Effect of ternary transition metal sulfide FeNi2S4 on hydrogen storage performance of MgH2
2023-11-18YaokunFuLuZhangaYuanLiaSanyangGuoHanYuWenfengWangKailiangRenWeiZhangShuminHana
Yaokun Fu, Lu Zhanga,,∗, Yuan Lia,, Sanyang Guo, Han Yu, Wenfeng Wang,Kailiang Ren, Wei Zhang, Shumin Hana,,∗
a State Key Laboratory of Metastable Materials Science and Technology, Yanshan University, Qinhuangdao 066004, China
b Hebei Key Laboratory of Applied Chemistry, School of Environmental and Chemical Engineering, Yanshan University, Qinhuangdao 066004, China
Received 6 July 2021; received in revised form 5 November 2021; accepted 29 November 2021
Available online 27 January 2022
Abstract Hydrogen storage is a key link in hydrogen economy, where solid-state hydrogen storage is considered as the most promising approach because it can meet the requirement of high density and safety. Thereinto, magnesium-based materials (MgH2) are currently deemed as an attractive candidate due to the potentially high hydrogen storage density (7.6 wt%), however, the stable thermodynamics and slow kinetics limit the practical application. In this study, we design a ternary transition metal sulfide FeNi2S4 with a hollow balloon structure as a catalyst of MgH2 to address the above issues by constructing a MgH2/Mg2NiH4-MgS/Fe system. Notably, the dehydrogenation/hydrogenation of MgH2 has been significantly improved due to the synergistic catalysis of active species of Mg2Ni/Mg2NiH4, MgS and Fe originated from the MgH2-FeNi2S4 composite. The hydrogen absorption capacity of the MgH2-FeNi2S4 composite reaches to 4.02 wt% at 373 K for 1 h, a sharp contrast to the milled-MgH2 (0.67 wt%). In terms of dehydrogenation process, the initial dehydrogenation temperature of the composite is 80 K lower than that of the milled-MgH2, and the dehydrogenation activation energy decreases by 95.7 kJ·mol–1 compared with the milled-MgH2 (161.2 kJ·mol–1). This method provides a new strategy for improving the dehydrogenation/hydrogenation performance of the MgH2 material.
Keywords: Hydrogen storage materials; Magnesium hydride; FeNi2S4; Hydrogen storage kinetics; Catalysts.
1. Introduction
With the rapid development of society,energy consumption has been intensifying and is expected to reach a peak in 2035[1–3]. For the sustainable use of energy in the future, hydrogen energy has been regarded as one of the most promising alternative energy sources of traditional fossil fuels because of its high energy density (142 MJ·kg–1). However, achieving safe, efficient and economical storage of hydrogen has been a challenge for hydrogen application [4–7]. Magnesium based hydrogen storage materials (MgH2) have been considered to be one of the most promising solid hydrogen storage materials due to the high hydrogen storage capacity (∼7.6 wt%),good reversibility and low cost, but the high thermal stability of Mg-H bond leads to the high dehydrogenation temperature (above 623 K) and sluggish reaction kinetics of MgH2,which hinders its practical application as a hydrogen storage medium [8–11].
In recent years, researchers have made in-depth studies to improve the thermodynamic and kinetic properties of MgH2.The specific methods include alloying, nano-confining and catalysts addition,etc. [12–15]. Among them, the addition of highly active catalysts has been proved an effectively approach to reduce the activation energy of hydrogen desorption reaction,thus improving the dehydrogenation kinetics[16,17].The explored application of additives with forward catalytic activity include modified carbon materials [18–20], transition metals [21–23] and their oxides [24–26], sulfides [27–29],halides [30–32], and alloy compounds [33–36]. Thereinto,transition metals can effectively weaken the Mg-H bond because hydrogen atoms tend to form covalent bonds with transition metals rather than ionic bonds with magnesium atoms[37,38], where the covalent bond between 3d transition metal elements and H atoms is relatively weaker [39]. Therefore,transition metals have been deemed as the main catalytic active substances and widely used to improve the hydrogen absorption and desorption performance of Mg/MgH2hydrogen storage systems [40,41].
