Anticorrosive and antibacterial smart integrated strategy for biomedical magnesium
2023-11-18JinLingZhoHnRuiCuiZeYuGoYnZeBiZhenZhenDongYnLiCiQiWng
JinLing Zho, HnRui Cui, ZeYu Go, YnZe Bi, ZhenZhen Dong, Yn Li,c,∗,CiQi Wng,∗
aSchool of ChemicalScience, University of Chinese Academy of Sciences, Beijing, 100049, China
b School of Materials Science and Engineering, Beihang University, Beijing 100083, China
c Beijing Advanced Innovation Centre for Biomedical Engineering, Beihang University, Beijing 100191, China
Received 1 June 2021; received in revised form 21 September 2021; accepted 29 November 2021
Available online 6 February 2022
Abstract Biomedical magnesium is an ideal material for hard tissue repair and replacement. However, its rapid degradation and infection after implantation significantly hindersclinical applications. To overcome these two critical drawbacks, we describe an integrated strategybased on the changes in pH and Mg2+ triggered by magnesiumdegradation. This system can simultaneously offer anticorrosion and antibacterial activity. First, nanoengineered peptide-grafted hyperbranched polymers (NPGHPs) with excellent antibacterial activity were introduced to sodium alginate (SA) to construct a sensitive NPGHPs/SA hydrogel. The swelling degree, responsiveness, and antibacterial activity were then investigated,indicating that the system can perform dual stimulation of pH and Mg2+ with controllable antimicrobial properties.Furthermore,an intelligent platform was constructed by coating hydrogels on magnesium with polydopamine as the transition layer. The alkaline environment generated by the corrosion of magnesium reduces the swelling degree of the coatingso that the liquid is unfavorable for contacting the substrate, thus exhibiting superior corrosion resistance. Antibacterial testing shows that the material can effectively fight against bacteria,while hemolytic and cytotoxicity testing suggest that it is highly biocompatible. Thus, this work realizes the smart integration of anticorrosion and antibacterial properties of biomedical magnesium, thereby providing broader prospects for the use of magnesium.
Keywords: Biomedical magnesium; Anticorrosion; Antibacterial; Intelligent; Nanoengineered peptide-grafted hyperbranched polymers.

Specifications Table
1. The antimicrobial hydrogel with Mg2+and pH double response was prepared by combining the excellent antimicrobial properties of nanoengineered peptide-grafted hyperbranched polymers and the pH sensitivity of sodium alginate.
2. The antibacterial hydrogel was coated on the surface of biomedical magnesium. The sample not only had Mg2+and pH double response, but also excellent corrosion resistance and outstanding antibacterial ability.
3. Intelligent biomedical magnesium was achieved by combining anticorrosive and antibacterial strategies.
1. Introduction
Research on biomedical implant materials has gradually shifted from traditional inert materials to bioactive degradable materials [1]. Unlike traditional inert materials that play no role in the healing process and that need to be surgically removed upon recovery [2], bioactive degradable materials gradually degrade in the body and are eventually replaced by new tissues and organs [3]. Magnesium is a biodegradable metalin the human body and does not need to be surgically removed upon healing. Its degradation product, Mg2+, enhances metabolism and is harmless to the human body [4].Moreover, the mechanical properties of magnesium are comparable to human bones, which eliminates stress-shielding effects [5]. Therefore, magnesium has great potential for hard tissue repair and replacement[6].Unfortunately,the corrosion resistance of magnesium is poor, and premature degradation of the metal damages the implanted device, thus resulting in surgery failure [7]. Furthermore, the degradation of magnesium inside the human body leads to an alkaline environment that is rich in H2, which ultimately reduces the immunity of the surrounding tissuesand increases the risk of bacterial infection. In fact, studies have reported that magnesium-based implant devices are likely to incite infection within two weeks of implantation [8,9]. Therefore, magnesium cannot be used in medical applications unless it is coated with a material that renders it resistant to premature corrosion and eliminates the risk of bacterial infection.
