Serum microvesicle microRNAs in patients with polycystic ovary syndrome
2023-12-01,,,,
, , , ,
(1.Department of Reproductive Medicine, Department of Obstetrics and Gynecology, the Affiliated Hospital of Zunyi Medical University, Zunyi Guizhou 563099,China; 2.Key Laboratory of Maternal &Child Health and Exposure Science of Guizhou Higher Education Institutes, Zunyi Medical University,Zunyi Guizhou 563099,China)
[Abstract] Objective To detect serum microvesicles (MVs) concentrations and expression profiles of microvesicle microRNAs (miRNAs) in patients with polycystic ovary syndrome (PCOS) and control groups, and analyze their potential role in the pathogenesis of PCOS. Methods Fifty serum samples from PCOS patients and 50 age-matched serum samples from women seeking fertility treatment as controls were collected. MVs were isolated through differential centrifugation and characterized by transmission electron microscopy (TEM), Western blot, and nanoparticle tracking analysis(NTA). The differentially expressed miRNAs were screened by high-throughput sequencing methods, and the targets of these miRNAs were predicted. GO / KEGG pathway enrichment analysis were performed. Female ICR mice were injected with serum MVs suspension from PCOS patients to observe the estrus cycle and ovarian structure. Immunohistochemical methods were used to detect the expression and localization of NF-κB and WNT5A proteins related to PCOS inflammation in the ovary. Results We found that serum MVs levels tended to be higher in the PCOS group and were dominated by small-diameter vesicles. High-throughput sequencing identified 19 differentially expressed miRNAs, of which 8 were up-regulated and 11 were down-regulated(P<0.05). Five differentially expressed miRNAs were identified by qRT-PCR, mong which three miRNAs were identical with the sequencing results. Enrichment analysis revealed that possible pathways associated with PCOS include AGE-RAGE signaling pathway, MAPK signaling pathway, and TGF-β signaling pathways. Injection of PCOS serum MVs caused estrous cycle disturbance in female ICR mice, and inflammation related protein NF-κB was positively expressed in the nucleus. Conclusion Small diameter MVs were more abundant in the serum of PCOS patients, and the differentially expressed miRNAs may be involved in multiple PCOS-related pathways. PCOS serum MVs can induce a mild PCOS-like phenotype in female mice.
[Key words] microvesicles; polycystic ovary syndrome; miRNA; follicular development
Polycystic ovary syndrome (PCOS) is one of the most common endocrine disorders in gynecological practice, with a worldwide incidence estimated between 4 % and 21 %[1]. Previous studies have shown that PCOS and its ovulatory dysfunction are closely related to metabolic factors such as high androgen levels, insulin resistance (IR), inflammation, abnormal glucose metabolism, follicle granulosa proliferation, and apoptosis[1-2]. However, its specific mechanism remains unclear[3]. Microvesicles (MVs, 100-1 000 nm), a type of nanometer-sized membrane-bound vesicles, are intercellular communication vehicles that can carry regulatory molecular and genetic information[4]. MV-encapsulated cargoes include RNAs (mainly miRNAs), proteins, lipids and cellular metabolites, which are found in various body fluids and are particularly abundant in serum[5-6]. The cargo in MVs may affect the expression of target cell genes, thereby affecting their functions and biological activities[4, 7-8]. Previous studies have shown abnormally high levels of MVs in the blood or follicular fluid of PCOS patients, and the number of these MVs was reduced after administration of the corresponding intervention, while MVs levels were not significantly altered in healthy women[9].
Several studies have suggested that abnormal internal microenvironment and follicle growth arrest in early follicular development may be critical factors in the pathogenesis of PCOS and that miRNAs may play an important role in this process[10]. It is a promising diagnostic marker and potential therapeutic target for PCOS[11-12]. In addition to miRNAs within germ cells, miRNAs in serum, plasma and follicular fluid MVs have also been shown to play a role in the development of PCOS as novel communication vehicles carrying miRNAs[12]. Studies have shown that MVs can cross the blood-follicular fluid barrier through capillary sheaths and may play a role in follicle development[13-14]. Jiang et al[15]found that plasma exosomal miR-18a-3p, miR-146a-5p, and miR-126-3p were significantly correlated with luminal follicle count and anti-Müllerian hormone (AMH) concentration in PCOS patients. Previous studies have shown that PCOS patients are generally in a state of chronic non-specific inflammation. Proinflammatory factors can be activated through Akt/NF-κB signaling pathway activation, which is involved in the pathogenesis of PCOS by modulating the inflammatory response[16]. MVs can act as carriers of pathogenic information to activate NF-κB signaling pathway producing inflammatory factors, which in turn activate the WNT signaling pathway to regulate follicle growth and development[17]. Currently, there is a paucity of studies on MV miRNAs in PCOS serum. In this study, we performed high-throughput sequencing and bioinformatics analysis of MVs from PCOS serum to identify any differentially expressed miRNAs related to these pathways. The objective was to explore whether MV miRNAs might be investigated as potential biomarkers for PCOS. The second objective was to explore whether MVs in PCOS serum can induce PCOS-like phenotype in female ICR mice.