One of the effective transition metal catalyst for Mg/MgH2system is nickel (Ni). The catalytic effect of Ni on hydrogen storage performance of MgH2has been extensively studied.For instance, Chen et al. reported that Ni nanofibers exhibited enhanced catalytic effect on the dehydrogenation of MgH2,which rapidly released 7.02 wt% H2within 11 mins at 598 K[42].Lan’s group showed that through Ni@rGO nanocomposites modification, the apparent activation energy of dehydrogenation of MgH2was reduced by 60.4 kJ·mol-1[43].Except for metal Ni, its compounds, especially nickel sulfides, are also beneficial to the hydrogen absorption and desorption performance of Mg/MgH2system. Wang et al. reported that the hydrogen absorption kinetics of Mg/MgH2system had been significantly improvedviathe addition of NiS2owing to the in-situ formed catalysts of MgS and Mg2NiH4phases during heating process [15]. In Xie’s work, they found that NiS reduced the activation energy of hydrogen desorption of MgH2by 64.71 kJ·mol-1[44]. In addition, transition metal iron (Fe)is also emerged as a highly effective catalyst for hydrogen absorption and desorption of Mg/MgH2system [45,46]. As reported by the deepgoing study of Zhang’s group, both Fe3S4and FeS2could remarkably increase the re/dehydrogenation kinetics of Mg/MgH2system and lower its initial dehydrogenation temperature [47,48]. In details, the initial dehydrogenation temperature of MgH2was reduced by 90 K and 102 K, respectively, after Fe3S4and FeS2were added, and Fe3S4and FeS2changed the rate control steps and promoted H diffusion of MgH2during hydrogen absorption. Despite the progress made to these transition metal catalysts, there still lack suitable catalysts that facilitate the fast hydrogen absorption and desorption for Mg/MgH2system, particularly for the operation at low temperatures.
Recently, studies have pointed out that ternary transition metal sulfides,e.g.FeNi2S4, NiCo2S4and FeCo2S4, are with excellent catalytic activity owing to the synergistic effect between different 3d transition metals, which have been confirmed in the fields of supercapacitors, oxygen and hydrogen evolution reactions [49–52]. Along this line, it is valuable to reveal whether the ternary transition metal sulfides promote hydrogen absorption and desorption properties of Mg/MgH2system. Therefore, herein, we reported the effect of ternary transition metal sulfides of FeNi2S4on hydrogen storage performance of MgH2for the first time, where the hydrogen absorption capacity of MgH2-FeNi2S4composite at 373 K ups to 4.02 wt%, about six times than that of pure MgH2(0.67 wt%), and its initial dehydrogenation temperature is 80 K lower than that of the MgH2. Furthermore, the phase transformation process of the in-situ formation of MgS, Mg2NiH4and Fe species by the reaction of FeNi2S4with Mg/MgH2and the catalytic mechanism of these catalytic active substances are discussed in detail.
2. Experimental section
2.1. Synthesis of FeNi2S4
All materials and reagents in the experiment were used as received without any further purification. FeNi2S4was synthesized by a facile one-step solvothermal method.First, 2 mmol FeSO4·7H2O (Aladdin, ≥99.0%), 4 mmol Ni(CH3COO)2·4H2O (Aladdin, 99.0%) and 16 mmol Lcysteine (Aladdin, 99.0%) were dissolved in a 100 mL mixed solution of deionized water and ethylene glycol (Tianjin Kaitong Chemical Reagent Co. LTD, 99.0%) with a volume ratio of 1:1 and magnetically stirred rapidly for 30 min. The solution was then transferred to a 200 mL Teflon-lined stainless steel autoclave and reacted at 433 K for 8 h. Finally,the black precipitate FeNi2S4was washed several times with deionized water followed by ethyl alcohol and dried at 333 K for 12 h in vacuum.
2.2. Preparation of MgH2-FeNi2S4 composite
The synthesized FeNi2S4was directly mixed with commercial Mg powder (Aladdin, 99.0%) at a mass ratio of 1:10 and hydrogenated at 673 K at 4 MPa for 20 h to form a MgH2-FeNi2S4composite as in our previous study[53].After partial Mg was hydrogenated, high energy ball milling was carried out with a ball-to-powder ratio of 40:1 at 400 rpm for 5 h,during which intermittent method is used (milling for 15 min and then pause for 15 min) to avoid friction overheating. The hydrogenation and ball milling processes were alternately repeated three times to ensure the complete conversion of Mg to MgH2. For comparison, the Mg powder without FeNi2S4was treated under the same condition and denoted as-milled MgH2. All samples’ transfer operations were carried out in a glove box filled with high purity argon (H2O and O2content less than 0.1 ppm). Schematic diagram of synthesis process model of the MgH2-FeNi2S4composite is as shown in Fig.S1.
2.3. Materials characterization
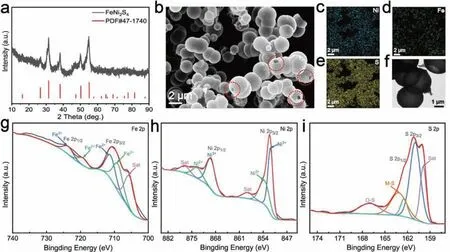
Fig. 1. Microstructure and composition of the FeNi2S4. (a) XRD pattern, (b) SEM images and corresponding elemental mappings of (c) Ni, (d) Fe, and (e)S, (f) TEM image, XPS spectra of (g) Fe 2p, (h) Ni 2p, and (i) S 2p.
