Restructuring of 4H Phase Au Nanowires and its Catalytic Behavior toward CO Electro-oxidation
2023-11-08XuxuYeBingyuLiuDaZhouYanXiaChen
Xuxu Ye,Bingyu Liu,Da Zhou,Yan Xia Chen
Department of Chemical Physics and Hefei National Research Center for Physical Science at the Microscale,University of Science and Technology of China,Hefei 230026,China
Au nanowires in 4H crystalline phase(4H Au NWs) are synthesized by colloid solution methods.The crystalline phase and surface structure as well as its performance toward electrochemical oxidation of CO before and after removing adsorbed oleylamine molecules (OAs) introduced from its synthesis are evaluated by high-resolution transmission electron microscopy (HR-TEM),Xray diffraction (XRD),underpotential deposition of Pb (Pb-upd) and cyclic voltammetry.Different methods,i.e.acetic acid cleaning,electrochemical oxidation cleaning,and diethylamine replacement,have been tried to remove the adsorbed OAs.For all methods,upon the removal of the adsorbed OAs,the morphology of 4H gold nanoparticles is found to gradually change from nanowires to large dumbbell-shaped nanoparticles,accompanying with a transition from the 4H phase to the face-centered cubic phase.On the other hand,the Pb-upd results show that the sample surfaces have almost the same facet composition before and after removal of the adsorbed OAs.After electrochemical cleaning with continuous potential scans up to 1.3 V,CO electro-oxidation activity of the 4H Au sample is significantly improved.The CO electro-oxidation activity is compared with results on the three basel Au single crystalline surfaces reported in the literature,possible origins for its enhancement are discussed.
Key words: Phase engineering,Crystalline phase transition,4H phase Au nanowires,Pb under potential deposition,CO electro-oxidation
I.INTRODUCTION
Many strategies such as alloying,doping,shape-controlled synthesis,and phase engineering can be used to alter catalyst properties [1-8].Among them,phase engineering is just recently proposed in last decade and has not been widely investigated so far [4-8].Phase engineering changes the atom stacking sequence of the catalysts,which will result in modulating the surface structure and electronic properties of the catalyst and consequently its catalytic performance toward specific reactions.For example,recent studies demonstrate that the face-centered tetragonal (FCT) PtCo intermetallic compound as well as the hexagonal PtNi alloy displays higher activity and stability for oxygen reduction reaction (ORR) and hydrogen evolution reaction (HER)than their counterparts such as nanoparticles (NPs)with face-centered cubic (FCC) structure [8,9].Recently,Zhanget al.successfully synthesized the 4H phase Au nanowires (NWs) with a characteristic packing sequence of ‘ABCB’ along the close-packed [001]4Hdirection by a wet-chemical method [10].Other transition metals with 4H phase have also been synthesized by the epitaxial growth of them onto the 4H phase Au NWs,forming 4H phase core-shell structures (Au@M,M representing the transition metals) such as Au@Ru,Au@Pd,and Au@Ir [11-16].Preliminary studies demonstrate that such materials have excellent catalytic activity toward many electrocatalytic reactions.For example,the 4H phase Au@Ru and Au@Pd showed high activity in HER and methanol oxidation reaction(MOR),respectively [12,14].However,there are still not many systematic studies aiming to unveil its structure-activity relationship of those electrocatalytic reactions.
For Au,the 4H phase is a metastable phase with higher energy compared to its FCC phase.Several experiments have demonstrated the occurrence of phase transformation from the 4H phase Au NWs to the more stable FCC phase under high-temperature and highpressure treatment [17-20].For instance,with the environment transmission electron microscope (E-TEM),the 4H phase Au NWs are found to be only stable under annealing at 327 ℃ and eventually transfer to the FCC phase after annealing at 800 ℃ accompanying with a slight distortion of its morphology.This is inconsistent with predictions by molecular dynamics simulations [18].On the other hand,electrochemical reactions often proceed in very harsh environments such as strong acidic or alkaline solutions,in addition,many reactions run at high anodic or cathodic potentials [21-24].Catalyst surface reconstruction and morphology changes take place frequently as evidenced by recent studies using well-defined single crystal electrodes [25] and shape-controlled nanocrystal surfaces [26,27].Therefore,it is important to collect the information on the real crystalline phase and surface structure of 4H Au NWs under electro-catalytic reaction before deducing the structure-activity relationship.