1 Materials and methods
1.1 Sample collection and Microvesicles isolation
The study was conducted with the approval of the institutional review board/Ethics Committee of Zunyi Medical University, written informed consent was obtained, approval number was KLLY-2020-015. And follow the guidelines of the Declaration of Helsinki. Data were collected after obtaining informed consent of all subjects. A total of 100 women were included in this study: 50 in the PCOS group and 50 in the healthy control group. Patients diagnosed with PCOS at the Affiliated Hospital of Zunyi Medical University between August 2019 and March 2021 were eligible for inclusion in the PCOS group. Fifty age-matched women seeking fertility treatment at fertility centers for tubal infertility served as controls.
The diagnosis of PCOS was based on the 2003 Rotterdam Consensus criteri[3]. Any two of the following criteria must be met: (1) sporadic ovulation and/or anovulation (menstrual cycle <35 days or history of ≤ 8 menstrual cycles in a year); (2) Clinical manifestations of hyperandrogenemia and/or biochemical hyperandrogenemia; (3) Morphological ultrasonography of the ovary showing characteristic polycystic features (≥ 12 follicles with a diameter of 2-9 mm in one or both ovaries) or ovarian volume ≥ 10 ml. The diagnosis of PCOS is based on qualifying any two of these three criteria, and exclusion of other causes of ovulation disorders or high androgen level/clinical manifestations of hyperandrogenemia. Patients with Cushing’s syndrome, adrenal/ovarian tumors, hyperthyroidism, diabetes, hypertension, or other conditions that may cause elevated androgens, insulin, and polycystic ovarian changes were excluded. All women in the control group had normal menstrual cycles (21 to 35 days), normal ovarian morphology (more than 12 follicles, 2-9 mm in diameter; ovarian volume <10 ml), and normal circulating total testosterone levels. Five ml of peripheral blood was collected on days 3-5 of the menstrual cycle, and the serum was collected for storage at -80 ℃ . All serum samples were freeze thawed only once.
Frozen serum was placed on ice after thawing in a 25 ℃ constant temperature water bath. Serum was diluted three-fold with 4 ℃ phosphate buffered saline (PBS, pH 7.4). Subsequently, it was centrifuged at 500 × g for 10 min, at 2 000 × g for 15 min, and at 5 000×g for 15 min to remove cells and cell debris. The supernatant was centrifuged at 20 000×g for 1 h, and the supernatant was removed. The wall of the centrifuge tube was rinsed extensively with 1 ml PBS, and the vesicle suspension was resuspended in 20 000 × g for 1 h. Subsequently, the supernatant was removed and the cells were resuspended in 100 μl PBS to resuspend the pellet and stored at -80 ℃ on ice.
1.2 Morphological characteristics and quantitative analysis of serum microvesicles
1.2.1 Transmission electron microscopy(TEM) and Western blot MVs samples were thawed on ice, and the mixed MVs resuspension was gently mixed at room temperature. Mixing 20.0 μl drops on a copper grid and allowed to stand for 2 min before staining with negative stain (30 μl 3 % phosphotungstic acid solution) for 5 min, air dried and then placed the copper grid into the sample tank, followed by image acquisition under TEM. The total protein was extracted from 100 μl frozen MVs using RIPA Lysis buffer and separated by 12 % sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE). After electrophoresis, the proteins were transferred to 0.22 μl PVDF membrane (360 mA, 90 min). The PVDF membrane was cleaned by Tris-Buffered Saline containing Tween 20 (TBST, 10 min×3/cycle) every time, and placed in 1×TBST containing 5 % skimmed milk for 2 h at room temperature. Subsequently, it was reacted with CD63 (1∶1 000, Mouse#H5C6; NOVUS) and primary antibodies overnight at 4 ℃.
1.2.2 Nanoparticle tracking analysis The size distribution of isolated MVs was determined by a nanoparticle tracking analyzer (Nanosight NS300, Malvern, UK). 20 μl serum MVs samples with PBS were taken, and the concentration of the samples and the distribution of different vesicle size subgroups was analyzed by Nanosight NS300 software. The concentration and size distribution of serum MVs between the two groups were compared and analyzed.