X-ray diffraction (XRD) was performed on a Rigaku Smaratlab X-ray diffraction powder diffractometer using Cu Kαradiation (λ= 1.5418 ˚A) in a range of 10°–90° (2θ) at a scanning rate of 5° min–1. X-ray photoelectron spectroscopy(XPS) was conducted on a Thermo ScientificTMK-AlphaTM+spectrometer equipped with a monochromatic Al KαX-ray source (1486.6 eV) operating at 100 W. All peaks would be calibrated with C1s peak binding energy at 284.8 eV for adventitious carbon. The microscopic morphology and element distribution of the samples were studied through scanning electron microscope (SEM, SUPRA 55) with energy spectrometer (EDS) and transmission electron microscopy (TEM,JEM-2010). The particle size distribution was measured from the corresponding SEM images by the Nano Measurer 1.2 software. Inductively coupled plasma-atomic emission spectrometer (ICP) was performed on an iCAP-6300. Differential scanning calorimetry (DSC, STA449C) was used to measure the thermal decomposition of the samples at different heating rates of 5, 10, 15 and 20 K·min–1from room temperature to 823 K under high purity argon condition.
The hydrogen absorption and desorption properties of the MgH2-FeNi2S4and as-milled MgH2samples were measured by Sievert-type pressure-composition-temperature (PCT, Beijing General Research Institute of Nonferrous Metals) volumetric apparatus. The operating pressure of hydrogen absorption and desorption should be higher than the equilibrium pressure of hydrogen absorption of Mg and lower than the equilibrium pressure of hydrogen desorption of MgH2, respectively. Therefore, the hydrogen absorption and desorption pressures were set as 3 MPa and 0.01 MPa. The temperatureprogrammed desorption (TPD) test was conducted from room temperature to 823 K at a heating rate of 5 K·min–1.
3. Results and discussion
3.1. Characteristic of the synthesized FeNi2S4
Fig. 1(a) shows that the XRD pattern of the synthesized FeNi2S4is a cubic structure with a space group Fd-3 m(JCPDS No. 47–1740) in pure phase. The FeNi2S4is corroborated as a hollow balloon structure by the SEM studies(Fig. 1(b)), where damaged mouths of the hollow balloons are observed as marked in red circles. EDS results further demonstrate the evenly distributed Fe, Ni and S elements in the FeNi2S4(Fig.1(c-e)),and the ICP result reveals an atomic ratio of Fe and Ni of approximately 1:2 (Table S1), verifying that material’s formula of FeNi2S4. The same EDS elemental mapping was done on the other parts of the FeNi2S4, and all the elements were overlaid with their corresponding colors,where uniform distribution of the elements could be clearly odserved (Fig. S2). TEM image in Fig. 1(f) also confirms that FeNi2S4has a hollow balloon, of which the diameter is∼2 μm, as attested in the particle size distribution test (Fig.S3).
XPS was further performed to identify the elements of FeNi2S4. The survey spectrum of FeNi2S4proves the presence of Fe, Ni and S, and the appearance of oxygen may be related to the air adsorbed on surface or impurities in the compound containing oxygen groups (Fig. S4). In the Fe 2p spectrum (Fig. 1(g)), there are two main peaks at 711.3 eV and 723.6 eV, which can be attributed to Fe 2p3/2of Fe3+and Fe 2p1/2of Fe2+, respectively. For the Ni 2p spectrum(Fig. 1(h)), the two main spin-orbit peaks 2p3/2(852.0 eV,869.4 eV) and 2p1/2(853.5 eV, 869.4 eV) indicate the presence of nickel oxidation states of Ni2+and Ni3+, respectively.The peak centered at 858.5 eV and 877.0 eV correspond to two shakeup satellites peaks of Ni 2p [50]. The exchange of electrons between different valence bands may provide an environment for electron movement on the phase surface, leading to higher catalytic activity [29, 54]. Fig. 1(i) shows the S 2p spectrum, where the binding energies of 161.1 eV and 162.5 eV correspond to the S 2p3/2and S 2p1/2, and the satellite peak of 163.3 eV corresponds to the metal-sulfur bond in the FeNi2S4; besides, the weaker peak at 167.0 eV is due to the O-S bond. The XPS results further confirm the chemical formula of the ternary transition metal sulfide.
