A multifunctional osteogenic system of ultrasonically spray deposited bone-active coatings on plasma-activated magnesium
2023-11-18HuiFangShichengZhouXiaoyunQiChenxiWangYanhongTian
Hui Fang, Shicheng Zhou, Xiaoyun Qi, Chenxi Wang, Yanhong Tian
State Key Laboratory of Advanced Welding and Joining, Harbin Institute of Technology, Harbin 150001, China
Received 8 July 2021; received in revised form 18 October 2021; accepted 20 October 2021
Available online 6 December 2021
Abstract Biomimetic bone-active coatings composed of inorganic nano-hydroxyapatite(i.e.,nHA)and organic silk fibroin(i.e.,SF)are layer-by-layer deposited on Mg-Zn-Ca alloy by a controllable ultrasonic spray method. Meanwhile, plasma activation is developed as a promising strategy to pretreat magnesium surfaces, which facilitates the direct adhesion of coatings with enhanced bonding interfaces. In this work, we engineer the nHA/SF composite coatings with excellent mechanical properties and adhesion force. The optimized parameters of ultrasonic spray bring significant influence on the surface morphologies of coatings. Assisted by hybrid plasma of oxygen and nitrogen (i.e., O2/N2 plasma), the activated Mg-Zn-Ca surfaces are uniformly covered by a robust and compact nHA/SF composite coating, establishing a multifunctional system with superior corrosion resistance and biological performance. Interestingly, secondary oxygen plasma treatment of nHA/SF coatings(A-nHA/SF) promotes the hydrophilicity, leading to a rapid self-repair effect from surface damage. The improvement of anti-corrosion and self-repair provides a dependable platform for better cell adhesion, proliferation, spreading and differentiation. These favorable factors contribute to the preferable in vivo biocompatibility and the promotion of newly formed bones for the A-nHA/SF-coated Mg implants.This study lays important foundations for coating strategy on biomedical magnesium alloy as multifunctional osteogenic system in bioactive implantable applications.
Keywords: Magnesium alloy; Hydroxyapatite; Silk fibroin; Biocompatibility; Self-repair.
1. Introduction
At present, human beings are rapidly aging, and a large number of patients are facing orthopedic diseases every year,such as fracture,arthritis,osteonecrosis and so on[1–3].Ideal bone repair materials are expected for treatment of various orthopedic diseases. Magnesium and its alloys possess low density, excellent mechanical properties and good biocompatibility, which are considered as promising biodegradable implants in orthopedics [4–6]. Compared with other medical metals, such as stainless steel, titanium and cobalt chromium alloys, the elastic modulus of magnesium alloy is quite close to that of human bone, thus reducing the stress shielding effect caused by the mismatching difference [7–9]. Moreover,its degradability provides temporary mechanical support in the early stage of recovery, and subsequently allows gradual degradation in physiological environment, which avoids suffering the second surgical operation [10,11]. However, the serious and over-quick corrosion of magnesium alloy along with local alkalinity and hydrogen evolution has been commonly emerging, the surface treatment of which has to be developed [12–14]. Coating technology is generally accepted to be applied on magnesium alloy surface as a protective barrier, which has been variously designed to delay its degradation rate [15,16]. Following the pace of coating strategy,extensive attempts including natural organics (chitosan (CS)[17], collagen (Col) [18], silk fibroin (SF) [19]), bioactive inorganics (dicalcium phosphate-dihydrate (DCPD) [20], tricalcium phosphate (TCP) [21,22], hydroxyapatite (HA) [23])and synthetic polymer (polylactic acid (PLA) [24], poly(latic-co-glycolic) acid (PLGA) [25], polycaprolactone (PCL)[26]),even their combinations,have been implemented to protect magnesium substrates against corrosion. There are still some challenges in the aspects of material design, surface pretreatment and coating preparation.
Inspired by the components of natural bone tissue, inorganic hydroxyapatite (HA, Ca10(PO4)6(OH)2) and organic collagen protein (Col) play important roles in bone matrix,which determines the osteogenic properties. HA accounts for about 65%∼70% of the total mass, which generally exists as needle or columnar crystals in nanostructure [27,28]. Due to excellent osteoinductivity and osteoconductivity, HA is regarded as one of bioactive ceramic materials, and has been widely studied in biomaterials field [29]. It has been reported that Mg-based alloys (such as ZA31 [30] and ZA91 [31])with the deposited HA coating have showed improved bioactivity via radio-frequency magnetron sputtering method. The well-known collagen is the most abundant protein component of extracellular matrix (ECM) of many tissues such as skin, bone and muscle, which makes up 90%–95% of the organic ECM, osteocalcin, osteopontin, osteonectin, bone sialoprotein, hyaluronan and proteoglycans [32]. Protein framework of bone tissue is supported by collagen with the structure of fibers, and its synthesis and decomposition conduct as the specific indicators of bone tissue formation and absorption [33]. Collagen possesses outstanding biological activity; however, it is easy to be absorbed by human body after being decomposed into small molecules blaming on its fast degradation rate and insufficient mechanical stress intensity.Instead, silk fibroin (i.e., SF), as another important natural fibrous protein, can be abundantly derived from Bombyx mori silkworms,spiders and beetles with diverse functions in abundance, and also endowed with unique mechanical properties,tunable biodegradation rate and highly biocompatible characteristics [34–36]. To date, it has been confirmed that SF was talented in triggering the expression of the early and late differentiation markers of osteoblasts [37,38]. Thus, SF considered as a potential candidate for Col, possibly becomes a better companion with HA used in bone tissue engineering.It is expected that blending nano-hydroxyapatite (i.e., nHA)with SF solution to prepare biomimetic bone-active coatings could achieve the improvement of corrosion resistance and osteogenic function, even possessing some unexpected capabilities [39,40].
Unfortunately, the designed nHA/SF biomimetic coatings cannot tightly adhere to the surface of magnesium substrates without any pretreatment, which has been reported as a common issue in many cases. The three most commonly used pretreatment processes are, respectively, preparing chemical conversion layer [41,42], micro arc oxidation (MAO) layer[23,43] and intermediate layer [25,44] basing on magnesium surfaces, increasing the deficient functional groups thus improving the adhesion force. Chemical conversion layers, such as magnesium hydroxide and magnesium fluoride coatings,are easy to generate by alkaline (NaOH) [45] and acid (HF)[39], respectively, which lack of long-term protection and biosafetyin vivo. MAO processes are widely used to enhance the adhesion of coatings on substrates, commercially applied in surface pretreatment of magnesium alloy.On the downside,another coating needs to be subsequently prepared to seal the holes on the brittle MAO layers, so that preventing corrosive ions from diffusing into magnesium surfaces. There are also some processes to directly immerse magnesium substrates into the adhesive solution mainly composed of polydopamine(PDA) or 3-amino-propyltriethoxysilane (APTES) with abundant functional groups bonding with outer coatings [46,47].Their biocompatibility and degradation behaviors need further confirming. In this case, plasma surface activation can replace the formed middle layers fabricated by those above pretreatment, achieving direct adhesion of nHA/SF coatings on magnesium alloy and avoiding more possibility of prepared interfaces cracking. Inductive coupling plasma includes charged and neutral particles generated from partially ionized gas, which might exhibit changed surface states after activation. The plasma system is a vacuum compatible system. Various particles in the plasma have certain energy and high activity. Therefore, when the material is immersed in the plasma atmosphere, these active particles can interact with the material surface to produce surface reaction and make the surface change biophysically and chemically to realize surface modification. Moreover, chemical traces and adsorbed other impurities in the grinding of magnesium alloy can be removed by the low-temperature treatment compared to conventional methods. Surface modification of plasma activation possesses many superiorities, such like strong space homogeneity, uniform surface treatment, handling complex shape materials and disinfecting the substrates, which is very suitable for prior treatment of medical devices [48–50]. Moreover, the interaction between the low-temperature plasma and substrates only involves the shallow surface (less than tens of nanometers), and will not damage the original characteristics of magnesium alloys [51,52]. In our previous studies,two-step plasma activation of oxygen followed by nitrogen plasma has been investigated as an environmentally friendly method to pretreat magnesium alloys, successfully fabricating SF-coated Mg structure without any middle layer [53]. However, the one-step activation process of oxygen and nitrogen hybrid plasma (i.e., O2/N2plasma) is easier to be precisely controlled and realized in actual production operation, which hopefully provides analogous effect for the improvement of adhesion force more effectively.