1.3 Establishment of serum microvesicle miRNA profiles by a high-throughput sequencing method and bioinformatics analysis
1.3.1 Microvesicles RNA extraction Total RNA of MVs was extracted by Trizol method. RNA-free 1.5 ml EP tubes were used for each step. After thawing 100 μl MVs on ice, 1 ml Trizol was added. 200 μl chloroform was added to each 1 ml Trizol, shaken for 15 s and upside-down for 10-20 times, then allowed to stand for 5 min at room temperature. After centrifugation at 12 000 r/min at 4 ℃ for 15 min, the top transparent liquid was carefully absorbed, and 1.5 volume 70 % ethanol was added and mixed. After standing for 5 min, and centrifugation at 12 000 rpm at 4 ℃ for 10 min, all the ethanol was carefully aspirated. DEPC water (20 μl) was added according to the amount of RNA to dissolve RNA. Subsequently, it was sealed and stored in -80 ℃ refrigerator for later use.
1.3.2 Microvesicles library construction and sequencing After the samples passed the quality inspection, the QIASEQ miRNA Library Kit was used to construct the library. The RNA quality was assessed by Agilent 2100 Bioanalyzer (Applied Biosystems, Carlsbad, CA). Using the special structure of small RNA with hydroxyl group at the 3’ end and complete phosphate group at the 5’ end, specific joints was directly added to both ends of small RNA. Then cDNA was synthesized by reverse transcription using primers with Umi (Unique Molecular Index). Then PCR amplification and purification were performed, and target DNA fragments (170-190 bp fragments) or target fragments were separated by PAGE gel electrophoresis, which was the Small RNA library. The concentration and integrity of the extracted total RNA were estimated by Qubit 3.0 Fluorometer (Invitrogen, Carlsbad, California), and Agilent 2100 Bioanalyzer (Applied Biosystems, Carlsbad, CA), respectively. The library quality and concentration were assessed by utilizing a DNA 1000 chip on an Agilent 2100 Bioanalyzer. Accurate quantification for sequencing applications was determined using the qPCR-based KAPA Biosystems Library Quantification kit (Kapa Biosystems, Inc., Woburn, MA). Each library was diluted to a final concentration of 2 nmol/L and pooled equimolar prior to clustering. Fifty bp single-end (SE) sequencing was performed on all samples.
1.3.3 Serum microvesicles microRNA target gene prediction and pathway analysis For miRNA expression analysis, identification and quantification of miRNAs were carried out using excerpt software. Genome annotation of miRNAs was also performed. In addition, novel miRNAs were identified with miRDeep2. TMM (trimmed mean of M-values) was used to normalize the gene expression. Differentially expressed genes were identified using the edgeR program. MiRNAs showing significantly altered expression (P<0.05) and | log2fold change |> 1.5 were considered differentially expressed. The multiMiR tool consisting of prediction databases (8 including miRanda and miRDBmiRTarBase), experimental validation databases and disease drug databases was used as candidate target genes of miRNA. Screening criteria: (1) for the predicted miRNA-gene, we only selected the top 10 % output with the highest probability of each tool; (2) Only conservative miRNA-gene relationships were selected (at least in one of miRanda, Pita and Targetscan databases);(3) Present in at least 5 prediction tools or in at least 2 validation databases. The target miRNAs obtained after screening were used for GO/KEGG analysis clusterProfiler was used to perform the GO and KEGG enrichment analysis.
1.4 Quantitative qRT-PCR Real-time PCR validation was performed using miRcute miRNA qPCR kit (SYBR Green,RiboBio, Guangzhou,China) based on specific stem ring RT primers and reverse primers, polyadenylation and reverse transcription. All miRNA primers were purchased from RiboBio (Guangzhou,China). Detection was performed by real-time fluorescence quantitative instrument (QuantStudio TM3, ThermoFisher, USA). The PCR reaction consisted of a quick start of 10 min at 95 ℃ followed by 45 cycles of amplification, each of which consisted of denaturation at 95 ℃ for 2 s and annealing at 60 ℃ for 20 s and 70 ℃ for 10 s. U6 expression was used as the standard for normalization of relative microRNA levels in each sample. The qualitative and specificity of PCR products were verified by melting curve analysis. Gene expression was analyzed by 2-ΔΔCTmethod. Each experiment was repeated three times.
1.5 In vivo injection of PCOS serum microvesicles into female mice
1.5.1 Effect of PCOS serum MVs on female mice
The study was conducted with the approval of the institutional review board/Ethics and Animal Use and Care Committees of Zunyi Medical University[NO:KLLY(A)-2020-120]. And this study conformed to the “3R” principle and conformed to the principles of animal protection as well as ethical requirements such as laboratory animal welfare. ICR females ICR at 8-9 weeks were divided into PCOS group, non-PCOS group, and negative control group. Approximately 40 μg MVs were re-suspended in 100 μl PBS and injected into mice via caudal vein. PCOS group received serum MVs sourced from PCOS patients, non-PCOS group received serum MVs from normal women, while the negative control group received the same amount of PBS.