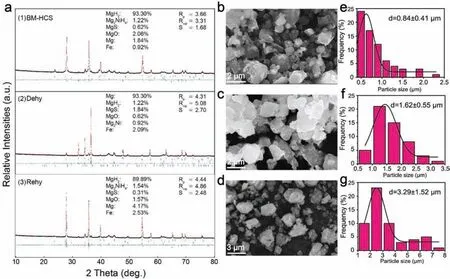
Fig. 2. Microstructure and composition of the MgH2-FeNi2S4 composite after ball-milling, dehydrogenation and rehydrogenation. (a) Rietveld refinement patterns of the XRD patterns of MgH2-FeNi2S4 composite after ball-milling and hydrogenation combustion process (BM-HCS), rehydrogenation and dehydrogenation, where the experimental data, calculated results, Bragg diffraction positions, and the difference profile are marked with black dots, a red curve,blue vertical bars, and a green curve, respectively, and the blue vertical bars from top to bottom belong to the phases listed in the corresponding figures in a same order; SEM images of the (b) ball-milling, (c) dehydrogenation and (d) rehydrogenation samples; Particle size distributions of the (e) ball-milling, (f)dehydrogenation and (g) rehydrogenation samples.
3.2. Phase composition and morphology characterization of MgH2-FeNi2S4 composites
The FeNi2S4compound was composited with Mg powder by 10 wt%to synthesis a MgH2-10wt%FeNi2S4composite by ball-milling and hydrogenation combustion processes (BMHCS). The schematic diagram of the preparation process of the MgH2-FeNi2S4composite is shown in Fig. S1. During the hydrogenation and ball milling processes, the chemical bond of the FeNi2S4will break and react with Mg and H2,and then in-situ generates new phases of MgH2, Mg2NiH4,MgS and Fe, thereby establishing a hydrogen storage system of MgH2/Mg2NiH4-MgS/Fe. Fig. 2(a) compares Rietveld refinement of the XRD patterns of the MgH2-10wt%FeNi2S4composite obtained by the BM-HCS, and after dehydrogenation and rehydrogenation processes. Results show that the obtained MgH2-10wt%FeNi2S4composite after BM-HCS composes MgH2, Mg2NiH4, MgS, MgO, Mg and Fe phases in contents of 93.30 wt%, 1.22 wt%, 0.62 wt%, 2.06 wt%,1.84 wt% and 0.92 wt%, respectively. The emergence of the Mg2NiH4,MgS and Fe phases proves the formation of heterogeneous catalytic system. Moreover, no new compound forms after rehydrogenation compared with the original composite,however the contents of Mg2NiH4(89.89 wt%), Mg (4.17 wt%) and Fe (2.53 wt%) increased slightly while the contents of MgH2(1.54 wt%), MgS (0.31 wt%) and MgO (1.57 wt%) decreased after multiple hydrogenation. Noteably, Mg is found in the XRD patterns for both samples, with a content of 1.84 wt% and 4.17 wt% respectively, which is caused by the inherent nature of Mg powder and the short time of experimental data collection. The appearance of MgO is inevitable because the sample is briefly exposed to air during testing,while Mg is very easily corroded by oxygen and water vapor in the air.
Based on the XRD characterization of hydrogen absorption, the possible phase transformation reaction of FeNi2S4with Mg during hydrogenation can be described as follows:

Fig. 3. Hydrogen absorption performance of the MgH2-FeNi2S4 composite and ball-milled MgH2. (a) Isothermal absorption kinetics at different temperatures(3.0 MPa); (b) Hydrogen absorption capacity at different temperatures (3.0 MPa); (c) Isothermal absorption kinetics at 473 K (3.0 MPa); (d) Isothermal absorption kinetics at 373 K (3.0 MPa).
For dehydrogenation (Fig. 2(a)), MgH2and Mg2NiH4transform into Mg and Mg2Ni respectively. The diffraction of MgS and Fe still exist, and their contents increase to 1.84 wt% and 2.09 wt%, respectively, which may be that the reaction in Eq. (1) has not stabilized yet. Fig. 2(b-d) shows the SEM images of the above samples, where we find that the particle size of the sample noticeably increases after dehydrogenation and rehydrogenation, which are 1.62 μm and 3.29 μm, respectively, but just 0.84 μm for the original composite (Fig. 2(e-g)). One reason is that in the process of hydrogen absorption, the entry of hydrogen leads to the lattice expansion of the in-situ generated Mg and Mg2Ni. Table S2 shows that the cell volumes of Mg and Fe increase significantly after rehydrogenation, and the expansion rates were 0.252% and 0.516%, respectively. With the increase hydrogen absorption cycles, the lattice continues to expand, and the grain spacing gradually increases, eventually leads to the increase of particle size in a certain degree.However,the most important reason is that the agglomeration effect of small particles causes the increase of particle size, which is inevitable in the reaction process.