Conventional preparation for coating deposited on magnesium substrate include simple immersion, dropping, spinning, sol–gel process, electric spraying and thermal spraying methods [21,23,42,53]. Among them, there are some inconvenient factors for large-scale application, including low material utilization, poor manufacturing efficiency and unevenly rough surfaces, which is not advantageous for manufacturing productions with diversified shapes and complex coating system. To solve the above problems, an interesting technique,ultrasonic spray deposition is developed to prepare various coatings on different surfaces, showing its unique features such as being large scale,simple operation and cost efficiency[54,55]. There are two main processes in ultrasonic spray deposition technology, that is, ultrasonic atomization and spray deposition. Ultrasonic atomization is to break liquid into the gas phase under the effect of ultrasonic wave to form tiny fog droplets. Ultrasonic spraying mainly converts the highfrequency oscillating electromagnetic energy into the mechanical properties of the liquid through piezoelectric materials to make the liquid vibrate. Under the ultrasonic wave, the liquid is broken to form tiny fog droplets with micron size.Tiny droplets guided by inert gas, finally reach the surface of the substrate. Even for multi-component coating system,the ultrasonic spray device can provide a controllable layer with uniform morphologies after optimizing the process parameters. Micron-sized droplets are formed by atomization of liquid, which possess larger contact area infiltrating into the substrate surfaces, not only providing superior surface homogeneity but promoting the adhesion of coating. For the engineered nHA/SF composite coating, it is a better choice to prepare by two-fluid ultrasonic spray deposition method, involving the gas and liquid [56]. Because the nano particles of hydroxyapatite do not tend to agglomerate under the vibration frequency of the ultrasonic nozzle, the blended nHA/SF solution prefers smooth spurt at the appropriate gas and liquid flow rates, evenly transported on the surface of plasma activated magnesium alloy. The intact coatings are formed on substrates after complete solvent evaporation, and the desired patterned morphology can even be achieved by specific masks for certain biological functions.
In this study, we considered three aspects including material design, surface pretreatment and coating preparation. The ultrasonic spray deposition was introduced to directly fabricate bone bionic nHA/SF composite coatings on biomedical magnesium alloy assisted by plasma activation pretreatment,aiming at a multifunctional osteogenic coating system. The optimum proportion of nHA and SF in composite coatings was explored to obtain excellent mechanical properties. Prior O2/N2plasma activation was expected to improve the adhesion force, and its influences on long-term corrosion process and biocompatibility were investigated. Moreover, the appropriate ultrasonic spray parameters were conducted to prepare dense and uniform coatings, which deposited on the plasma activated magnesium substrates layer-by-layer with distinctive corrosion resistance. The bonding model for the interaction among nHA, SF and Mg were clarified as well as the corrosion mechanism. To seek rapid and efficient self-repair capability, the secondary plasma activation process of oxygen was conducted on prepared coatings. It is believed to be a prospective strategy that could be applied in bone tissue engineering.
2. Materials and methods
2.1. Materials and coatings
The extruded Mg-2.0 wt%Zn-1.0 wt%Ca alloy was cut into specimens with dimensions ofφ12 × 2 mm in size,and ground by SiC paper with different grit sizes from 800 to 2000 successively. Polished specimens were ultrasonically rinsed with acetone and ethanol for 15 min,and dried in flowing nitrogen. Lyophilized silk fibroin sponges (Simatech Inc.,Suzhou, China) were directly dissolved in deionized water with the solution final concentration of 2.0 wt%. Nano hydroxyapatite particles (99%, ∼20 nm, Macklin) were used to mix into silk fibroin solution with the mass ratio of 1/5(nHA/SF5), 1/10 (nHA/SF10) and 1/20 (nHA/SF20), respectively, and then stirred until the solution was well mixed for the preparation of SH composite coatings.
2.2. Plasma surface activation
Plasma is a partially ionized gas containing charged and neutral particles, which includes positive and negative ions,neutral radicals, atoms or molecules, and UV radiations [51].The plasma can induce physical and chemical effect on the substrate surfaces. During prior activation process, the Mg-Zn-Ca substrates were placed in the plasma chamber, which was evacuated using a vacuum pump. The selected incoming O2and N2were controlled at each flow rate of 0.5 L/min.The power and frequency of the discharge were 200 W and 13.56 MHz, respectively. The plasma was generated by discharge between the ion-trapping metal plate and electrode.Batch samples were treated for 120 s before subsequent coating process (Fig. S1A).
2.3. Ultrasonic spray deposition
Two-fluid ultrasonic spray deposition system mainly is composed of four modules including gas flow, liquid flow,spray nozzle and ultrasound generator, as shown in Fig. S1B.A distance of 8 cm was maintained between the nozzle and substrate, and the ultrasonic spray coating was performed using a 50 kHz ultrasonic spray nozzle. The gas flow module delivered the high purity argon to the spray nozzle with speed range from 2 L/min–25 L/min. The incoming liquid velocity was controlled from 0.1 mL/h–300 mL/h. The incoming gas velocity, liquid velocity and spray process cycle are important parameters for the formation of uniform and compact coatings. At the appropriate liquid velocity, the mixed solution can be atomized smoothly. Under the carrier of optimum gas flow, the mixed solution is taken to the substrate surfaces.The real-time monitoring of the specimen was performed by quantitative delivery during the spray process cycle.
2.4. Characterization methods
Transmission electron microscopy (TEM, Tecnai G2 F30,FEI Co. USA) was used to analyze the microstructures of the nHA particles blended with SF, and the morphologies of coating surface were observed by scanning electron microscopy (SEM, MERLIN Compact, ZEISS). Atomic force microscopy (AFM, Dimension Fastscan, Bruker) was applied for more microscopic morphology and surface roughness of coating surface in size of 2 × 2 μm2. Flourier transform infrared (FT-IR, Nicolet is 50) and X-ray diffraction (XRD,X’PERT) were conducted to investigate the chemical interaction between nHA and SF, and X-ray photoelectron spectroscopy (XPS, Thermo Fisher Scientific) were carried out to analyze the chemical states of the coated samples. Additionally, the mechanical properties of coatings were measured by nanoindentation test using Agilent Nano Indenter G200 to assess the hardness and modulus.The surface approach velocity, depth limit and surface approach distance were 10 nm/s,800 nm and 3000 nm, respectively. The maximum indentation depth was holding for 15 s to eliminate the influence of material creep. To investigate the adhesion force between coating and substrate, the nanoscratch test was performed to estimate the bonding strength at the interface. An increased force from 0 mN to 100 mN was loaded onto coated samples,penetrating the coatings and leaving 300 μm scratch observed by SEM. The scratch velocity was 30 μm/s. At least three measurements were carried out on each coated sample during both tests.
2.5. Corrosion behavior
2.5.1. Electrochemical measurement
The electrochemical workstation (CHENHUA CHI660E,Shanghai) was used to investigate the corrosion performance of bare and three coated samples (pure nHA coating, pure SF coating and nHA/SF composite coating) by a standard threeelectrode system. The samples, the saturated calomel electrode and the platinum electrode were respectively applied as working electrode, reference electrode, and counter electrode in Hank’s solution (KCl 0.4 g/L, NaCl 8.0 g/L, CaCl20.14 g/L, KH2PO40.06 g/L, Na2HPO4•12H2O 0.12 g/L,MgSO4•7H2O 0.2 g/L, NaHCO30.35 g/L, pH = 7.4) at 37 °C. The exposed sample surface area in solution was 0.28 cm2. After all the samples were stabilized in the electrolyte environment, the electrochemical impedance spectroscopy (EIS) measurement was conducted at a frequency range of 105Hz to 10-2Hz with a single amplitude of 10 mV.Fitting curves were performed with the ZSimpwin 3.30 software, and the error rate of fitting impedance value was controlled less than 10%.
2.5.2. Long-term immersion
Immersion experiments were conducted as long as 28 days in Hank’s solution at 37 °C, and the samples were divided into four groups of bare, SF and nHA/SF-coated samples, as well as nHA/SF-coated samples without prior plasma activation (nHA/SFD). After immersing for 1, 7, 14, 21 and 28 days,the surface and cross-sectional morphologies of samples were observed by SEM and detected by the equipped energy dispersive X-ray spectroscopy (EDS). During immersion, the pH value and hydrogen released volume of all groups were measured, as well as the weight loss. The degradable rate was estimated according to the following Eq. (1), wherewis the degradable rate, Δmis the weight loss,tis the immersion time andSis the exposed area. The immersed samples were raised by the solution of 10 g/L AgNO3and 200 g/L CrO3to remove the corrosion products, subsequently rinsed with deionized water and then dried in air. FTIR and XRD were used to analyze the composition of corrosion products after long-term immersion test.
2.6. Cytocompatibility assessment
2.6.1. Indirect cell assay
Murine MC3T3-E1 pre-osteoblast cells were cultured in a cell culture medium with alpha-minimum essential medium(α-MEM, Gibco, USA) containing 10% fetal bovine serum(FBS, Gibco, USA) and antibiotics (100 units/mL penicillin and 100 μg/mL streptomycin) in a humidified incubator with 5% CO2at 37 °C. All the samples were sterilized under UV radiation for 1 h before biocompatibility measurements. For the indirect cell assay, the sterilized samples were immersed in non-FBSα-MEM for 72 h with an extraction ratio of surface area to volume of 1.25 cm2/mL in the same culture environment according to ISO10993-12. The obtained extracts were preserved at 4 °C prior to the indirect cell experiments.The seeded MC3T3-E1 cells were cultured in a 96-well plate at a density of 1 × 104cells per well, and then incubated in a humidified incubator with 5% CO2at 37 °C for 24 h.After that, the culture medium was replaced by the preserved extracts with equal volume, and cultured for 1, 3, 5 and 7 days. Cell viability was evaluated using a cell counting Kit-8 solution (CCK-8, 10 μL, Solarbio, Beijing) was added to each well and continued culturing for 4 h under the same conditions according to the manufacturer’s instructions. The absorbance was measured at a wavelength of 450 nm to obtain the optical density value (OD), which was used to calculate the cell viability according to Eq. (2). The culture medium group was set as blank control. Another four groups were the same with those in immersion experiments.