Magnesiumis commonly coated with a protective film using a variety of methods to reduce its corrosiveness.Examples includethe anodizing [10], organic coating [11], electroplating [12], and laser treatment [13]. Although some of these methods are highly efficient, most of them are designed for industrial applications and cannot be adapted for the stringent conditions of the medical environment. Biodegradable materials are often applied to the surface of magnesium to build an anticorrosive coating [14–17]. Although the coating delays direct contact between the metal and the corrosive medium,this passive protection method is insensitive to changes in the external medium [18]. For example, the presence of microcracks or micropores in the coating can aggravate corrosion[19]. The rapid development of smart materials has inspired new solutions to old problems [20]. Changes in pH and Mg2+concentration caused by the degradation of magnesium can lead to an intelligent coating that delays magnesiumcorrosion. The design concept currently adopted for an intelligent and protective membrane for magnesium [21,22] contains a non-biocompatible component (corrosion inhibitor [23]) and a heavy metal salt [24]. This method is suitable for use in industry but is not compatible with biological applications.Therefore, the construction of a biocompatible and intelligent anticorrosion platform on the surface of magnesium is a key research direction [25].
The coating materials used for biomedical magnesium should also minimize the risk of bacterial infectionsin addition to reducing the corrosiveness of metals. The component responsible for antibacterial activity is usually a drug directly deposited on the surface of magnesium via blending, electrochemical deposition, and other processes. However, drugs operate through diffusion, which makes it difficult to control the release rate of the antibacterial component [26]. If the drug delivery platform is sensitive to changes in pH and ionic strength caused by magnesium degradation, it is possible to achieve controlled drug release [20]. On the other hand, multidrug resistance is an increasingly prominentproblem [27–29],and researchers are using antimicrobial agents that are not prone to drug resistance in magnesium systems [30–32]. Antimicrobial peptides (AMPs), the natural antimicrobial agents derived from the human immune system [33]), have an amphiphilic structure with cationic amino acids and nearly half of the hydrophobic side chain amino acid sequence. Unlike conventional antibiotics that act on specific targets within bacteria, cations of AMPs can bind to the anions of pathogenic bacteria through electrostatic interactions. The hydrophobic side chains of AMPs cross the bacteria membranecausing exudation of intracellular substances to ultimately kill the bacteria[34,35]. This antibacterial mechanism of physical destruction reduces the likelihood of bacterial AMPs becoming resistant.
Although AMPs have brought new hope to solving the crisis of drug-resistant bacteria [35,36], they cannot be effectively used due to their limited resources, complex extraction process, and high cost. In addition, large concentrations of antibacterial peptides have been shown to induce toxicity in mammalian cells [37,38]. To overcome these problems, scientists have attempted to simulate the amphiphilic amino acid chain structure of antimicrobial peptides by chemical synthesis [39,40]. The effects of different topologies on the antimicrobial properties of polypeptides have been explored. The branched structure ensures the positive charge density needed to achieve higher antibacterial activity in the local environment of the segmenteven at lower concentrations. Nanostructures can cause greater damage to the extracellular membrane due to their small scale [41,42]. We previously prepared a new type of cationic-rich nanoengineered peptide-grafted hyperbranched polymers (NPGHPs) that can effectively fight against bacteria [43]. This study investigates the efficiency of a biomedical magnesium intelligent material constructed from NPGHPs loaded on pH-sensitive sodium alginate (SA) [44].SA was used as carrier of NPGHPs to prepare antibacterial hydrogel with pH and Mg2+double response. The mechanism is driven by the electrostatic interaction between the two components as well as chelation with the calcium ion.The effects of pH and Mg2+concentration on drug release and controlled antibacterial properties were studied. The hydrogel was then coated on magnesium using polydopamine as a transition layer to build up a multifunctional biomedical magnesium. The corrosion resistance, antibacterial property,and biocompatibility of the hydrogel-coated magnesium were evaluated. This smart strategy combines corrosion inhibition and antibacterial function.
2. Experimental methods
2.1. Experimental materials
We used high-purity magnesium (99.99%, Yantai Haoyi Biomaterials Co., Ltd.), HNO3(65%, Beijing Chemical Plant), as well asNaHCO3, KCl, MgCl2•6H2O, Na2SO4, Tris,K2HPO4•3H2O, SA, APTES, CaCl2, Na2HPO4•12H2O, and NaH2PO4•2H2O (Sinopharm Group Chemical Reagent Co.Ltd.).Sodium alginate was from BR(Chinese medical grade).