3.3. Hydrogen absorption and desorption kinetics
Fig. 3 displays the isothermal absorption kinetics curves of the MgH2-FeNi2S4composite and the as-milled MgH2at different temperatures. As can be seen from Fig. 3(a), the hydrogen absorption capacity of the ball-milled MgH2is higher than that of the MgH2-FeNi2S4composite at high temperatures (573 K–623 K), because the theoretical capacity of the composite is reduced after the addition of FeNi2S4and a small amount of Mg has been converted to MgS during hydrogenation. However, with the decrease of operating temperature,the hydrogen absorption of the ball-milled MgH2drops off in a cliff-like manner, while the MgH2-FeNi2S4shows excellent low temperature hydrogen absorption performance(Fig.3(b)).The MgH2-FeNi2S4composite exhibits faster hydrogen absorption rate and higher hydrogen absorption capacity when the temperature is below 523 K. At 473 K, the hydrogen absorption capacity of the MgH2-FeNi2S4composite in 1 h reaches 5.8 wt%, about 3.1 times than that of the pure Mg(1.89 wt%), and it can rapidly absorb 4.7 wt% H2just within 60 s (Fig. 3(c)). Further, as the temperature reduces to 373 K,the composite still absorbs 4.02 wt%H2in 1 h,while the pure Mg can only absorb 0.67 wt% H2(Fig. 3(d)).
The improvement on the hydrogen desorption performance of MgH2viacompositing with FeNi2S4is also reflected in the rate and capacity. The dehydrogenation rate of the composite is much faster than that of MgH2at 623 K (Fig. 4(a)),moreover, when the temperature is lowered to 573 K, the ball-milled MgH2almost no longer desorbs hydrogen, while the MgH2-FeNi2S4composite still releases 1.92 wt% H2(Fig. 4(b)). The initial hydrogen desorption temperature is another important indicator to measure the hydrogen desorption property of hydrogen storage materials. Fig. 4(c) shows that the initial hydrogen release temperature of the MgH2-FeNi2S4composite is ∼540 K, which is ∼80 K lower than that of the ground MgH2(∼620 K). In addition, the desorption peak temperature of the composite is ∼622 K (Fig. 4(d)), ∼50 K lower than that of the ball-milled MgH2(672 K). The above results indicate that the FeNi2S4has outstanding catalytic activity on dehydrogenation of MgH2, because Mg2NiH4can dehydrogenate in advance of MgH2, and Fe and MgS catalysis can work synergistically on the dehydrogenation of MgH2[55,56].

Fig. 4. Hydrogen desorption performance of the MgH2-FeNi2S4 composite and ball-milled MgH2. Isothermal desorption kinetics of the MgH2-FeNi2S4 composite and ball-milled MgH2 at (a) 623 K (0.01 MPa) and (b) 573 K (0.01 MPa); (c) TPD and (d) differential curves of the MgH2-FeNi2S4 composite and ball-milled MgH2.

Table 1.Theoretical kinetic models and goodness of fitting for the sorption kinetic data of MgH2-FeNi2S4. α= reacted fraction, t = time, k = reaction rate constant[59].
To clarify the catalytic mechanism of FeNi2S4on hydrogen absorption and desorption reactions of MgH2, the kinetic mechanism of hydrogenation and dehydrogenation of the composite are analyzed. Theoretical mechanical equations of contracting volume (CV) model and Johnson-Mehl-Avrami(JMA) model are used to fit the isothermal hydrogen absorption and desorption data (Table 1), where the Johnson-Mehl-Avrami (JMA) model is suitable for the case that the nucleation and growth of the new phase randomly start from the bulk phase, but nucleation begins at the surface and continues to grow from the surface to bulk in the contracting volume(CV) model [57].
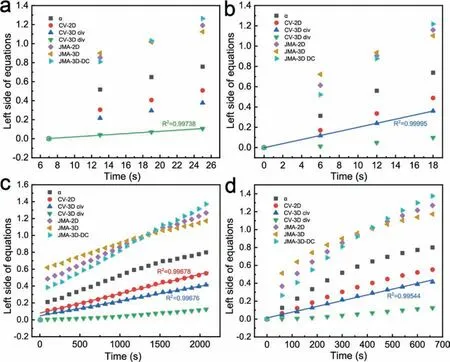
Fig.5. JMA model and CV model fitting curves for isothermal hydrogen absorption and desorption data of the MgH2-FeNi2S4 composite.Hydrogen absorption at (a) 598 K, (b) 473 K and (c) 373 K; (d) Hydrogen desorption at 598 K.