Alkaline phosphatase (ALP) activity is considered as a reference for osteogenic differentiation potential of cells,which was measured according to the manufacturer’s protocol (Jiancheng, Nanjing, China) for the osteogenic differentiation studies. MC3T3-E1 cells were seeded for 1, 3, 5, and 7 days, and the ALP activity were measured. Briefly, after culturing osteoblast cells for designative time, cells were lysed and performed spectrophotometrically by mixing the colorimetrical production of p-nitrophenol (p-NP) via p-nitrophenyl phosphate (p-NPP)/endogenous ALP enzymatic reaction. After mixing and incubating at 37 °C for 90 s, the ALP activity was measured by calibrating the zero with distilled water at the determination wavelength of 405 nm, and continuously monitoring the absorbance change by reading the absorbance values at 30 s and 2 min, respectively. The results of ALP activity was normalized against total protein concentration, and cell differentiation activity with respect to the control group could be calculated as Eq. (3).
Cellular live/dead staining assay provides intuitionistic image for the differences of cell viability and propagation. For live/dead cell assay, osteoblast cells were cultured in 48-well plates for 1, 3, 5, and 7 days at a density of 3 × 104cells per well with the indirect extracts, and were stained, respectively. Briefly, the 10 × assay buffer was diluted 10 times to obtain 1 × assay buffer (phosphate-buffered saline, PBS).Staining reagent was obtained by adding 5 μL Calcein-AM(2 mM)and 15 μL PI solution (1.5 mM)into 5 mL PBS. The cells were washed using PBS three times, and then incubated with the prepared fluorescent dye for 15 min at 37 °C in the humidified incubator. Fluorescent microscopy images of dyed cells were taken by fluorescence microscope(Nikon DSU3, Japan). Furthermore, the cultured cells in 24-well plates(1 × 105cells per well) were washed by PBS for once after incubating with the indirect extract solution for 12 and 24 h,respectively. 2 ml 4% (w/v) paraformaldehyde was added into each group of wells and fixed at room temperature for 30 min.Subsequently,the cells were washed for three times with 0.1%PBS as long as 5 min for each time.To observe the cytoskeleton and cell spreading, 1: 100 diluted actin tracker green and 1: 50 diluted DAPI solution were used to stain the cellular actin and nuclei for 1 h and 5 min, respectively, which were incubated at room temperature and away from light. Again,cells were washed for three times with PBS as long as 5 min for each time and imaged by fluorescence microscope.
2.6.2. Direct cell assay
The cells (5 × 103cells per well) were directly seeded on samples, which were placed in 48-well plates and then incubated in 10% FBS+89% DMEM/F12+1% penicillin/streptomycin for 2 h at 37 °C and 5% CO2. After cell adhesion, the culture medium was resupplied to 500 μl per well, and the cells were incubated for 12 and 24 h. The samples were rinsed by PBS for three times and fixed with 2.5%glutaraldehyde solution for 1 h at room temperature, subsequently dehydrated in a gradient ethanol with increasing concentrations of 50, 60, 70, 80, 90, 95 and 100% for 10 min each and treated with hexamethyldisiloxane (HDMS) before drying. All the samples were sputter-coated with gold layer before SEM observation.
2.7. Self-repair capability
2.7.1. Secondary plasma activation
Plasma surface treatment can effectively produce a large number of free radicals in the surface layer of polymer materials. The newly generated free radicals can further add specific functional groups (such as oxygen-containing functional groups –OH and –OOH, etc.) to affect various surface properties of materials. In this view, O2plasma was used to treat the coating surface for the improvement of free radicals, aiming at the efficient and rapid self-repair capability. The fabricated SF and nHA/SF-coated samples were placed in the plasma chamber again, which was evacuated to 40 Pa by vacuum pump. The flow rate of oxygen gas was 1.0 L/min.The power and frequency of the discharge were 200 W and 13.56 MHz, respectively. The coated samples were activated for 60 s before scratch tests, as shown in Fig. S1C. The wettability and functional groups on the coating surface before and after secondary plasma activation were investigated by measured water contact angles (DataPhysics, OCA 25-HTV)and tip enhanced laser confocal Raman spectroscopy system(Raman, inVia-Reflex, Renishaw), respectively.
2.7.2. Scratch test
Artificial scratches were performed on the coated samples using Agilent Nano Indenter G200 with a diamond cone tip.A constant load of 100 mN was applied on the coating surfaces,which could penetrate the coatings reaching the magnesium substrates. The obtained scratches had the same path length of 300 μm, width of 20 μm, and depth of 7.5 μm. These scratched samples were subsequently immersed in Hank’s solution at 37 °C for 2, 4 and 6 h to allow their self-repairing process. The damaged area on the coated samples were observed by three dimensional (3D) ultra-depth of field optical microscope(VHX-1000E,KEYENCE),which can exhibit and measure the changed 3D size of the scratches before and after repairing by images. Three scratches were conducted on each sample.
2.8. Animal experiment
The animal experiments comply with the requirements of the University Ethics Committee of the Harbin Institute of Technology. The fabricated rod implants (φ2.5 × 8 mm)of uncoated, SF-coated and nHA/SF- coated samples were used to put in the right thighbone of three New Zealand White rabbits (4.0 kg–4.5 kg), respectively. The rabbits were anaesthetized with Ketamine (35 mg/kg) to predrill with a 2.5 mm hand-operated drill. The surgical site was disinfected by penicillin injection for observation during 3 weeks after implantation. A micro-computed tomography device (Micro-CT, SkyScan-1176, BRUKER, Germany) was conducted to scan the implants with the taken-out thighbone. The corrosion performance and growth of the new bone around the implants were observed in transverse view, coronal view and three-dimensional (3D) view, respectively.
2.9. Statistical analysis
All data in this study were presented as mean ± standard deviation (SD) with at least 3 trials for statistical significance.The statistical analysis of quantitative experiments was performed using SPSS 22.0. Statistical significance was defined asp< 0.05 and performed by one-way analysis of variance(ANOVA).
3. Results
3.1. Coating optimization and characterization
3.1.1. TEM microstructure of nHA blended with SF

Fig. 1. TEM analysis of (A) nHA, (B) nHA/SF5, (C) nHA/SF10 and (D) nHA/SF20 samples. (A1) (B1) (C1) (D1) and (A2) (B2) (C2) (D2) are magnified TEM field, (A3) (B3) (C3) (D3) are HRTEM images and (A4) (B4) (C4) (D4) are the corresponding SAED patterns.
Fig. 1 shows the magnified TEM field, high-resolution TEM (HRTEM) images and the corresponding selected area electron diffraction (SAED) patterns of the nHA before and after blending with SF. The original nHA particles were in the shape of short rod and needle with an average diameter of 20 nm, as presented in Fig. 1A1,A2. The interplanar spacing of 2.72 ˚A in the HRTEM image (Fig. 1A3) belonged to the (300) orientation of nHA phase, while SAED patterns(Fig.1A4)indicated that distinct diffraction spots of the(002),(300), (310), (222), (213), and (004) crystal planes. Notably,the nHA particles were coated by SF when blending, forming clear boundaries (Fig. 1B1,C1) between nHA and SF,however, the interconnected boundaries became unapparent in nHA/SF20sample due to the excessive mass of SF. It was more and more obvious that SF with small bubble morphologies covered on nHA, as shown in Fig. 1B2,C2,D2 as the increased SF mass. The bubble morphologies were caused by the high energy electron beam bombarding and breaking the fragile protein surface during TEM observation. Meanwhile, the diffraction spots in Fig. 1B4,C4,D4 also became less distinct after blending, which was attributed to the bonding and coverage of SF with nHA. The nHA/SF5, nHA/SF10and nHA/SF20samples showed the same spacing of 2.72 ˚A in the HRTEM images (Fig. 1B3,C3,D3) as the original nHA sample, which suggested that the crystal structure of nHA did not change after blending with SF, and the inherent biological activity of nHA was retained as bioactive ceramic materials.