2.2. Preparation of nanoengineered peptide-grafted hyperbranched polymers (NPGHPs)
Synthesis of antimicrobial polymeric peptides was based on our previous work [43]. First, H-PAMAM,carbobenzyloxy-lysine N-carboxyanhydrides, and valine Ncarboxyanhydrides were synthesized according to the literature[45–47].Cbz-Lys-NCA and Val-NCA were grafted on the H-PAMAM core by ring-opening polymerization with terminal amino groups as reaction sites. The products were labeled as Cbz-NPGHPs. Finally, NPGHPs were obtained by removing the carbobenzyloxy protection groups. H–NMR: 2–4 ppm(H-PAMAM),4.2 ppm(a,CO–CH–HN),1–1.74 ppm(b,CH2of Cbz-L-Lys-NCA), 2.7ppm(c, -CH2NH3), 1.8–2.02 ppm (d,CH–CH3of DL-Val-NCA), 0.8 ppm (e, CH3),8.2 ppm (f,NH); GPC (Mn:45,000 Da, PDI=1.29).
2.3. Preparation of NPGHPs/SA mixture andNPGHPs/SA hydrogel
Solid SA was weighed and slowly added to hot water. The powder was stirred and dissolved to 4 wt.%. The NPGHPs were weighed and dissolved into an aqueous solution, and the NPGHPs solution was mixed with an equal volume of 4 wt.% SA solution. A small amount of aminopropyltriethoxysilane (APTES) (50 μL/mL mixed solution) was added to the mixed system. The mixture was then transferred to a cylindrical mold after being stirred evenly. After standing for 0.5 h, it was immersed in 5% calcium chloride solution for further crosslinking and molding. Some of the resulting NPGHPs/SAmixture was used for the coating of magnesium,and the other was cross-linked with 5% CaCl2solution to form NPGHPs/SA hydrogels.
2.4. Construction of MG, MG-PDA, and MG-PDA-NPGHPs/SA
Polished magnesium sheet (10 mm x 10 mm x 2 mm)was smoothed with water and 240/120/3000/5000 mesh sand paper. The polished magnesium was subjected to alkaliheat treatment. The samples were immersed in 1 M NaOH solution for 2 h at room temperature, and the alkali-treated samples were then placed in a vacuum oven at 60 °C for heat treatment for 6 h. The alkali heat-treated samples were immersed in a 2 mg/ml, dopamine ethanol solution at pH 10 for 12 h. The sample was rinsed with absolute ethanol and then blown dry; the sample was labeled as Mg-PDA.The resulting Mg-PDA sample was then immersed in the NPGHPS/SAmixture, and the sample was immersed in a 5%CaCl2solution for crosslinking. The samples were labeled as Mg-PDA-NPGHPS/SA.
2.5. Study on stimuli responsiveness of NPGHPs/SA hydrogel
The pH of the medium solution was set as 7 and 9, and 0.2 mM MgCl2was added into two different pH media to investigate the influence of the pH of the medium and the addition of Mg2+on the swelling behavior of samples. Next,0.5 g of the hydrogel was separately added to a centrifuge tube containing the above four different media (20 mL), transferred to a constant temperature shaker,and incubatedat 37°C.At fixed time points, each hydrogel was removed, and the mass of the hydrogel at that time point was weighed and recorded. The final degree of swelling of the hydrogel at each time point was calculated. The release test of NPGHPs is the same as the method of swelling. The difference is that the samples were transferred to a constant temperature shaker and released at 37 °C. Samples were collected, and the fluorescence emission spectra were tested. The concentration and release of NPGHPs were calculated according to the fluorescence intensity measured at each time point.
2.6. Antibacterial properties of MG, MG-PDA, and MG-PDA-NPGHPs/SA
The samples of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA were immersed in 10 mL liquid medium inoculated withE.coli(1 × 104CFU/mL).The samples were transferred to a 35 °Cincubator for 48 h, and then the number ofE.coliin each sample was counted by the plate colony counting method to evaluate the antibacterial properties of each sample.
2.7. Corrosion inhibition performance of MG, MG-PDA, and MG-PDA-NPGHPs/SA
Corrosion inhibition was characterized by an immersion test and an electrochemical test. The in vitro immersion test mainly characterized the corrosion of the sample by monitoring the pH change of the medium and the amount of H2released. The sealed samples to be tested were placed in a centrifuge tube, 20 mL of SBF simulated body fluid was added,and the solution was transferred to a 37 °C constant temperature water bath. The changes in pH and H2with soaking time were periodically recorded.
The electrochemical test used a three-electrode system.The samples were the working electrode, a graphite rod was the counter electrode, and a saturated calomel electrode was the reference electrode.The test was conducted in a constant temperature water bath at 37°C using SBF as an electrolyte solution. For the polarization curve (I/E) tests, the self-corrosion potential was set from-500 mV to 500 mV,and the scan rate was5 mV/s.EIS testing was typically performed from high frequency to low frequency with a frequency range of 100 mHz to 100 kHz.