1.5.2 Detection of vaginal exfoliated cells in ICR female mice From 9∶00-10∶00 am daily, vaginal exfoliated cells were detected in ICR female mice. 40 μl of PBS at room temperature was aspirated and slowly injected into the vagina of female mice, waiting for the PBS to flow out and then aspirated back in; the process was repeated 2-3 times. After the clear PBS had become turbid, it was injected into the corresponding numbered wells of the 96-well plate. The vaginal exfoliated cells were then observed under an inverted microscope.
1.5.3 HE staining and immunohistochemical methods After 48 h of MVs injection, both ovaries were taken, washed twice with PBS, and fixed with 4 % tissue fixative. Subsequently, the ovarian tissues were dehydrated and paraffin-embedded using tissue dehydrator TP1020 and paraffin embedding machine EG1150 according to the procedure, and serially sectioned by tissue slicer RM2245 at 5-mm thickness. Ovarian sections were stained with hematoxylin-eosin using an automatic staining machine ST5010. In the immunohistochemical method, anti-WNT5A and anti-NF-κB p56 [E379] were incubated and developed using DAB chromogenic reagent after completion, which were observed under the microscope while developing color and terminated immediately after the appearance of brownish yellow color.
1.5.4 Expression of WNT5A and NF-κB protein in ovary was detected by immunohistochemistry The sections were dewaxed after natural rewarming at room temperature. Antigen repair was carried out by boiling citrate repair solution at high temperature. After the closure, the antibodies were incubated with diluted WNT5A and NF-κB antibody, stained at room temperature with DAB chromogenic reagent, and observed under the microscope. When yellow coloration was observed in the cytoplasm, the chromogenic process was terminated by washing, and the nucleus was stained with hematoxylin. Then, they were allowed to dehydrate according to standard ethanol concentrations, air dried, and sealed with neutral gum.
1.6 Statistical analysis SPSS 20.0 statistical software was used for data processing. Continuous variables are expressed as mean ± standard deviation and pairedttest was used for comparison. Fisher’s Exact test was used to evaluate the biological functions of the statistically significant overlap between the differentially expressed gene set and the functional gene set (e g, GO/KEGG).Pvalue<0.05 was considered indicative of statistical significance.
2 Results
2.1 Morphological characteristics and quantitative analysis of serum microvesicles MVs can be isolated from the serum of PCOS patients by differential centrifugation, shown in Figure 1. Under TEM (Figure 1A), these were mostly oval membranous vesicles or cup-shaped vesicles of varying sizes with a double layered membrane structure. They are distributed singly or in clusters, and MVs with diameters between 100 and 500 nm are seen more often. CD63 signals were detected by Western blot (Figure 1C), confirming the presence of MVs in serum pellets after differential centrifugation in this experiment.