3.1.2. Chemical interaction between nHA and SF
由于Simulink可以有效的对非线性时变系统进行仿真分析,因此本文提出了利用Simulink中的Power Systems的模块库来搭建三电平逆变器的仿真模型,如图1所示。
Fig. 2A presents the surface functional groups of magnesium alloy substrates before and after plasma activation and coating preparation. An increased absorption around 1400 cm-1–1500 cm-1(corresponding to free hydroxyl groups) and decreased absorption band at 1632 cm-1(related to C=O stretching vibration) indicated the improved surface hydrophilicity and removed organic contaminants [57,58].This modification on magnesium alloy could provide more adhesion position with sufficient hydrophilic functional groups to bond with coatings, which was conducive to enhance the adhesion force between coatings and substrates. Besides, the peaks at 566.8 cm-1and 1040 cm-1(assigned to PO43-),876 cm-1(assigned to PO42-),1632 cm-1(assigned to OH-),1400 cm-1–1450 cm-1and 1636.8 cm-1(assigned to CO32-)belonged to nHA, which also appeared in the FTIR spectra of nHA/SF5, nHA/SF10and nHA/SF20samples. Moreover,C–N stretching vibration (amide III), N–H bending vibration(amide II) and C=O stretching vibration (amide I) were exhibited at the peaks of 1228 cm-1,1509 cm-1and 1623 cm-1,respectively in pure SF coating, which significantly changed after blending with nHA [59,60]. As the increased mass of nHA, the characteristic peaks at 566.8 cm-1and 610 cm-1were corresponded to PO43-and were enhanced as shown in Fig. 2A1, suggesting the formed chemical bonds between SF and nHA. Meanwhile, the intensity of absorption peak at 1040 cm-1increased significantly with the addition of nHA(Fig. 2A2), which might be attributed to the hydrogen bond interaction between PO43-in HA and -NH2in SF. Fig. 2A3 shows the decisive changes of amide I and amide II when SF and nHA interacted with each other, that is, the absorption bands of amide I and amide II were weaken and moved to the position of 1522 cm-1and 1632 cm-1, respectively. This indicated that there was a strong interaction between nHA particles and SF, meanwhile, the improved and broadened absorption peaks at the wavenumber of 3500 cm-1–4000 cm-1(Fig. 2A4) were a remarkable sign of the formation of a large number of hydrogen bonds.
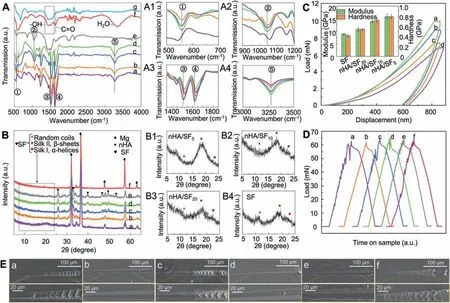
Fig. 2. (A) FTIR and (B) XRD patterns of prepared samples of (a) SF, (b) nHA/SF20, (c) nHA/SF10, (d) nHA/SF5, (e) nHA, (f) bare Mg, and (g) plasma activated Mg samples, respectively. (C) Load-displacement curves, hardness and modulus of (a) nHA/SF5, (b) nHA/SF10, (c) nHA/SF20 and (d) nHA/SF samples. (D) Load-time curves and (E) scratch SEM of (a) un-activated SF, (b) SF, (c) un-activated nHA/SF10, (d) nHA/SF10, (e) nHA/SF20 and (f) nHA/SF5 samples.
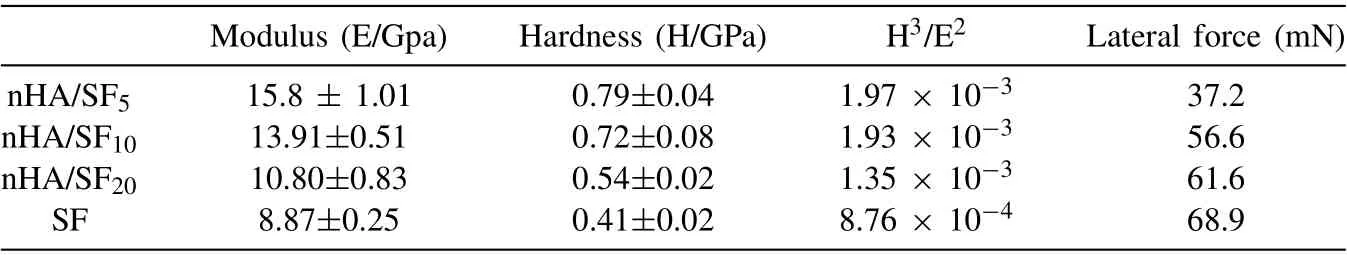
Table 1 Modulus, hardness and lateral force of nHA/SF5, nHA/SF10, nHA/SF20, and SF coatings.
Fig. 2B shows the XDR results of the sample surfaces with the scanning range was 5°–65°, exhibiting metal magnesium peaks at 32.2°, 36.6°, 48.1°, 57.5° and 63.1° on the substrates [61]. Diffraction peaks of the HA phase were observed at 2θ= 25.9°, 32.8°, 39.8°, 46.7°, 49.5° and 53.2° on the pure nHA and nHA/SF coatings, which were performed through matching with the standard ICCD file (ICDD card No. 00-009-0432). The phase identification of HA agreed with the crystallographic texture and orientation of SAED patterns in TEM analysis [23]. However, the intense characteristic peaks belonging to magnesium also widened and weakened after coating, and the intensities of the HA peaks decreased along with the increased mass ratio of SF. Because the magnesium substrate interference of XRD diffraction was obvious,and the main characteristic peaks of SF crystal structure were concentrated in the range of 2θ=5°–25°,the small angle diffraction method (diffraction angle = 1°) was used to investigate the structural transformation of SF, as shown in Fig. 2B1–B4. The detected diffraction peaks at 12.1°, 19.7°and 23.9° were related to the random coils, Silk II (β-sheets)and Silk I (α-helices) structures, respectively [62,63]. Generally, Silk I (αhelices) and Silk II (β-sheet) structures were more stable and corrosion-resistant than random coils, which profited the cell adhesion and spreading according to previous studies. With the increase of nHA content, the characteristic peak of random coil structure gradually weakened, while the silk II,β-sheet structure at 19.7° diffraction peak increased,indicating the interaction between SF and nHA.Therefore,the addition of nHA made SF fold into a more stable secondary structure,which was good for the improvement of mechanical properties and corrosion resistance of the coatings.
3.1.3. Mechanical property and adhesion force of nHA/SF composite coating
Fig. 2C shows the load-displacement curves of indentation testing, which were smooth without any pop-in or pop-out behavior, indicating that the coatings were smooth and nocracking during the loading process. Moreover, the nHA/SF5and nHA/SF10coatings possessed the smaller displacement under the same load compared to the pure SF coating, which exhibited stronger resistance to external load and had better resistance to plastic deformation. In other words, when the same pressure was applied to 800 nm depth, the greater maximum load was required with the increase of the proportion of nHA,suggesting the enhanced ability to resist deformation.The hardness and modulus of various coatings were described in Fig. 2C. The hardness (H) and modulus (E) of the composite coating improved with the increase of reinforcing nHA phase. The modulus of composite coating reached 15 GPa–20 GPa when the mass ration of nHA/SF = 1/5–1/10. The relatively high modulus can balance the stress-shielding effect between human bone (3 GPa–20 GPa) and Mg alloy substrates (40 GPa–50 GPa) [64]. The strategy of increasing the ratio of the reinforcing phase in the coating was commonly used to improve the strength and modulus of the material.The higher the modulus was, the coating was not easy to deform under external load, but the toughness was worse. Thus,the ability of coating to resist external force can be evaluated by H3/E2, which was concluded in Table 1. A higher H3/E2value implies that the coating can resist failure due to the strain for a longer period of time, allowing redistribution of applied loads over a large area, thereby delaying failure of the coating [65]. It was significantly improved for nHA/SF10coatings in H3/E2value compared to pure SF coating, which indicated that nHA/SF coatings had better mechanical properties in resisting deformation from external force.
Fig. 2D shows the load curves of scratch testing, in which the abrupt changes always reflected the poor adhesion of coatings on substrates, and the lateral forces were used to estimate the adhesion force [66]. The detailed lateral force at critical load during scratching process were listed in Table 1. Enhanced adhesion force both in pure SF coating and nHA/SF10composite coating was achieved by O2/N2plasma activation due to the formed robust chemical bonds at the interface. This was attributed to the improved hydrophilic functional groups on plasma-activated clean surfaces, which can react with a large number of amino groups from SF.Furthermore, nanoscratch SEM images of coated samples presented different morphologies without and with plasma activation. Obvious tearing and peeling could be observed on the directly coated samples, as shown in Fig. 2Ea,c, while smooth and undamaged nanoscratch morphologies exhibited in Fig. 2Eb,d indicated the excellent adhesion of coatings.More importantly, the interaction between nHA and SF increased with the addition of nHA, while the adhesion force between composite coating and substrate decreased. The adhesion force of nHA/SF5coatings deteriorated suddenly(Fig. 2Ef), which might be caused by the undulation of the surface morphology when the nHA content was too much,resulting in the gaps between the coating and the substrate.These emerging obstacles impeded the movement of indenter due to the insufficient adhesion force, which led to peeling and shedding. Therefore, combined with mechanical property and adhesion force investigation, nHA/SF10composite coating was preferable as the protective barrier for the magnesium substrates in long-term service.