2.8. Biocompatibility of MG, MG-PDA, and MG-PDA-NPGHPs/SA
The cytotoxicity of the samples was determined by indirect extraction. First, the extract of the samples to be tested was prepared, and the sample was sterilized and immersed in a 37 °C incubator for 24 h usingα-Minimum Essential Medium (α-MEM). After leaching, the supernatant was removed, and the extracts at 30% and 50% concentration were prepared usingα-MEMand placed in a refrigerator at 4 °C for 12 h. Cell culture was performed after extractpreparation:30%, 50%, and 100% sample extracts were used as the experimental group;α-MEM was used as the blank control.The above solutions were separately transferred to the same volume of the cell suspension in a 96-well plate, and cultured in a 37 °C incubator at 5% CO2for 1 d and 3 dNext, 100 μL of 5% MTT solution was added to each well and placed in a constant temperature incubator for an additional 6 h. Finally,100 μL of DMSO was added to fully dissolve the product,and the absorbance of the solution at 570 nm in each well was measured using a microplate reader.
Fresh anticoagulant rabbit blood was used to evaluate the hemolysis of the sample to mammalian red blood cells. The samples were immersed inthe system using a direct method including fresh anticoagulant rabbit blood; hemolysis was detected after co-culture. Next, 10 mL of physiological saline,10 mL of sterile distilled water, and 10 mL of physiological saline with samples were used as the negative control group,positive control group, and experimental group, respectively.Fresh anti-coagulated rabbit blood (0.2 mL) was added to the above three groups of samples, gently shaken and mixed, and then placed in the 37 °C incubator for 2 h. Subsequently, the mixture was centrifuged at 3000 rpm for 10 min, and the absorbance of the samples supernatant at 540 nm was measured using an ultraviolet-visible spectrophotometer to calculate hemolysis.
The hemolysis rate was calculated according to the following formula:
whereODnis the OD540value of the negative control group;ODpis the OD540value of the positive control group;ODsis the OD540value of the experimental sample group.
3. Results and discussion
3.1. Construction of NPGHPs/SA hydrogel
We previously designed and synthesized a new type of cationic-rich antimicrobial polypeptide with a nano branched structure (NPGHPs) (Fig. 1a), simulating the amphiphilic amino acid chain structure of AMPs to effectively fight bacteria [43] ((Fig. 1a, Figure S1). Nanostructures can cause greater damage to the extracellular membrane due to their small scale, and the branched structure can effectively ensure the positive charge density needed to achieve higher antibacterial activity even at lower concentrations. The antibacterial mechanism of physical destruction reduces the likelihood of AMPs becoming resistant.
A hydrogel of antibacterial NPGHPs in the SA carrier was prepared. The two components were held together by electrostatic interactions between SA and NPGHPs as well as chelation with calcium ions. A mixture of SA and NPGHPs was cross-linked with Ca2+to form a milky hydrogel. The poreswere 100–500 nm in the hydrogel as observed by scanning electron microscopy(SEM)(Fig.1b).Infrared(IR)spectroscopy was used to check the structure of the prepared hydrogel (Fig. 1c). SA shows absorption bands near 3199,1598, and 1404 cm-1, which correspond to the stretching vibration of -OH, -COO- (asymmetric), and -COO- (symmetric), respectively [48]. NPGHPs have obvious characteristic peaks at 3280, 1630, and 1545 cm-1corresponding to N–H stretching vibration, amide I, and amide II, respectively. The characteristic peak at 2965 cm-1corresponds to the vibration of the -CH3group in valine [49]. With the formation of hydrogel, the intensity of SA characteristic absorption peaks located at 1598 and 3199 cm-1increased. Moreover, the absorption band of the primary amine cation (-NH3) at nearly 2060 cm-1disappeared, suggesting the electrostatic force between NPGHPs and SA. In ion-crosslinked NPGHPs/SA, the absorption bandwidth and intensity (-OH and NH stretching vibration) at 3361 cm-1are significantly stronger than SA[50]. This indicates that there is a stronger intermolecular interaction between SA and NPGHPs. This also confirms the formation of a NPGHPs/SA hydrogel.

Fig. 1. (a) The animated maps and molecular formulas of NPGHPs. (b) The microstructure of the NPGHPs/SAhydrogel (freeze-dried sample). (c) IR of the SA, NPGHPs and NPGHPs/SA hydrogel.