Nanoparticle Tracking Analyzer (Figure 1B) showed that the total concentration of MVs in the PCOS group was 9.58×108particles/ml and the average diameter was 141 nm. In control group, the total concentration of MVs was 5.12×108particles/ml and the average diameter was 290 nm. The total concentration of MVs in the serum of the PCOS group was 1.87 times higher than that in the normal control group. The results indicated that the nanoparticles in the serum samples of PCOS tended to increase, but the diameter was slightly smaller. The data showed that the main diameter of MVs in both groups was in the range of 100-500 nm, while that in the PCOS group was mainly in the range of 100-250 nm (Figure 1B).
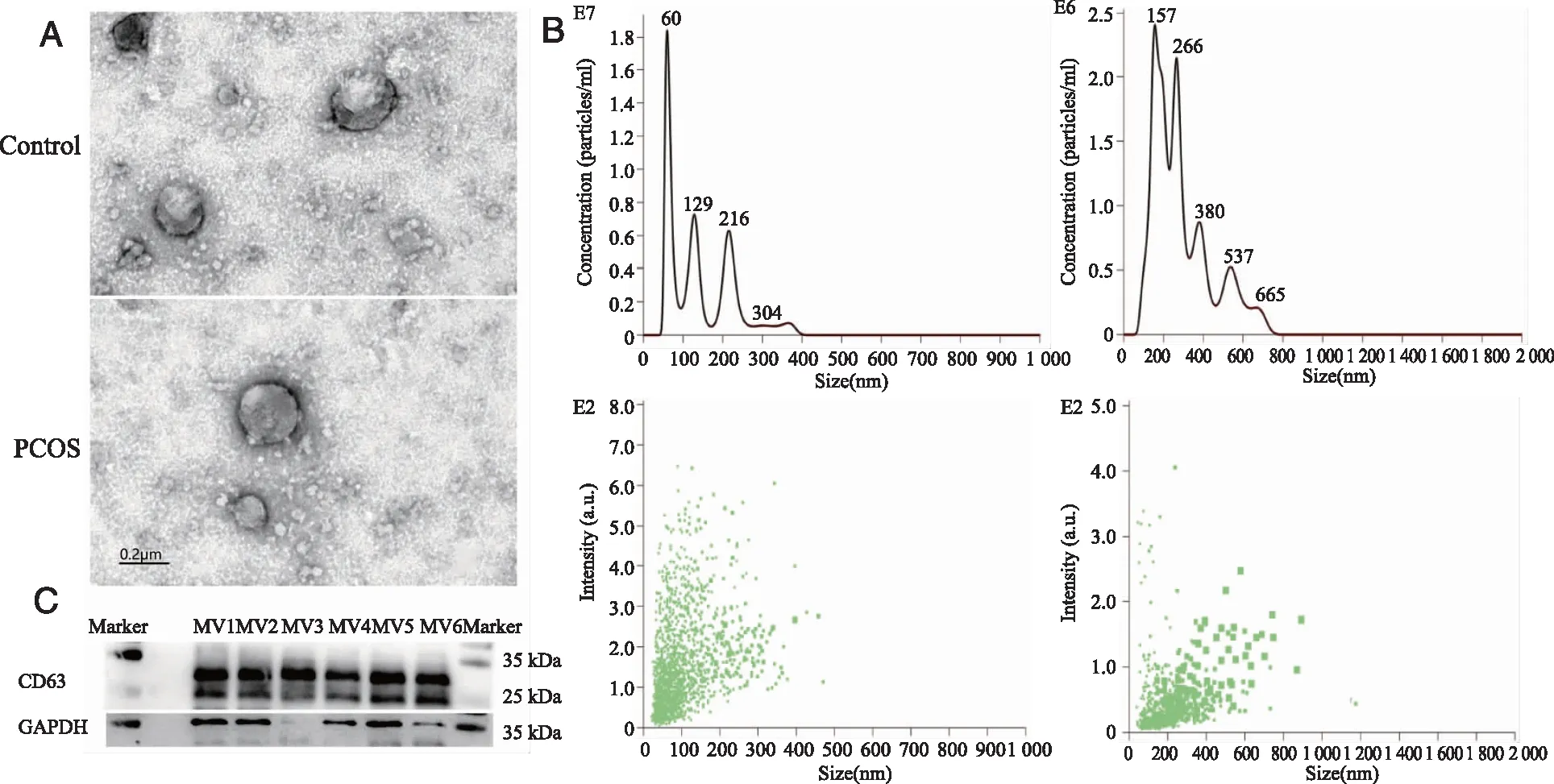
A: TEM demonstrated that the MVs isolated from PCOS group and control group were oval membranous vesicles or cup-shaped vesicles (scale bar: 0.2 μm); B: Size distribution of PCOS group and control group serum MVs, as determined by NTA; C: Western blots showing the expressions of CD63 in six independent MVs samples of both groups(MV1-3:PCOS group,MV4-6:control group).Figure 1 Quality control of isolated serum MVs
2.2 Analysis of miRNAs in serum MVs
2.2.1 Sequence analysis of serum MV miRNA in PCOS The total RNA concentration of MVs in PCOS group was higher than that in normal control group as assessed by the Agilent 2100 BioAnalyzer. Total RNA had a distribution of 22 nt in both groups.
Initially, miRNA deep sequencing revealed a total of 730 miRNAs in the serum MVs. Finally, we identified 19 significantly differentially expressed miRNAs (| log2fold change |>1.5,P< 0.05) for subsequent analysis. Of these, 8 miRNAs were upregulated and 11 miRNAs were down-regulated (Table 1). The heat map of the 19 differentially-expressed miRNAs (P<0.05) is shown in Figure 2A.
2.2.2 GO and KEGG Analysis of the Differentially-Expressed miRNAs in PCOS GO analysis of the target genes of the above miRNAs was performed from three aspects: molecular function, biological process, and cellular component. The functional prediction of differentially expressed miRNAs in PCOS was mainly based on the intersection number (>3) of differentially-expressed gene sets and GO term and the significance ofPvalue(Figure 2). We selected 7 miRNAs for further analysis(hsa-miR-539-5p, hsa-miR-106a-5p, hsa-miR-495-3p, hsa-miR-33b-5p, hsa-miR-125b-2-3p, hsa-miR-885-3p and hsa-miR-486-3p ) (P<0.05).Our study researched 182 GO terms(Figure 2A-C). The main molecular function, biological process and pathway analyses determined by GO analysis of target genes of the 7 differentially-expressed miRNAs are shown in Figure 2D-F.
Twenty-two related pathways were obtained by KEGG enrichment analysis, and the possible pathways related to the development and progression of PCOS were as follows: AGE-RAGE signaling pathway in diabetic complications, MAPK signaling pathway, cellular senescence, TGF-β signaling pathway, Circadian rhythm and cancer (Figure 2G).