3.1.4. Ultrasonic spray deposition optimization and coating characterization
In ultrasonic spray deposition process, appropriate parameters including gas flow rate, incoming liquid velocity and spray cycle play important roles in preparing uniform and compact coating. The SEM morphologies and inserted sample images of SF coating exhibited the uniformity of coating surface under different gas flow rates (2.5, 5 and 7.5 L/min for A1, A2, and A3) and incoming liquid velocities (18, 27 and 36 mL/h for B1, B2, and B3) as shown in Fig. 3A and B, respectively. The coating surface was uneven when the liquid velocity was too fast as well as gas flow rate, due to the droplet aggregation with too fast atomization velocity. When the gas flow rate was too slow and the motive force was insufficient, the atomized droplets could not fully blow to the substrate surface, resulting in the uneven coating.Moreover, the pores on all the three coatings (nHA1∼nHA3,SF1∼SF3 and nHA/SF1∼nHA/SF3 coatings) became smaller and smaller with the spraying cycle increasing from one to three (Fig. 3C), especially for the composite coating. The nHA/SF coating was very dense after spray for 3 cycles,and the surface roughness measured by AFM was smaller(Ra = 3.7 nm) compared to the other coatings as shown in Fig. 3D. The rough nHA coating surface had Ra value of 41 nm while the surface roughness of SF coating was 13.3 nm. nHA particles could be regarded as a nanofiller,which fulfilled and smoothen the micropores on SF coating.Dense and uniform nHA/SF composite coating with smaller roughness was in favor of possessing excellent anti-corrosion property, which could prevent the invasion of corrosive ions.

Fig. 3. SEM images of SF coatings fabricated by (A) gas flow rates of (A1) 2.5, (A2) 5 and (A3) 7.5 L/min and (B) incoming liquid velocities of (B1) 18,(B2) 27 and (B3) 36 mL/h. (C) SEM and (D) AFM images of nHA, SF and nHA/SF coatings after spraying for 3 cycles.
The quantitative XPS peak analysis was further performed to investigate the chemical states on the nHA,SF and nHA/SF coatings (Fig. 4). For Mg alloy substrates, the peaks for C 1 s of plasma activated Mg were relatively lower compared to bare Mg, which was agreed with the disappearing absorption band of organic contamination (C=O/C–O) from FTIR patterns. Moreover, the trendy of decreased Mg-O-H bonds with increased Mg-O and generated =N–H/=N–O- for activated Mg might be attributed to the break of O–H under the combined bombardment of oxygen and nitrogen plasma[42,67]. These formed chemical bonds were advantageous to react with a variety of amino acids carried by the coating of silk fibroin,leading to more robust bonding interface.Obvious Mg 2p peak in nHA coating indicated the porous morphology, which was in accord with the SEM observation. The SF and nHA/SF coatings without Mg 2p spectra were relatively dense, and tightly coated on the surface of magnesium alloy substrates. For nHA coatings, the Ca 2p spectra were fitted using two components corresponding to Ca-P(351.96 eV)and Ca-OH (353.96 eV), while the P 2p spectrum presented two components related to P-O (133.26 eV) and P-N (136.66 eV),respectively [68]. It was interesting that the Ca-OH bonds disappeared and Ca-C bonds appeared after adding nHA in SF coating, which suggested that there was chemical reaction between nHA and SF. nHA coating and SF coating showed significant difference in the C 1 s, N 1 s and O 1 s spectra. Moreover, the fitted peaks for C 1 s, N 1 s and O 1 s showed slight differences in C–C/C–O bonds, C–N/N–H bonds and C–O-C/C=O bands of SF and nHA/SF coatings[42,69], indicating the structural transformation of SF caused by interaction between nHA and SF.
3.2. Corrosion resistance
3.2.1. Electrochemistry test
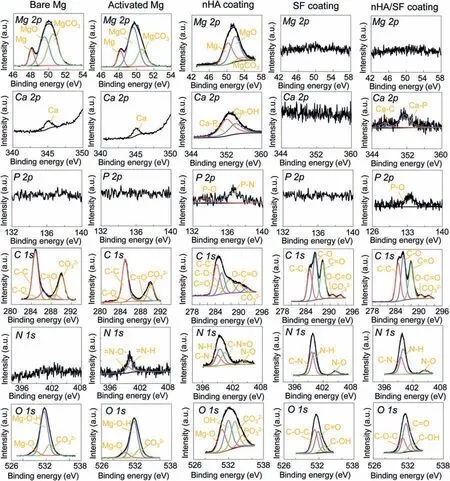
Fig. 4. XPS analysis of bare Mg, activated Mg, nHA coating, SF coating and nHA/SF coating.

Table 2 Fitted parameters of EIS results for all groups of samples.

Fig. 5. (A) and (B) Nyquist plots, (C) and (D) Bode plots, (E) equivalent circuit, (F) polarization resistance of bare, nHA, SF and nHA/SF samples.
Fig.5F shows the calculatedRpvalues,and higherRpvalue indicated lower corrosion rate. The magnesium alloy coated by nHA/SF coating had the lowest corrosion rate and the best corrosion resistance, presenting the highestRp(6.26 × 104Ωcm2).Therefore,the thickness and density of the coating were both improved when spraying for 3 times, which can protect the substrate from corrosion. The corrosion resistance of coated samples could be controlled by applying different spray cycles, and there were significant advantages for the improvement of nHA/SF coating.
3.2.2. pH value and H2 release
In the long-term immersion test, the nHA/SF-coated samples without plasma pretreatment (nHA/SFD) were considered as control to compare with the other samples. Fig. 6A shows the optical images of the immersed samples during corrosion process. Both SF and nHA/SF-coated samples exhibited a small amount of degradation after 28 days, while the nHA/SFD coating was peeled off the substrates partly in the middle period of immersion. Even worse, the corrosion of nHA/SFD coating increased markedly just like bare samples at the end of immersion test. The pH value and released of H2volume of bare samples exhibited a sharply increased trend, while SF and nHA/SF coated samples had much lower level, as shown in Fig. 6B,C. Generally speaking, smaller pH changes promotes the cell growth, which is more conductive to the cell viability. Thus, the lowest pH level of nHA/SFcoated samples could reflect the potential cytocompatibility[44]. nHA/SFD coating possessed less corrosion resistance than the nHA/SF-coated samples, which was attributed to the poor adhesion between coatings and substrates. Moreover,the nHA/SF coated samples had the slowest degradation rate(0.059 mg/day•cm2), which was the 1/8 of that for bare magnesium alloy according to the calculated results in Table 3.The significantly delayed degradation rate of nHA/SF coating was acceptable compared to that human body can bear(0.145 mg/day•cm2).
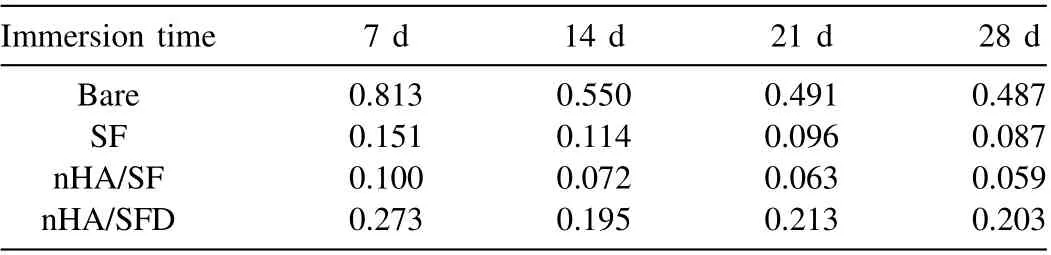
Table 3 Degradation rate (mg/day•cm2) for all groups of samples.
3.2.3. Surface and interface morphologies
The surface and cross-sectional morphologies of immersed samples were respectively detected by SEM and EDS analysis (Fig. 7A,B). Bare magnesium was seriously corroded,and the surface was rough and uneven, showing obvious pits and cracks. Moreover, the pits were expanding into substrates and products were accumulated on the surfaces with a nonuniform corrosion mode.For SF and nHA/SF-coated samples,a few corrosion products were formed on the surface of the coating little by little, and a thin corrosion product layer appeared. However, the surface and cross-sectional morphologies of nHA/SFD-coated samples lost the protective coatings with the immersion, which showed a similar corrosion behavior with bare samples at the later stage. The poorly adhesive nHA/SF coating peeled off the substrate due to the absence of plasma activation, indicating that the robust bonding strength at the interface was indispensable in the long-term reliability.Furthermore, the EDS results of corroded surfaces exhibited the largest P element on bare samples, while relatively lower P for nHA/SF coating reflected the delayed degradation of the coated samples. A large amount of corrosion products was presented on the bare and nHA/SFD samples, while a thinner corrosion product layer were detected on the EDS mapping for nHA/SF coated samples. Thus, the coated samples prepared by plasma activation possessed excellent corrosion resistance during immersion process.