3.2. pH and Mg2+ Triggered drug release from NPGHPs/SAhydrogel
The swelling behavior of the NPGHPs/SA hydrogel was studied under different pH and Mg2+concentrations(Fig.2a).In our previous work, the swelling ability and the drug release behavior of the NPGHPs/SA hydrogels were investigated at under acidic environments (pH=5) and physiological environments (pH=7.4) in the absence (0 mM) and presence(5 mM) of magnesium ions, respectively [51]. The results showed that in the presence of magnesium ions and physiological conditions (Mg2+=5 mM, pH=7.4), the swelling degree of the hydrogel is higher, and the NPGHPs drugs are more easily released from the gel network. In this work, our target is to design a suitable strategy of intelligent biomedical magnesium with controlled-release platform. After biodegradable magnesium is implanted into the organism, the body fluid pH changes very little. But in the part of the material, the tightly bound parts with magnesium may especially produce a relatively high pH situation, so we designed the drug-controlled release test under pH as 7 and 9 respectively.At the same time, when magnesium is implanted in the human body, under the action of the human circulatory system,the magnesium ions concentration in the body fluid can only rise slightly with the degradation of magnesium [52, 53]. Too high magnesium concentration can cause adverse reactions in human body. Therefore, in this study we used a solution with a Mg2+concentration of 0.2 mM as the environment for drug release testing of NPGHPs/SA hydrogels.The swelling degree of the hydrogel under neutral conditions is significantly higher than that under alkaline conditions, and the addition of Mg2+slightly promoted the swelling.This is because the SA molecular chain has more negative chargesunder alkaline conditions,and the electrostatic interaction with the NPGHPs molecule is stronger. The NPGHPs/SA hydrogel is also relatively tight in the macroscopic state. Under neutral conditions, the number of negative charges on the SA molecular chain is small, and the electrostatic interaction with NPGHPs molecule is weak.In addition, although Mg2+cannot cause cross-linking of SA,it may compete with Ca2+and NPGHPs, weaken the degree of cross-linking of SA hydrogel, and increase the swelling due to electrostatic interactions between SA and NPGHPs.

Fig. 2. (a) Swelling ratio of NPGHPs/SA under different conditions. (b) Responsive release of NPGHPs. (c) Mechanism of responsive release of NPGHPs.
The effects of pH and Mg2+concentration on the release of NPGHPs in the hydrogel were also evaluated(Fig. 2b). Under alkaline conditions, the release rate of NPGHPs in the hydrogel was slower than that under neutral conditions, and the addition of Mg2+promoted the release of NPGHPs. Diffusion control was mainly adopted in the early stage of NPGHPs release, and the release was relatively stable and slow. The hydrogel reaches swelling equilibrium over time, and its pores become relatively large leading to accelerated release of NPGHPs. Under alkaline conditions, the degree of swelling of the NPGHPs/SA hydrogel is small, and diffusion is hindered, which leads to low release rates. Under neutral pH conditions,the weakening of the electrostatic interactions leads to an increase in the degree of swelling, which facilitates diffusion and the release of NPGHPs.High concentrations of Mg2+may weaken the degree of SA cross-linking and the electrostatic interaction between SA and NPGHPs,thus promoting the release of NPGHPs (Fig. 2c). Therefore,the effects of pH and Mg2+concentration on the release of NPGHPs are consistent with the swelling test results of theNPGHPs/SA.
In summary, the NPGHPs/SA hydrogel is responsive to pH and Mg2+. Neutral conditions and the addition of Mg2+promote the release of NPGHPs. The hydrogel intelligently responds to changes in external conditions and achieves controlled drug release. The next step was to evaluate whether the hydrogel-coated magnesium is sensitive to changes in pH and Mg2+concentration caused by magnesium corrosion.
3.3. Construction and characterization of MG, MG-PDA,and MG-PDA-NPGHPs/SA
The antibacterial system was applied on the surface of magnesium as shown in Fig. 3a. Dopamine was graft polymerized to form a transition layer of polydopamine on the surface of the alkali heat-treated magnesium. On this basis,the antibacterial coating is constructed with amino and hydroxyl as reactive functional groups.