The absorption and desorption kinetic curves of the MgH2-FeNi2S4composite is calculated based on the data within the capacity of 0–80 wt%[31].Notably,the rate-limiting step differs at different temperatures during hydrogenation (Fig. 5(ac)). At 598 K, the rate-limiting step is a three-dimensional growth and a diffusion-controlled process by the decreasing interface velocity (CV 3D div) (Fig. 5(a)). When the temperature drops, the corresponding theoretical kinetic model changes. At 473 K, the rate-limiting step can be described by a three-dimensional growth model with constant interface velocity (CV 3D civ) (Fig. 5(b)), while at 373 K, the best equation describing the hydriding rate of the composite becomes uncertain (Fig. 5(c)). As can be seen in Fig. 5(c), the fit results calculated using the CV 2D formula and the corresponding formula for CV 3D civ are extremely close, with R2of 0.99678 and 0.99676, respectively. Due to the stubborn thermodynamic barrier of MgH2, the H diffusion rate and nucleation rate of the hydrogen storage system are affected as the temperature decreases.The above results suggest that temperature is an important factor limiting the hydrogen absorption rate except for the catalyst,which is due to the inherently stable thermodynamic properties of MgH2. For dehydrogenation of the composite, the rate-limiting step at 598 K is consistent with the three-dimensional growth model of contracting volume at constant interfacial velocity (Fig. 5(d)), which demonstrates that the dehydrogenation reaction starts from the surface, and the reaction is controlled by the interfacial speed of the grain boundary transformation phase. In other words,due to the large particle size of the hydride, hydrogen ion diffusion is the rate-limiting step during dehydrogenation reaction. It is worth noting that the dehydrogenation reaction is initiated by the H-anion binding, namely the decomposition of the Mg-H bond, but the strong Mg-H bond has a high energy barrier, therefore it is necessary to study the weakening effect of FeNi2S4on the Mg-H bond [57,58].
3.4. Dehydrogenation kinetics and thermodynamics
Dehydrogenation activation energy (Ea) is an important index to evaluate the kinetic performance of hydrogen storage materials, which can be calculated according to parameters obtained from the DSC test based on Kissinger method [63].Fig.6(a)shows the DSC curves during dehydrogenation of the MgH2-FeNi2S4composite. Apparently, the endothermic peak temperature of the composite is much lower than that of the ball-milled MgH2(Fig. S5), which is consistent with the TPD results.Moreover,the DSC curves of the MgH2-FeNi2S4composite present two endothermic peaks, which correspond the hydrogen release processes of Mg2NiH4and MgH2, respectively. TheEavalue is calculated by the following Kissinger’s equation as Eq. (2):

Fig. 6. Dehydrogenation activation energy and cycling performance of the MgH2-FeNi2S4 composite. (a) DSC dehydrogenation temperature profiles; (b)Kissinger plots compared with MgH2; (c) Kinetic curves of isothermal hydrogen absorption and desorption at 598 K for within 10 cycles; (d) XRD patterns after cycling 10 times.
whereαis the heating rate,Tmis the peak temperature, and R is the gas constant. TheEavalue of the ball-milled MgH2is 161.2 kJ·mol–1(Fig.6(b)),but it decreases to 90.9 kJ·mol–1and 65.5 kJ·mol–1for the MgH2-FeNi2S4composite, respectively, for the two stages of hydrogen desorption as circled by green ellipses and marked as 1 and 2 in Fig.6(a).TPD curves should also show two platforms corresponding the two hydrogen desorption stages according to the reports in the literature[29],but it is not obvious in this study.The appearance of two endothermic peaks may be due to that the hydrogen desorption of Mg2NiH4and MgH2is simultaneous in the first stage,but Mg2NiH4is dominant. In the second stage, as the temperature rises, most of MgH2absorbs heat and releases hydrogen. Meanwhile, in order to further explore the improvement of thermodynamic properties of MgH2by adding FeNi2S4,the dehydrogenation enthalpy change of MgH2-FeNi2S4composite was measured by integrating DSC peaks. The dehydrogenation enthalpy change of MgH2-FeNi2S4composite is determined to be 1552 J·g–1H2, which is lower than the ballmilled MgH2, indicating that FeNi2S4has a positive effect on the improvement of the thermodynamic properties of MgH2.In addition, it is worth noting that compared with the hydrogen storage system doped with other transition metal sulfides in related literatures as shown in Table 2, theEavalue of the MgH2-FeNi2S4composite is at an upstream level. In conclusion, the addition of transition metal sulfide FeNi2S4reduces the energy barrier for the binding of hydrogen anions in thesystem, thus reducing the hydrogen desorption activation energy of MgH2to a large extent.
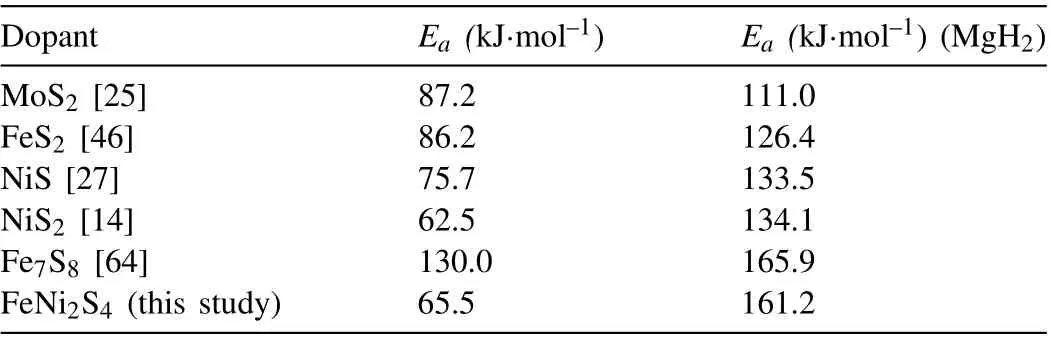
Table 2.The apparent activation energy(Ea)values of several MgH2-catalyst systems.