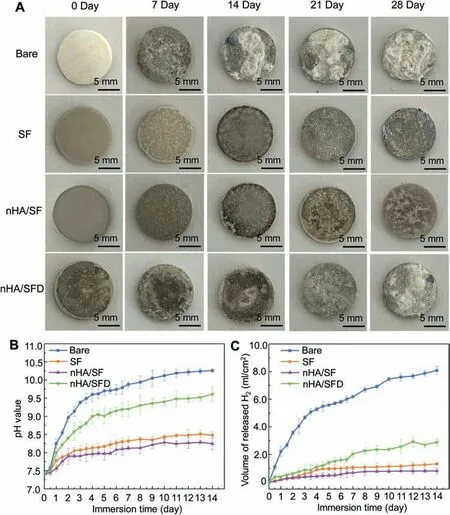
Fig. 6. (A) Optical images, (B) pH value and (C) released hydrogen of degraded bare, SF, nHA/SF and nHA/SFD samples along with immersion time.
3.2.4. Corrosion products
The composition of formed corrosion products on the immersed sample surfaces is shown in Fig. 7C,D. The amide characteristic peaks belonged to SF were detected after immersion for 28 days, which indicated that the coatings were still relatively complete after long-term corrosion.The peaks of OH-groups appeared at 1591 cm-1and 1656 cm-1, and small peaks at 1420 cm-1suggested the generated CO32-groups. These emerged peaks implied that the corrosion product of Mg(OH)2might absorb the CO2in air and partially converted into MgCO3. Moreover, the peaks at 528 cm-1and 872 cm-1(assigned to HPO42-) of nHA/SF-coated samples were distinctive compared to the others with the PO43-at 561 cm-1and 1000 cm-1[39,40,42],and more intense PO43-peaks indicated the more serious corrosion of bare and nHA/SFD-coated samples. Based on XRD patterns, the peaks of both Mg(OH)2and Mg3(PO4)2were exhibited on all the tested samples. This indicated that the corrosion process of pure SF-coated MgZnCa accorded with our previous study [50]. And the porous SF coating could provide passageway for corrosive ions to contact Mg substrates. The corrosion products of [Mg(OH)2/Mg3(PO4)2]were gradually formed on the sample surface. However, the composition of nHA/SF-coated samples showed the newly generated diffraction peaks of 10.8° corresponding to the(100) crystal planes of Ca10(PO4)6(OH)2, which was different from the original nHA phase. Moreover, the peaks of CaHPO4•2H2O was detected at 2θ=28.6°,which the mixture of [Mg3(PO4)2/Mg(OH)2/Ca10(PO4)6(OH)2/CaHPO4•2H2O]corrosion products. The formed phosphate products of calcium on nHA/SF-coated samples were beneficial to improve the surface activity after corrosion, thus promoting the adhesion of osteoblasts and the formation of new bone.
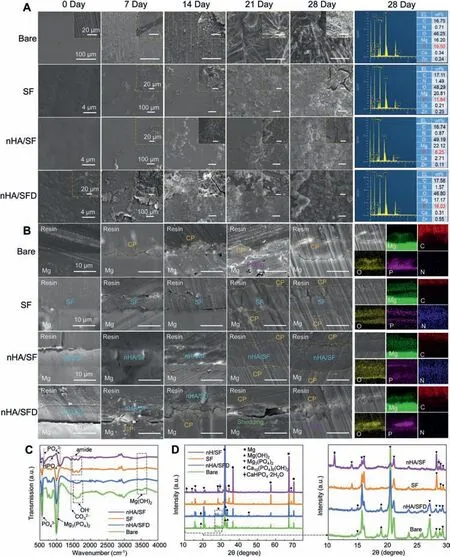
Fig. 7. (A) Surface and (B) cross-sectional morphologies of degraded bare, SF, nHA/SF and nHA/SFD samples along with immersion time. (C) FTIR and(D) XRD patterns of corrosion products.
3.3. Biocompatibility studies
3.3.1. CCK-8 viability and ALP activity
The CCK-8 and ALP results were expressed as average activity with standard deviation, as shown in Fig. 8A,B. After 1-day culturing, the cell viability of each group exhibited no significant difference for both CCK-8 viability and ALP activity. At the 3rd day, the cell viability of coated samples was improved, which showed significant difference compared with black control. nHA/SF-coated samples possessed the best cell viabilities and significant difference compared to bare magnesium alloy when culturing for 7 days, reaching the CCK-8 viability of 128% ± 3.2% and ALP activity of 452% ± 38.8%, respectively. For SF group and nHA/SF group, there were significant differences from the third day of culture period. The CCK8 viability of composite coating was improved due to the presence of nHA. Moreover, the ALP activity of nHA/SF-coated samples showed significant superiority with that of nHA/SFD-coated samples with the increase of culture time. Combining these results, it indicated that nHA/SF and SF-coated samples were better than the bare magnesium alloy and nHA/SFD-coated sample groups in cells proliferation and osteogenic differentiation potential of cells,which was attributed to the excellent biocompatibility of silk fibroin and robust interface performance. More importantly,when culture time point came to the seventh day, the ALP activity of nHA/SF coating was significantly improved compared with pure SF coating. This confirmed the value of nHA in the composite coating with the best cell viability performance, which was attributed to the combination of organics and inorganics as well as the changes of the second silk fibroin structures.

Fig. 8. (A) CCK-8 viability, (B) ALP viability and (C) live/dead staining of cultured cells with the extracts of each group of samples at 1, 3, 5 and 7 days.∗Represents p < 0.05, ∗∗represents p < 0.01, ∗∗∗represents p < 0.001.
3.3.2. Live/dead cell behavior
Fig. 8C shows the live/dead staining images of all groups of samples, and coincided well with those revealed with cell viability tests. More live cells presented after culturing with the extracts of coated samples compared to bare magnesium alloy. As the culture time increased, more and more live cells could be observed in nHA/SF-coated sample groups, which showed remarkable advantages than bare samples. The differences among these groups were the best significant especially when prolonging the culture time for 7 days, which agreed well with the results in CCK-8 and ALP measurements. This revealed that the coated samples fabricated by plasma activation was noncytotoxic, and nHA/SF-coated samples could even promote cell activity and proliferation especially with the prolonged culture time. This also confirmed that the composite coating with organic SF and inorganic nHA performed better biocompatibility.
3.3.3. Cytoskeleton and cell spreading
The MC3T3-E1 osteoblast cells were cultured with extracts for 12 h and 24 h, respectively, and stained as shown in Fig. 9A. Obviously, the numbers of stained cells in different groups varied significantly after being cultured whether for 12 h or 24 h, which was also reported by similar studies. [39,40,42,44] More cells were observed in condition of culturing with the SF and nHA/SF extracts compared with bare magnesium alloy extracts. Additionally, nHA/SF-coated samples possessed more propagated cells than SF-coated samples as well as nHA/SFD-coated samples after being cultured for 24 h. In higher magnification of top right images,well-spread-out and fusiform cells appeared on both SF and nHA/SF-coated groups, extending in various directions and exhibiting rearranged orders. However, bare magnesium alloy samples had the cells with polygonal morphology, which showed worse propagation and differentiation ability at the early stage. This suggested that cells indirectly cultured with nHA/SF-coated sample extracts could be living and better developed for further adhesion on coating surfaces.
3.3.4. Cell adhesion

Fig. 9. (A) The fluorescent images of the cytoskeleton and (B) SEM images of adhered cell morphologies after culturing for 12 and 24 h on bare, SF,nHA/SFD and nHA/SF samples.
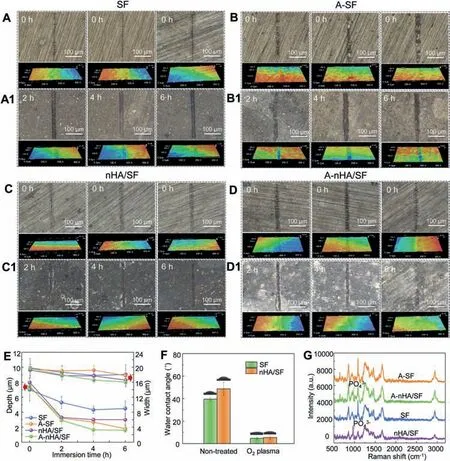
Fig. 10. Scratch morphologies during self-repair process (A–D) before and (A1-D1) after immersion. (E) Depth and width of scratch along with immersion time; (F) Water contact angle and (G) Raman spectra of SF, nHA/SF, A-SF and A-nHA/SF samples.