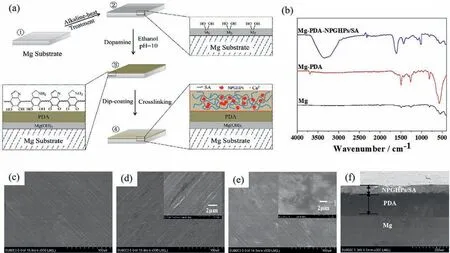
Fig. 3. (a) Preparation process of Mg-PDA-NPGHPs/SA coating; (b) IR of Mg, Mg-PDA, Mg-PDA-NPGHPs/SA; (c–e) SEM of Mg, Mg-PDA, Mg-PDANPGHPs/SA;and (f) The cross section of Mg-PDA-NPGHPs/SA.
The chemical structure and morphology of each sample(Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA) was analyzed by IR spectroscopy and SEM. The results show that the surface of bare magnesium is flat with some scratches (Fig. 3c). The sample has no obvious characteristic absorption peaks on the IR spectrum,indicating the absence of organic components on the surface (Fig. 3b). Polydopamine-coated magnesium (Mg-PDA) has a relatively flat and smooth surface where in the original scratches on the Mg surface are covered by the PDA(Fig. 3d and Figure S2). The IR spectrum of Mg-PDA exhibits an absorption peak of the phenolic hydroxyl group near 3694 cm-1. The absorption peak near 1492 cm-1is attributed to the C = C bonds in the benzene ring; the peak at 1261 cm-1corresponds to the C–N bond (Fig. 3b). These results indicate proper deposition of a polydopamine layer on magnesium. Finally, SEM and FTIR analyses were conducted on the Mg-PDA-NPGHPs/SA sample(Fig.3e). A cross-sectional view of this surface in SEM shows that the thickness of each layer (PDA and NPGHPs/SA) is relatively uniform; the layers are closely connected. The thickness of the PDA layer is approximately 150 μm and the NPGHPs/SA layer is approximately 50 μm (Fig. 3f). The IR spectrum of the Mg-PDA-NPGHPs/SA shows an absorption peak near 3300 cm-1corresponding to the -OH groupas well as two peaks at 1601 cm-1and 1431 cm-1corresponding to the carboxylic acid groups (Fig. 3b). The absorption peak at 1080 cm-1corresponds to the C–O-C group (Fig. 3b). These results confirm the presence of both dopamine and antibacterial layers on the magnesium surface.
3.4. Antibacterial properties of NPGHPs/SA hydrogel, MG,MG-PDA, and MG-PDA-NPGHPs/SA
The excellent antibacterial properties of NPGHPs have been demonstrated [43]; here, we compared the antibacterial properties of NPGHPs/SA hydrogel, Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA samples by exposing them toE. coli. The number ofE. colibefore and after addition of the investigated samples was determined using the plate colony method, and the antibacterial performance of each sample was evaluated.
First, the responsive antibacterial properties of the NPGHPs/SA hydrogel were studied and evaluated based on the diameter of the antibacterial area (Fig. 4a and Figure S3).The NPGHPs/SA hydrogel showed the most obvious bacteriostatic effect. The diameter of the antibacterial area was found to be directly proportional to the amount of NPGHPs released in solution. Neutral pH conditions promote the release of NPGHPs versus alkaline conditions,which ultimately enhances the antibacterial activity of the hydrogel (Fig. 4a and Section 3.2). Under the same pH conditions, the addition of Mg2+slightly increases the bacteriostatic area (Fig. 4a).Therefore, the antibacterial activity of the NPGHPs/SA hydrogel can be adjusted by manipulating the pH and Mg2+ion concentration of the surrounding environment.

Fig. 4. (a) Responsive antibacterial properties of SA and NPGHPs/SA hydrogels; (b) and (c) Antibacterial properties of Mg, Mg-PDA, and Mg-PDANPGHPs/SA.
Next, antibacterial tests were also performed on Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA samples (Fig. 4b, c). The proliferation of bacteria of Mg and Mg-PDA samples was only minimally inhibiteddue to changes in pH and ion concentrations triggered by the corrosion of magnesium. This shows that even though the thickness of the PDA layer is greater than that of the NPGHPs/SA gel layer (Fig. 3f), the PDA layer alone cannot effectively improve the antibacterial ability of Mg. However, only a small amount (3.68%) of bacteria survived in the Mg-PDA-NPGHPs/SA sample. This shows that the Mg-PDA-NPGHPs/SA coating has a significant antibacterial effect.
3.5. Corrosion inhibition performance of MG, MG-PDA, and MG-PDA-NPGHPs/SA
The corrosion degree of the samples can be indirectly reflected by detecting changes in the pH of the solution in which they are immersedas well as changes in the amount of H2released. The results are shown in Fig. 5.