3.5. Cycling performance

Fig. 7. Structure and Morphology of the MgH2-FeNi2S4 composite after hydrogenation and dehydrogenation. (a) TEM image, (b) SAED pattern, and (c)HRTEM image after hydrogenation; (d) TEM image, (e) SAED pattern, and (f) HRTEM image after dehydrogenation.
In order to study the cycle stability of the MgH2-FeNi2S4composite,the isothermal hydrogenation and dehydrogenation within 10 cycles were carried out at 325 K and 3.0 MPa. As shown in Fig. 6(c), the capacity and rate of hydrogen absorption and desorption of the MgH2-FeNi2S4composite hardly decay within 10 cycles, indicating its good cycle stability.However, the hydrogen desorption capacity of the composite is 1.5 wt% lower than the hydrogen absorption capacity on average(Fig.S6).This is due to the inherent stable thermodynamic performance of MgH2. The XRD patterns of the composite after hydrogenation and dehydrogenation at the 10thcycle are shown in Fig. 6(d), where no significant change occurrs compared with the previous XRD. Since the temperature of the hydrogen absorption and desorption cycle is 598 K and the period is only 30 min, the hydrogen absorption and desorption processes are not fully completed, so Mg and MgH2are inevitably found in the diffractions after hydrogenation and dehydrogenation, respectively. SEM shows the morphology of the MgH2-FeNi2S4compositeviahydrogenation after cycling 10 times (Fig. S6). Notably, the particles slightly agglomerate due to Van der Waals attraction after cycling [65,66], which is consistent with the previous description (Fig. 2(c)). In terms of element distribution, MgS, Fe and Mg2NiH4are distributed uniformly in the MgH2system;thereinto, the continuous catalytic action of MgS and Fe is the important factor for maintaining the cyclic stability of the composite.
3.6. Catalytic mechanism
To further investigate the catalytic reaction mechanism of FeNi2S4in Mg/MgH2system, TEM study was carried out. Fig. 7(a) and (d) show the TEM images of the MgH2-FeNi2S4composite after hydrogenation and dehydrogenation.As shown in the corresponding SEAD patterns, the diffraction rings of MgS and Fe appear in both hydrogenation and dehydrogenation samples, and the MgH2and Mg2NiH4in the hydrogenation sample become Mg and Mg2Ni after the hydrogen is released (Fig. 7(b) and (e)). The lattice fringes corresponding to the (111) plane of MgH2and the (220)plane of Mg2NiH4with interplanar spacing of 0.2194 nm and 0.2289 nm, respectively, are also clearly observed from HRTEM (Fig. 7(c)). After dehydrogenation, the interplanar spacings of 0.2451 nm and 0.2247 nm of the sample corresponding to the Mg (111) plane and the Mg2Ni (200)plane are observed in Fig. 7(f). The crystal planes of MgS(200) and Fe (110) remain the same after hydrogen absorption and desorption, but there is a slight measurement deviation for the lattice spacing. These results as well as the corresponding SAED pattern are consistent with the previous XRD results, revealing the reaction process of FeNi2S4in Mg/MgH2system. More importantly, we can observe that the catalytic active of MgS and Fe species disperse among the main phase Mg/MgH2from the HRTEM images. Such dispersion state is conducive to improving the hydrogen absorption and desorption performance of the composite. At the same time, Mg2Ni and Mg2NiH4, as inducible phases, can absorb and desorb hydrogen prior to Mg/MgH2system because of their lower hydrogenation and dehydrogenation temperatures, so they can be considered as “hydrogen pump” in the composite. Besides, XPS spectra of Fe, Ni and S elements of the composite after multiple rehydrogenation and dehydrogenation processes also confirm the stable catalytic system, where the peak location and peak strength are almost the same, and no significant changes in valence states(Fig. S7).
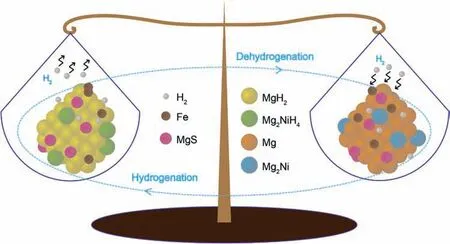
Fig. 8. Schematic diagram of the hydrogenation/dehydrogenation processes of the MgH2-FeNi2S4 composite.