For the cell morphologies of direct culturing observed by SEM, Fig. 9B shows the distinctive adhesion behavior on different groups. It could be found that the adhered cell morphologies were almost accordant with the cytoskeleton. There are significant differences between polygonal and spreading morphologies. Some cells with spherical shape morphology exhibited on bare magnesium alloy surfaces, while prolonged cells with spreading shape morphology adhered on coated sample surfaces showing obvious filopodia at high magnification. More specifically, big cracks appeared on bare magnesium alloy after just 12 h and 24 h culturing due to the poor corrosion resistance.The release of hydrogen and the increase of pH during corrosion were not conducive to cell adhesion, resulting in the near-round dead cells on the surfaces.For nHA/SFD-coated samples, there were also some cracks presenting on the coatings, which was attributed to the weak adhesion force at the bonding interface. These defects could cause rapid Mg ions release,which were potential risks for inflammation and implantation failure. However, the cells could still spread on the surface of complete coating, which profited from the good biocompatibility of nHA and SF. Additionally,the SF and nHA/SF-coated sample surfaces without obvious defects attracted more cells with both fusiform-spreading morphologies and elongated filopodia, and these cells adhered tightly to the substrates thanks to the prior O2/N2plasma activation. This revealed that the coated samples with strong adhesion force were preferable for cells spreading, and this prior plasma activation was feasible for implant application.
3.4. Self-repair property
3.4.1. Scratch morphology
The scratched samples were immersed in Hank’s solution at 37 °C after monitoring predetermined periods of 0 h–6 h,which were investigated by optical microscope with 3D images for depth and width measurement (Fig. 10A–D). There was no obvious difference for SF-coated samples within 6 h,while scratches with irregular edges formed on the secondary activated SF coating (A-SF). It was evident that shorter, narrower, and shallower scratches exhibited on nHA/SF-coated samples no matter whether they were activated by oxygen plasma. Compared to other groups, the scratch on the secondary activated nHA/SF coating (A-nHA/SF) was almost complete, showing efficient and superior self-repair ability.According to the reported works, the general time for repairing coating to similar surface integrity was 12 h–24 h even longer for inorganic coatings [39,40,42], while the oxygen plasma activation shortened the significantly shorten the repair period with 6 h. This suggested the high efficiency of oxygen plasma activation in surface modification.
3.4.2. The effect of secondary plasma activation
Fig.10E exhibited significant decline in both scratch height and width for A-nHA/SF sample, which could be shortened to 1.60 μm ± 0.20 μm in depth and 16.03 μm ± 0.81 μm in width after immersing for 6 h. These changes were attributed to the self-repair property of the nHA/SF coating itself, on the other hand, it was due to the effect of secondary oxygen plasma activation. The effect of secondary plasma activation was investigated by surface wettability and chemical states,as shown in Fig. 10F and G, respectively. The water contact angles for both SF and nHA/SF-coated samples were dramatically decreased under oxygen plasma treatment for 60 s,which implied significantly improved wettability of coating surface. Moreover, Raman spectra also presented more intense hydrophilic functional group at 2750 cm-1–3000 cm-1region of the Raman shift. For nHA components, representative PO43-group at 963 cm-1was retained after oxygen plasma activation. The peak at 1670 cm-1assigned to amide I (β-sheets) and a complex amide III region with multiple peaks at 1084 cm-1and 1244 cm-1–1276 cm-1were enhanced in SF and nHA/SF coating after the second treatment[73,74]. These increased hydrophilic functional groups could attract more reactive ions in immersion solution and further promote the deposition products assembling on the defects with bare magnesium substrates. Especially when the crack or damage formed on the coatings, magnesium ions and calcium ions were precipitated, resulting in the increase of pH value. Due to the reversible structure of silk fibroin,the stable Silk II was response to the increase of pH, and the phosphate ions in nano-hydroxyapatite were released from the prior interaction with SF, which participated in deposition reaction and block the development of defects. Furthermore, implant surface hydrophilicity plays an important role in cell adhesion and spreading, as well as proliferation [75,76]. Poor surface hydrophilicity limits the application for tissue engineering,but cell growth followed up with the improved hydrophilicity[77].The viability of bio-film or coating with hydrophilic surface can be improved several folds compared with hydrophobic surface, which profits the excellent biocompatibility [78].Similar hydrophilicity effect of secondary plasma activation could be verified by N2plasma and Ar plasma despite placed in the atmospheric environment for a period of time. (Please refer to Fig. S2)
3.5. In vivo study
Fig. 11 shows the Micro-CT analysis of uncoated, ASF-coated and A-nHA/SF-coated Mg alloy implants after 3 weeks’period.The cross sections and 3D models were reconstructed and visualized for better presentation of the corrosion performance and surrounding tissues. On one hand, uncoated Mg and A-SF-coated Mg implants both showed obvious corrosion pits on the surface morphologies. A-nHA/SF-coated implants possessed a more uniform degradation mode during the implantation. On the other hand, more new bone tissues were formed on the interface between the implants and bones (Fig. 11C). This indicated that the addition of nHA could promote the formation of new bone due to its bioactivity as bone-active material. The fabricated A-nHA/SF-coated MgZnCa implants had superior biocompatibility compared with uncoated and A-SF-coated MgZnCa. More importantly,this confirmed the feasible fabrication method of plasma activation combined with ultrasonic spraying is a promising strategy for orthopedic applications.
4. Discussion
4.1. Bonding mechanism
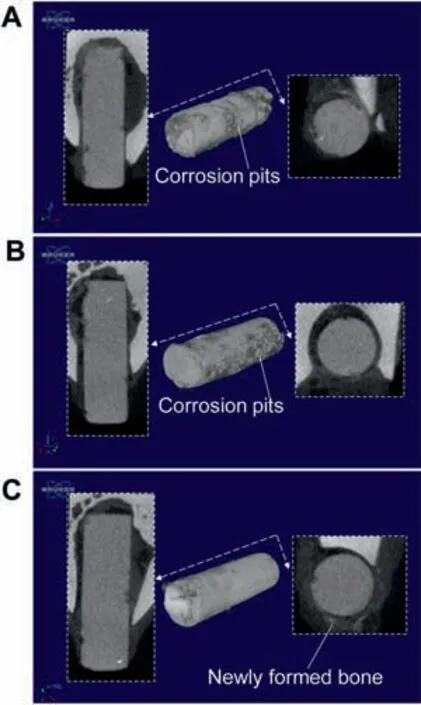
Fig. 11. 3D reconstruction micro-CT images for the (A) uncoated MgZnCa,(B)A-SF-coated MgZnCa and(C)A-nHA/SF-coated MgZnCa implants after implantation for 3 weeks.
The bonding behaviors in nHA/SF-coated Mg structure included two interaction processes of nHA-SF and nHA/SF-Mg.For composite coating, nano hydroxyapatite particles were coated by more stable silk fibroin Silk II structure, as shown in Fig. 12B. Hydrogen bond and chemical bonds were generated from the interaction between phosphate in HA and abundant amino groups in SF (belonging to the decisive changes of amide I and amide II) [62,63]. This bonding in composite coating caused the fold of silk fibroin, resulting in the improvement of mechanical properties and corrosion resistance. On the other hand, organic contaminants and natural oxide layer on Mg surface obstructed the effective bonding between outer coatings and Mg substrates. Moreover, there is little chemical bond connected between the organic protein coating and inorganic metal substrate, only depending on fragile physical adhesion.Based on the surface chemical composition, plasma always leads to a decrease in the content of organics on the material surfaces, eventually decomposed into water and carbon dioxide. Furthermore, for O2/N2plasma,both oxygen and nitrogen are reactive gasses, and it has been reported that a certain amount of nitrogen gas can effectively improve the activity of oxygen plasma [79]. Excitation and ionization of oxygen and nitrogen happens. Meanwhile, the ionization energy of nitrogen is greater than that of oxygen,thus both oxygen and nitrogen indispensable in the surface chemical states, introducing the corresponded polar groups.With the assistance of nitrogen, the oxygen plasma activity is enhanced, and the dissociation of nitrogen molecules are quite enough, so nitrogen-containing bonds are generated on the surface of magnesium as well as free hydroxyl groups(Fig. 12A). The ions produced by nitrogen ionization could react with oxygen rapidly during ionization of nitrogen and oxygen hybrid gas, which increases the activity of oxygen plasma and probably forms some free radicals or nitrogenrelated bonds on the surfaces in favor of enhancing the adhesion. On this basis, the schematic diagram of bonding mechanism for composite coating and Mg substrate using plasma activation is shown in Fig. 12C. The significantly improved hydrophilicity allows stronger mechanical interlocking effect.More importantly, the unique N-hanging bonds generated via hybrid oxygen/nitrogen plasma activation can easily react with abundant amino groups in the silk fibroin molecule to form strong chemical bonds at the interface, which significantly improves the adhesion force. Therefore, mechanical interaction and chemical bonding at the interface provide long-term corrosion resistance even excellent biocompatibility with the reliable interface after drying and dehydration.This confirmed that hybrid oxygen/nitrogen plasma activation is an effective method for the modification of magnesium surface and the improvement of coating adhesion. The bonding processes of nHA-SF and nHA/SF-Mg make the coated structure more reliable in clinical application.