An increase in pH was observed with increased soaking time for all three samples (Mg, Mg-PDA, and Mg-PDANPGHPs/SA), indicating that corrosion occurred. After soaking was begun, the pH increase rate of the Mg sample is the fastest, and the pH increase rate of the Mg-PDA-NPGHPs/SA sample is the slowest. After soaking for the same time, the pH of the soaking solution of the three samples is Mg>Mg-PDA>Mg-PDA-NPGHPs/SA, indicating that the coating of PDA can delay the corrosion of Mg, and the modification of the SA antibacterial coating further added increase the thickness of the coatingto delays the corrosion of the Mg substrate(Fig. 5a). The H2release experiments were consistentwith the pH experiment (Fig. 5b). After soaking for 48 h,the Mg sample released 30% more H2than either the Mg-PDA or Mg-PDA-NPGHPs/SA samples, which confirms the obviouslycorrosion inhibition effect of the Mg-PDA andMg-PDA-NPGHPs/SA.

Table 1 Corrosion potential and corrosion current density of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA.
To further analyze the effect of the PDA and NPGHPs/SA layer on magnesium corrosion,Tafel polarization curves of the Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA samples were recorded (Fig. 5c and Table 1). The results show that the potential of the Mg-PDA-NPGHPs/SA is greater than that of Mg-PDA, which in turn is greater than that of Mg. The current density values of these samples show the opposite trend,suggesting that the corrosion resistance of Mg was improved after modification.

Fig. 5. (a) pH change of solution; (b) The release of H2 in SBF. (c) Tafel polarization curves of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA; (d–f) Nyquist plots for electrochemical impedance spectroscopy of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA immersed in SBF.
The Nyquist diagram of the Mg, Mg-PDA, and Mg-PDANPGHPs/SA samples soaked in SBF and its analog circuit are shown in Figs. 5d–f. The Nyquist (Fig. 5d) and Bode plots(Figure S4) of Mg suggest that Mg has a small impedance in the initial state. As the immersion time is extended, the corrosion products cover the surface so that the interface resistance values increase .At the early stages of immersion,the impedance value of Mg increased rapidly, indicating that the corrosion rate was relatively fast. After three days of immersion,the change in impedance values gradually decreased,resulting in slower corrosion rates due to the accumulation of surface corrosion products that partially hinder the internal corrosion.
The capacitive arc diameter values were initially recorded upon immersion of the Mg-PDA sample and were significantly larger (two-fold) than that of the Mg sample (at the early stages).This suggests that PDA increases the impedance and delays the corrosion of Mg. Upon further soaking, the impedance values of Mg-PDA showed a uniform and steady increase (Fig. 5e), which implies that corrosion of Mg-PDA still occurs albeit at a slower rate than that of the pure Mg.The outer PDA coating is gradually destroyed as corrosion of the Mg-PDA sample continues; the impedance values of the sample then gradually decrease. The counteracting effects of the gradual destruction of the external PDA coating and the gradual accumulation of corrosion products results in an overall increase in impedance.

Fig. 6. (a-c) Cytotoxicity test of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA. (d) Hemolysis test of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA.
The impedance of the Mg-PDA-NPGHPs/SA recorded initially upon immersion lies between those observed for Mg and Mg-PDA (Fig. 5f). The impedance values of Mg-PDANPGHPs/SA increase rapidly within one day of immersion.The impedance values of the Mg and Mg-PDA samples were smaller than those of the Mg-PDA-NPGHPs/SA sample throughout the entire period of soaking (12 days). The overall performance is relatively stable. This indicates that the corrosion rate of Mg-PDA-NPGHPs/SA is relatively slow versus the other two samples. Thus, both of the PDA and NPGHPs/SA coating make the magnesium resistant to corrosion.
Our previous research results showed that the NPGHPs/SA hydrogel has sensitive environmental responsiveness. The swelling degree of the hydrogel under neutral conditions is significantly higher than that under alkaline conditions. When applied to the surface of the Mg-PDA,the resulting Mg-PDANPGHPs/SA intelligently responds to changes in pH. The alkaline environment generated by the corrosion of magnesium reduces the degree of swelling of the coating, which prevents contact of the liquid with the substrate and delays magnesium corrosion.Both the electrochemical and immersion tests show that they achieve the best corrosion resistance.