In the present study, 175 DEGs were meaningfully involved in the regulation of miRNAs, among which has-miR-106a-5p regulated most genes. Among them, Crot is a co-regulated gene of has-miR-106a-5p and has-miR-33b-5p(Figure 2B). The specific results are shown as follows: Five differentially expressed miRNAs (hsa-miR-33b-5p, hsa-miR-106a-5p, hsa-miR-125-1-3p, hsa-miR-486-3p, hsa-miR-885-3p) were randomly identified by qRT-PCR,where hsa-miR-33b-5p and hsa-miR-106a-5p showed opposite expression to the sequencing results; and hsa-miR-125-1-3p, hsa-miR-486-3p and hsa-miR-885-3p showed the same expression (P<0.05,Figure 2C).
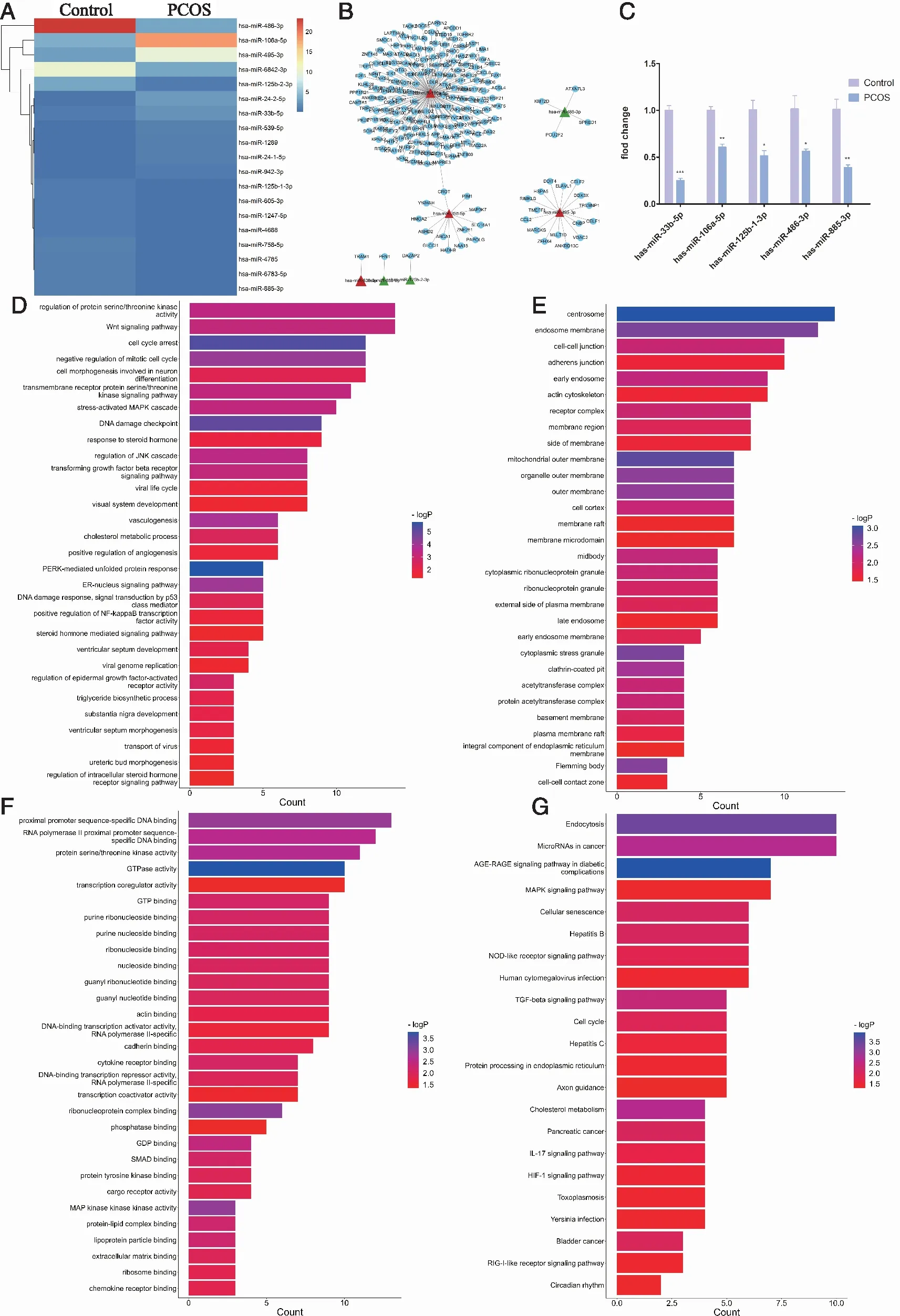
A: Heat map of 19 differentially-expressed miRNAs , P<0.05; B: Target miRNA-gene network; C:qRT-PCR, *: P<0.05, **: P<0.05, ***:P<0.001; D-F:Gene Ontology (GO) functional analysis bar chart for target genes of the 19 differentially-expressed miRNAs(D-Biological Process, E-Cellular Component, F-Molecular Function);X-axis: Gene Ratio; Y-axis: GO terms; G: The barplot the 22 statistically significantly enriched pathways identified by KEGG analysis (X-axis:KEGG pathway terms;Y-axis:-log2P, P is P-Value Corrected with Bonferroni step down). Figure 2 Expression of miRNAs in serum MVs from patients with polycystic ovary syndrome (PCOS) and controls

Table 1 High throughput sequencing results showed significantly differentially expressed miRNAs
Fold change is presented as miRNA expression in women with versus without polycystic ovary syndrome (PCOS); hsa =Homo sapiens; miRNA =microRNA.
