepared by wet media milling.–, 64 (1): 367-373, DOI: 10.1016/j.lwt.2015.06.007.
Zare, Y., 2016. Development of cubic orthogonal skeleton or three perpendicular plates system for prediction of Young’s modu- lus in polymer nanocomposites assuming the interphase., 294 (12): 2071-2078, DOI: 10.1007/s00396-016-3960-1.
Zeng, X., Zhang, Y., andNyström, A. M. J. B., 2012. Endocytic uptake and intracellular trafficking of bis-MPA-based hyper- branched copolymer micelles in breast cancer cells., 13 (11): 3814-3822, DOI: 10.1021/bm301281k.
Zhang, K., Huang, Q., Li, Y., Liu, L., Tang, X. F., and Tang, B., 2022. Maturation process and characterization of a novel ther- mostable and halotolerant subtilisin-like protease with high col-lagenolytic activity but low gelatinolytic activity., 88 (3): e02184-02121, DOI: 10.1128/aem.02184-21.
Zhang, Y., Ran, L., Li, C., and Chen, X., 2015. Diversity, struc- tures, and collagen-degrading mechanisms of bacterial colla- genolytic proteases., 81 (18): 6098-6107, DOI: 10.1128/AEM.00883-15.
Zhang, Z., and Su, D., 2009. Behaviour of TEM metal grids du- ringheating experiments., 109 (6): 766-774, DOI: 10.1016/j.ultramic.2009.01.015.
Zhao, X., Ng, S., Heng, B. C., Guo, J., Ma, L., and Tan, T. T. Y., 2013. Cytotoxicity of hydroxyapatite nanoparticles is shape and cell dependent., 87 (6): 1037-1052, DOI: 10.1007/s00204-012-0827-1.
(November 11, 2022;
February 8, 2023;
March 31, 2023)
© Ocean University of China, Science Press and Springer-Verlag GmbH Germany 2023
. Tel: 0086-532-82032400
E-mail: wuhaohao@ouc.edu.cn
(Edited by Qiu Yantao)
杂志排行
Journal of Ocean University of China的其它文章
- Effects of 5-Azacytidine (AZA) on the Growth, Antioxidant Activities and Germination of Pellicle Cystsof Scrippsiella acuminata (Diophyceae)
- Using Rn-222 to Study Human-Regulated River Water-Sediment Input Event in the Estuary
- Parameterization Method of Wind Drift Factor Based on Deep Learning in the Oil Spill Model
- YOLOv5-Based Seabed Sediment Recognition Method for Side-Scan Sonar Imagery
- A Metadata Reconstruction Algorithm Based on Heterogeneous Sensor Data for Marine Observations
- Large Active Faults and the Wharton Basin Intraplate Earthquakes in the Eastern Indian Ocean