4.2. Corrosion behavior
Fig. 12D illustrates the corrosion mechanism of bare and nHA/SF-coated samples in Hank’s solution.The solution contains various corrosive ions (especially chloride), and the degradation of magnesium always results in the hydrogen release and pH increase, which is closely related to bare magnesium samples. The bare surfaces are directly exposed to the solution, and phosphates are formed on the magnesium as the erosion of ions. A corrosion product layer composed of magnesium phosphate and magnesium hydroxide is accumulated on samples with the development of degradation period, as shown in Reaction (1) and (2). As a result, original defects are gradually deteriorated and serious pitting appears on the substrates, showing poor corrosion resistance.
For nHA/SF-coated MgZnCa, more compact composite coating could provide effective protection for substrates to resist corrosive ions. Furthermore, the robust bonding interface gives a secondary safeguard, thanks to the strong mechanical interlocking and chemical adhesion between coating and substrate. More diffusion energy is required for ions to pass through outer composite coating, and then resist robust chemical bonds at inner layer to contact with magnesium.Compared with our previous work [53], nHA/SF composite coating is more compact and resistive than pure SF coating with micropores. Less ions can corrode Mg alloy through the super-small sized porosity, which are controlled by ultrasonic spraying process. When the diffusion energy of ions is higher than bonding energy, tinny cracks were formed on the coating surface as well as the interface. Thus, the Mg substrates are gradually degraded with a low rate. Moreover, the thin product layer presents little by little, which is attributed to the delayed and uniform degradation process of SH coated samples.According to the FTIR and XRD analysis,the mixture of[Mg3(PO4)2/Mg(OH)2/Ca10(PO4)6(OH)2/CaHPO4•2H2O] corrosion products are formed on the coating surface. Except for the formation of Mg3(PO4)2and Mg(OH)2, H2PO42-is combined with H2O,and converts into HPO42-and PO43-(Reaction (3) and (4)). The Ca2+contained in nHA/SF composite coating can react with them to generate Ca10(PO4)6(OH)2and CaHPO4•2H2O in Hank’s solution, respectively as Reaction(5) and (6). The calcium containing corrosion products may play a positive role in the activity and differentiation of osteoblasts. Consequently, the corrosion behavior indicates that nHA/SF composite coating with the enhanced adhesion not only avoids serious pitting corrosion of the substrates, but also provide bioactive inorganic salts for cell adhesion.

Fig. 12. Schematic illustration for the (A) interaction of nHA and SF, (B) effect of hybrid oxygen/nitrogen plasma activation, (C) bonding mechanism of coating and substrate, (D) corrosion models, (E) cell behaviors and (F) self-repair process.
To further understand the factors influencing the cell behaviors upon material exposure,Fig.12E exhibits the possible performance of osteoblast cell adhesion and spreading.As Reaction (1), the bare samples possessed a worse anti-corrosion ability, and serious corrosion led to rapid increase in pH values, Mg2+concentration and hydrogen release. Moreover, a large number of corrosion products [Mg3(PO4)2/Mg(OH)2]was not bioactive materials for cell viability. High local pH,increased Mg2+concentration and released hydrogen bubbles could denature membrane proteins and destroy the disabling active ionic transport,resulting in spherical cells on bare samples with poor activity. However, for nHA/SF-coated samples, the composite coating was composed of organic protein and inorganic hydroxyapatite with excellent osteoinductivity and osteoconductivity, which had similar osteogenic properties with natural bone tissue. Noticeably, the silk fibroin and calcium salt significantly improved the cell biological responses on the surface-modified samples. Silk fibroin was considered as the promising matrix for bone regeneration due to its highly biocompatible characteristics.Hydroxyapatite had superior bioactive, which was the major component in bones. Thus, prolonged cells with obvious filopodia spread and adhered on nHA/SF-coated sample surfaces, showing preferable adhesion performance. On the other hand, the improved corrosion resistance delayed the rapid increase in pH values, Mg2+concentration and hydrogen release, which provided a stable platform for the proliferation and differentiation of osteoblasts. Additionally, the newly formed[Ca10(PO4)6(OH)2/CaHPO4•2H2O]in corrosion product layer was also benefit in enhancing bioactivity and biocompatibility, presenting relevant and bioactive properties to osteogenesis. Therefore, the outstanding cytocompatibility and osteogenesis activity of nHA/SF-coated samples were attributed to the above factors.
4.3. Self-repair process
Self-repair capacity is one of the main coating properties, which attracts focus in this study. Fig. 12F illustrates the speculated self-repair process when damage appeared on oxygen plasma activated nHA/SF coating. Generally, the selfrepair coating system is regarded as an ability to recover the structure integrity or functional performance with no external physical intervention[80].In this study,the secondary oxygen plasma was applied to assist the improved functional groups on coating surfaces, aiming at more efficient self-repair process.This second modification of coating produced the unique self-repair mechanism for the nHA/SF-coated samples. When the coating was damaged,the magnesium alloy substrates was corroded resulting in the increase of local pH value (Fig. 6B)and the release of Mg2+ions. Due to the reversibility of silk fibroin structure, the addition of hydroxyapatite promoted the transformation of random coils structure into stableβ-sheets structure, however, when the pH raised, the interaction between nHA and SF was disconnected. The nHA/SF coating would release the captured Ca2+and PO43-ions belonging to hydroxyapatite into the alkaline environment, because silk fibroin was response to pH and had residual random coils.Moreover, the silk fibroin with negative charge was more preferable for the deposition situation in an alkaline environment due to the disconnected the interactions. While in a microenvironment with high pH, these structures of the silk fibroin were of negative charge and the interactions between SF and nHA were disconnected, resulting in a likely rather elongated molecular conformation. Thus, more PO43-ions were released in the local defects (further illustrated in Fig.S3). With the assistance of improved free hydroxyl groups by oxygen activation, more cations were attracted in the environment, and react with released PO43-more rapidly. The released Mg2+/Ca2+ions would react with PO43-ions to yield the product Mg3(PO4)2(Reaction (2))/Ca3(PO4)2(Reaction (7)) as shown in the EDS results in Fig. S3. Generally,PO43-ions and the Mg3(PO4)2/Ca3(PO4)2salt are stable in an alkaline environment. Thus, the Mg3(PO4)2/Ca3(PO4)2salt could deposit a passive film on the defects,healing the barrier property and preventing further corrosion. Therefore, except for Reaction (2), the product of Ca3(PO4)2was formed on the defects(Reaction(7)),which further deposited as a barrier film on the surface little by little, achieving the so-called selfrepair process. Actually, the self-repair process could be controlled by different activation parameters according to various clinic requirements. Diversified chemical states were modified using different plasma, which might lead to distinctive self-repair mechanism. Furthermore, the richness of surface functional groups was related to the activation time, exhibiting manageable self-repair efficiency. This secondary plasma activation provided an expectable coating surface modification method for the development of multifunctional medical devices in the near future.
5. Conclusion
A multifunctional osteogenic system of bone-active coatings was layer-by-layer deposited on plasma-activated magnesium via ultrasonically spraying, and the bioactive composite coating was composed of inorganic nano hydroxyapatite(nHA) and organic silk fibroin (SF). The bonding of nHA-SF and nHA/SF -Mg provided the protective coating, improved adhesion and mechanical performance as well as supported the long-term corrosion resistance and excellent biocompatibility of coated magnesium alloy. The nHA/SF-coated Mg structure was preferable for cytocompatibility and osteogenic activity due to the superior surface performance with bioactivity of nHA and osteogenic potential of SF. Additionally, the more efficient self-repair ability was endowed with the help of the secondary activation process of oxygen plasma, which was benefited from the improved hydrophilicity and chemical modification of the coating surface.Combining the advantages of plasma activation and ultrasonic spraying, these results offer a highly expected producing for further developing the bone active modification of biomedical Mg alloy in view of material design, surface pretreatment and coating preparation.
Data availability statement
All data included in this study are available upon request by contact with the corresponding author.
Declaration of competing interest
The authors declare that they do not have any conflicts of interest.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Grant No. 51975151), the Heilongjiang Provincial Natural Science Foundation of China (Grant No.LH2019E041), and the Heilongjiang Touyan Team.
猜你喜欢
杂志排行
Journal of Magnesium and Alloys的其它文章
- Characterizations on the instantaneously formed Ni-containing intermetallics in magnesium alloys
- Corrosion resistance of Mg-Al-LDH steam coating on AZ80 Mg alloy:Effects of citric acid pretreatment and intermetallic compounds
- Gradient structure induced simultaneous enhancement of strength and ductility in AZ31 Mg alloy with twin-twin interactions
- In-depth analysis of the influence of bio-silica filler (Didymosphenia geminata frustules) on the properties of Mg matrix composites
- Effect of Cd on matrix structure ordering and aging precipitation evolution in a Mg-Gd-Cd solid-solution alloy
- Rolling texture development in a dual-phase Mg-Li alloy: The role of temperature