2.3 Effect of PCOS serum MVs on ICR female mice
2.3.1 Serum-derived MVs from PCOS patients can prolong the estrous cycle of ICR female mice The sloughed vaginal epithelial cells of ICR female mice for 14 consecutive days were detected, and the results showed that the complete estrus cycle length of female mice was approximately 4-6 days. Female mice injected with serum MVs from PCOS patients developed an estrus cycle disorder, characterized by prolonged estrus or diestrus and no complete estrus cycle. The estrus cycle in the control group and blank group was normal. Under the microscope, HE-stained ovarian sections showed preserved ovarian structure in each group, and there was no significant between-group difference in this respect(Figure 3A).
2.3.2 NF-κB and WNT5A protein expression were abnormal in female mice injected with serum-derived MVs from PCOS patients Examination of HE-stained sections showed no morphological abnormalities in ovaries in all groups, and the presence of follicles at all levels could be observed(Figure 3B). We examined the localization and expression of WNT5A and NF-κB in the ovaries of three groups of female rats by immunohistochemistry, and the results showed that Wnt5A and NF-κB positive signals were mainly localized in follicular granulosa cells, which were brown or yellow in color. In the PCOS group, strong positive signals for WNT5A and NF-κB were found in the granulosa cells of all follicle grades, and the more granulosa cells there were, the more pronounced were the signals. In the control and negative control ovaries, WNT5A protein was highly expressed in the mature follicles and less in the secondary and primary follicles. In the control and negative control groups, NF-κB protein was more abundant in the nuclei of granulosa cells of immature follicles in the PCOS group than in the negative control group. The NF-κB protein positive signal was less localized in the follicles of the negative control group, and intra-nuclear localization was rarely seen(Figure 3C).

A:Effect of serum microvesicles on the estrus cycle of ICR female mice (D: Diestrus; P: Proestrus; E: Estrus; M:Metestrus); B:Effect of serum MVs on ICR female mice ovaries,original magnification was ×40, Scale bar was 500 μm; C: Localization and expression of WNT5A and NF-κB in ovaries; original magnification was ×400, Scale bar was 0.2 μm;Left: luminal follicles; Right: primary and secondary follicles.Figure 3 Effect of PCOS serum MVs on ICR female mice
3 Discussion
Previous studies have shown an increasing trend of MV release from body fluids in PCOS patients, which is consistent with our findings[9, 13]. Studies have shown that vesicles can carry abnormal messages, traverse capillary sheaths, and transmit messages or substances between germ cells[12]. The follicle is the main functional unit of the ovary. Ovarian follicle development is influenced by the interplay of several factors including germ cells, blood vessels, and ovarian steroid hormones[17]. Abnormalities in any of the above links may potentially affect the developmental outcome of the follicle[15,18]. Intercellular interactions are important to the maintenance of homeostasis and normal function; cell free nanoparticles (proteins, small RNAs, MVs, exosomes, etc.) are important mediators of cell-cell interactions, including miRNAs within vesicles, and miRNAs within vesicles have been confirmed to play important roles in a variety of diseases[12, 19].
In the present study, we first observed the morphological characteristics of serum MVs under a microscope. The MVs were varied in size and were round or oval in shape with a double layered membrane structure. Serum MVs concentrations and size distribution were compared between PCOS patients and controls. In this experiment, the total concentration of serum MVs in the PCOS group showed an increasing trend. There were more microcysts (mainly 100-500 nm) with smaller microcyst diameters in the serum of PCOS patients, suggesting that PCOS may be more capable of promoting the release of small microcysts, the contents of which may differ from those of controls. Exosomes and MVs cannot be completely separated due to the limitations of the existing isolation techniques. A number of impurities (such as proteins or lipids) inevitably occur in the extracted samples, which also explains why our PCOS group had the highest peak at 60 nm. We suspect that this is related to abnormal lipid metabolism and lack of purification steps in PCOS[1]. This phenomenon was consistent with the results of “cholesterol metabolism” in terms of bioinformatics function prediction and pathway enrichment.