According to the above results, the hydrogen absorption and desorption schematic diagram of the MgH2-FeNi2S4composite is used to reveal the catalytic mechanism of FeNi2S4on the MgH2, which is as shown in Fig. 8. In fact, in the previous preparation process, the Mg/Mg2Ni-MgS/Fe hydrogenation system and the MgH2/Mg2NiH4-MgS/Fe dehydrogenation system has been successfully constructed. During hydrogenation, because of the ahead hydrogen absorption of Mg2Ni before Mg and the existence of MgS/Fe, the diffusion path of hydrogen atoms is changed, so that the system can quickly absorb hydrogen at a lower temperature.During dehydrogenation, on one hand, Mg2NiH4with low hydrogen desorption enthalpy induces MgH2to dehydrogenate at a lower temperature [29,67]. On the other hand, since the transition metals Fe and Ni have a strong affinity for H, in order to fill the 3d orbitals lacking electrons, the transition metals can easily absorb hydrogen atoms and release H2molecules from the hydrogen storage system [68–70]. In addition, the catalytic MgS exists stably in the hydrogen storage system, and provides more interfaces and channels for the rapid diffusion of H with other catalytically active substances. It is worth noting that MgS and Fe also play a synergistic catalytic role on the kinetics of de/hydrogenation performance of MgH2[47]. In summary, Mg2Ni/Mg2NiH4, MgS and Fe form a heterogeneous catalytic system, which has a multiphase interface and provides more active sites and diffusion channels for hydrogen atoms. Mg2Ni/Mg2NiH4, MgS and Fe serve as catalytic active substances, in which Mg2Ni/Mg2NiH4acts as the “hydrogen pump” driving Mg/MgH2hydrogen absorption and discharge, and MgS/Fe provides the catalytic active center to accelerate the transfer of H [71,72]. Therefore, the synergistic catalytic action of Mg2Ni/Mg2NiH4,MgS and Fe improves the hydrogen storage performance of Mg/MgH2.
4. Conclusion
In this study, a hydrogen storage material of the MgH2-FeNi2S4composite were prepared by hydrogenation combustion synthesis and ball milling method. The MgH2/Mg2NiH4-MgS/Fe heterogeneous catalytic system has been successfully constructed and shows excellent hydrogen storage performance and cycling stability. The MgH2-FeNi2S4composite can absorb 6.09 wt% H2and release 1.92 wt% H2in 1 h at 573 K. Noticeably, the composite is able to absorb 4.02 wt% H2at 373 K under 3.0 MPa H2, and the initial hydrogen desorption temperature is also reduced by about 80 K.In terms of desorption kinetics, the desorption activation energy of the composite with the addition of catalyst decreases from 161.2 kJ·mol–1of the pure MgH2to 65.5 kJ·mol–1. After 10 cycles of hydrogen absorption and desorption, the hydrogenation and dehydrogenation capacity of the composite still reach 98.9% and 99.7%, respectively. The in-situ generated Mg2NiH4/Mg2Ni during hydrogenation/desorptionation acts as “hydrogen pump” in the composite, while the stable MgS and Fe can be used to provide continuous catalysis for the system, therefore, the synergistic catalytic action of Mg2Ni/Mg2NiH4, MgS and Fe improves the hydrogen storage performance of Mg/MgH2.This work extends the horizon of ternary transition metal sulfides with special structures as catalysts to improve the hydrogen absorption and release performance of MgH2, and provides new ideas for the development of efficient and stable catalysts to enhance the hydrogen storage performance of Mg-based hydrogen storage materials.Based on FeNi2S4, the influence of other transition metal sulfides on MgH2will also be investigated in future studies, and the introduction of carbon materials will be considered to disperse the entire heterogeneous catalytic system and provide more catalytic active sites.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (grant numbers 52071281 and 51971197), the Natural Science Foundation of Hebei Province (grant numbers E2019203161, E2019203414 and E2020203081), and Science and Technology Major project of Inner Mongolia (2020ZD0012).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.sipas.2020.100009.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Characterizations on the instantaneously formed Ni-containing intermetallics in magnesium alloys
- Corrosion resistance of Mg-Al-LDH steam coating on AZ80 Mg alloy:Effects of citric acid pretreatment and intermetallic compounds
- Gradient structure induced simultaneous enhancement of strength and ductility in AZ31 Mg alloy with twin-twin interactions
- In-depth analysis of the influence of bio-silica filler (Didymosphenia geminata frustules) on the properties of Mg matrix composites
- Effect of Cd on matrix structure ordering and aging precipitation evolution in a Mg-Gd-Cd solid-solution alloy
- A multifunctional osteogenic system of ultrasonically spray deposited bone-active coatings on plasma-activated magnesium