In the Mg equivalent circuit (Fig. 5d), Rsis the solution resistance,CPE1is the electric double layer capacitance at the interface between the electrolyte and the corrosion product,R1is the corresponding charge transfer resistance,R2is the resistance of the corrosion product,and CPE2is the corresponding capacitance. In the equivalent circuit of Mg-PDA (Fig. 5e),Rsis the solution resistance, CPE0is the electric double layer capacitance at the interface between the electrolyte and the PDA coating, and R0is the corresponding charge transfer resistance. CPE1is the capacitance of the PDA coating, and R1is its resistance. R2is the resistance of the alkali heat treatment layer, and CPE2is the corresponding capacitance. In the equivalent circuit of Mg-PDA-NPGHPs/SA (Fig. 5f), Rsis the solution resistance; R0, R1, and R2are the resistances of SA coating, PDA coating, and alkali heat treatment layer,respectively; and CPE0, CPE1, and CPE2are corresponding capacitances. The fitting data are shown in Tables S1–S3.
3.6. In vitro biocompatibility evaluation of MG, MG-PDA,and MG-PDA-NPGHPs/SA
The cytotoxicity test results of Mg, Mg-PDA, and Mg-PDA-NPGHPs/SA are shown in Fig. 6a-6c. After one day of culture, only the complete extract (α-MEM) had a slight effect on the growth of MC3T3-E1 cells with an increase in the concentration of Mg extract; the survival rate of the cells in the test range was maintained over 80%. After three days of culture, the cells grew well and the survival rate was around 100% (Fig. 6a). The Mg-PDA sample had a slight effect on cell growth, and the activity of the cells cultured for three days is higher than those cultured for one day (Fig. 6b).The Mg-PDA-NPGHPs/SA sample also had no significant effect on cell growth (Fig. 6c). The extracts of the samples showed low cytotoxicity and did not harm mammalian cells.
The hemolysis results of Mg, Mg-PDA, and Mg-PDANPGHPs/SAare shown in Fig. 6d.The hemolysis rate of Mg is high (56.33%), but decreased upon coating with PDA or PDA-NPGHPs/SA (lower than the standard of 5%; 4.02%and 3.19% for Mg-PDA and Mg-PDA-NPGHPs/SA, respectively). Therefore, Mg-PDA and Mg-PDA-NPGHPs/SA are not expected to damage mammalian red blood cells.
4. Conclusion
In summary, we successfully fabricated an intelligent biomedical magnesium Mg-PDA-NPGHPs/SA by coating an antibacterial NPGHPs loaded SA hydrogel with polydopamine as the transition layer. The NPGHPs/SA hydrogel is responsive to changes in pH and Mg2+. Under neutral conditions,the electrostatic interaction between SA and NPGHPs is weak,which benefits the diffusion and release of NPGHPs. Alkaline conditions strengthened the electrostatic interactions between SA and NPGHPs, thus delaying the release of NPGHPs.Higher concentrations of Mg2+also promote the release of NPGHPs due to greater competition with chelating Ca2+. The NPGHPs/SA hydrogel showed obvious bacteriostatic effects.Its antibacterial activity can be controlled by manipulating the pH and Mg2+concentration of the surrounding environment.The smart Mg-PDA-NPGHPs/SA reduced the corrosion of magnesium and has good antibacterial activity; it can significantly inhibit the proliferation ofE.coli. It also has lower cytotoxicity and hemolysis rates versus pure metals well as excellent biocompatibility. Thus, we integrated the anticorrosion and antibacterial properties for biomedical magnesium.The unique capabilities of biomedical magnesium are based on a NPGHPs/SA hydrogel layer, thus making it a promising material for clinical applications.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was financially supported by the National Natural Science Foundation of China (no. 51671179, 51971014)and the Excellent teacher ability improvement project(E1E40308).
Supplementary materials
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jma.2021.11.030.
杂志排行
Journal of Magnesium and Alloys的其它文章
- Characterizations on the instantaneously formed Ni-containing intermetallics in magnesium alloys
- Corrosion resistance of Mg-Al-LDH steam coating on AZ80 Mg alloy:Effects of citric acid pretreatment and intermetallic compounds
- Gradient structure induced simultaneous enhancement of strength and ductility in AZ31 Mg alloy with twin-twin interactions
- In-depth analysis of the influence of bio-silica filler (Didymosphenia geminata frustules) on the properties of Mg matrix composites
- Effect of Cd on matrix structure ordering and aging precipitation evolution in a Mg-Gd-Cd solid-solution alloy
- A multifunctional osteogenic system of ultrasonically spray deposited bone-active coatings on plasma-activated magnesium