In our study, nineteen differentially expressed miRNAs were identified in the serum MVs of PCOS patients, and seven more meaningful miRNAs were further screened for bioinformatics analysis. Go and KEGG analyses predicted their target functions, suggesting that these miRNAs may be involved in AGE-RAGE signaling pathway in diabetic complications, MAPK signaling, TGF-β signaling pathways and circadian rhythm, regulation of cancer. Further literature review revealed that the bioinformatics functions of these differentially expressed miRNAs and their target genes may influence inflammation[20], abnormal glucose metabolism[21], abnormal lipid metabolism[22], and IR[21]in PCOS patients. These are closely related to the occurrence, development or abnormal follicular development in PCOS. For example, in the study by Jiang et al, the target functions of the differentially expressed miRNAs in PCOS exosomes included MAPK signaling, endocytosis, circadian rhythm, and cancer pathways[15]. This is similar to our results.Has-miR-33b-5p and hsa-miR-106a-5p were highly expressed in serum MVs sequencing of the PCOS group, andHMGA2 andABCA1 were the target genes of miR-33b-5p. Hsa-miR-106a-5p was up-regulated in sequencing and down regulated in qRT-PCR, and the two results presented an opposite trend of expression, which was similar to the results of other PCOS plasma exosome studies[15]. A previous study showed that miR-33b-5p was highly expressed in ovarian tissue of insulin resistant PCOS mice, and inhibition of miR-33b-5p may improve IR by targeting HMGA2 and inhibiting GLUT4 production[21]. In addition, miR-33b-5p is also up-regulated in hypercholesterolemic patients, and miR-33b-5p and miR-106a-5p can regulate the cholesterol geneABCA1 and are involved in cholesterol regulatory processes[22]. The inflammatory geneNLRP3 is a target gene of hsa-miR-106a-5p, and high androgen exposure can be mediated through the activation of the NLRP3 inflammasome[21, 23]. Other studies have also shown that NLRP3 expression is associated with the induction of chronic low inflammatory response, abnormal follicle development, as well as ovarian interstitial cell fibrosis[24].
Hsa-miR-885-3p is underexpressed in serum MVs of PCOS. As Zhang et al showed, low levels of miR-885-3p in the peripheral blood of T1D patients promoted TLR4 expression, which enhanced NF-κB in the signaling pathway activity, resulting in increased levels of proinflammatory cytokines[25].The predicted results indicate that hsa-miR-106a-5-p, hsa-miR-33b-5p, and hsa-miR-495-3p are involved in the age rage signaling pathway of diabetic complications. The AGE-RAGE system is closely related to PCOS. In vitro validation experiments confirmed that the AGE-RAGE system may be associated with hormonal imbalance and granulosa cell dysfunction[26]. The AGE-RAGE signaling pathway in diabetic complications in PCOS patients may be involved in promoting the initiation and progression of the inflammatory state[27]. For example, activation of the oxidative stress response by activating NOX-1 and decreasing SOD-1 expression was shown to result in a proinflammatory environment characterized by oxidative stress[27-28]. Differentially expressed miRNAs in serum MVs are involved in regulating the AGE-RAGE signaling pathway, possibly by increasing IR, and directly contribute to abnormal follicle development[29-30].
GO and KEGG analysis results suggested that these differentially expressed miRNAs were associated with MAPK signaling, TGF-β and TGFBR expression, Wnt signaling and other target functions and the relevant pathways. MAPK signaling pathway is one of the most typical insulin signaling pathways. The MAPK-ERK pathway may contribute to resistance to the metabolic effects of insulin, which is important for ovulation and weight regulation in PCOS patients[31]. For example, the p38 MAPK protein is expressed in oocytes and granulocytes and regulates maturation[31]. Previous studies have shown that the bioavailability of TGF-β1 is abnormally elevated in granulosa cells of women with PCOS[32]. TGF-β1 target genes are associated with PCOS oocyte and granulosa cumulus cell disorders. The expression of this gene may be involved in the regulation of follicle and oocyte maturation[33]. Upregulated TGF-β1 in PCOS patients may also be a major factor in ovarian interstitial fibrosis and capsular thickening[34]. WNT signaling molecules are important local ovarian factors regulating follicle development and ovulation, and components of the WNT signaling pathway are specifically expressed at different stages of follicle development[35]. WNT5A belongs to the WNT protein family and is essential for the normal maturation and developmental maturation of ovaries and follicles. Previous studies have shown that overexpression of WNT5A in PCOS patients can induce apoptosis via PI3K/Akt/NF-κB signaling, enhancing granulosa cell inflammation and oxidative stress[36]. Tail vein injection of serum MVs from PCOS patients in wild type ICR female mice was shown that could induce estrous cycle disorder.
The differentially expressed miRNAs in serum MVs of PCOS patients may be involved in the regulation of pathways related to abnormal glucose metabolism, lipid metabolism, insulin resistance, and inflammation. The differentially expressed miRNAs in serum MVs may be related to the occurrence and development of PCOS, and this study expands our understanding of PCOS disease and helps to further explore the pathogenesis of PCOS and identify new therapeutic approaches.