Another unsolved issue is the effect of adsorbed OAs on the 4H Au NWs surface,since the OAs are used as the surfactant during the synthesis process.It is well known that the surfactant plays a vital role in electrocatalysis,which could serves as inhibitors or promoters for different reactions [28-34].However,little attention is paid to the effect of adsorbed OAs in previous studies.In this work,in order to explore the properties of 4H Au nanowires with the surfactant-free surface,acetic acid cleaning andin situelectrochemical oxidation are used to remove adsorbed OAs on the 4H Au NWs [29,30,35].The morphology and structure of 4H Au NWs before and after the chemical and electrochemical cleaning procedures are characterized by the high-resolution transmission microscope (HR-TEM),selected area electron diffraction (SAED),and X-ray diffraction (XRD),underpotential deposition of Pb (Pb-upd).The surfacesensitive reaction,namely carbon monoxide (CO) electro-oxidation,is used as a probe reaction to investigate the catalytic properties of 4H Au samples before and after the chemical or electrochemical cleaning.
II.EXPERIMENTS
A.Materials
Gold (III) chloride hydrate (HAuCl4·aq,~50% Au basis),oleylamine (70%,technical grade),1,2-dichloropropane (99%),hexane (technical grade),ethanol(≥99.5%),5% Nafion (Item number: 274704) and the other chemicals used in the experiments without special mention are all from Sigma-Aldrich.The CO gas and Ar gas are from Jiangsu Shangyuan Special Gas Company China.All the reagents are used as received without further purification.
B.Synthesis of 4H Au NWs
The 4H Au NWs are synthesized following the methods invented by Hua Zhang’s group with several modifications [11,12,36].In brief,200 mg HAuCl4·3H2O is added to a 250 mL thee-neck flask in an N2box,then,100 mL oleylamine is added to the flask.After that,the flask is put on an 80 ℃ oil bath with a condenser under N2 flow for 15 min to remove the air upper the liquid under magnetic stirring.Then,5 mL 1,2-dichloropropane is rapidly injected into the mixed solution.The sample is magnetically stirred for 1 min and then the solution is maintained undisturbed at 80 ℃ for 6 h.The product is precipitated with 200 mL ethanol and washed with hexane for several times at 6000 r/min for 5 min until the supernate is colorless.Then,the product is dispersed in hexane and stored in the fridge.
C.The catalyst ink preparation
1 mL 4H Au NWs in hexane is dropped to 1 mL XC-72R Hexane solution (1 mg/mL) and the mixture is ultrasonicated for 30 min under ice water.Then the product is centrifugal separation and 3 mL acetic acid is added and kept at 70 ℃ for 2 h to partially remove oleylamine molecules adsorbed on the surface.The product is centrifuged at 3000 r/min and washed with ethanol 3 times.Then,a mixture of 750 µL H2O,250 µL isopropanol,and 10 µL 5%Nafion is added and ultrasonicated for 30 min under ice water to form a uniform catalyst ink.
To remove the small nanoparticles in the 4H Au NWs,the well-dispersed ink is centrifuged at 3000 r/min again,the liquid supernatant is discarded,the rest is redispersed in 1 mL XC-72R ethanol solution (1 mg/mL)and ultra-sonicated for 10 min to disperse 4H Au-NWs on the carbon.Then the solution is centrifuged at 6000 r/min for 5 min,and the precipitate is redispersed in the mixture (750 µL H2O+250 µL isopropanol,and 10 µL 5% nafion) followed by ultra-sonicated for another 30 min.The sample is named as 4H Au-H.
To remove OAs with diethylamine,the 4H Au nanowires mixed with carbon (1 mL 4H Au NWs in hexane NWs in hexane and 1 mL XC-72R hexane solution mg/mL)) are re-dispersed in 4 mL ethanol and 1 mL diethylamine is added to the mixture.Then the mixture is stirred at room temperature for 12 h and centrifuged to separate,the precipitate is washed by 20%acetic acid and ethanol for 3 times to remove residual diethylamine.Lastly,the precipitate is redispersed in the mixture (750 µL H2O+250 µL isopropanol,and 10 µL 5% nafion) followed by ultra-sonicated for another 30 min.The sample is denoted as 4H Au-DEA.
D.Electrochemical measurement
The CO oxidation experiments are carried out with a pine rotating instrument using a glass carbon (GC) rotating disk electrode as the electrode substrate (diameter: 5 mm).Before the electrochemical test,the GC electrode is carefully polished with 50 nm alumina powder and thoroughly ultrasonicated for 1 min in Milli-Q water for 3 times,then it is washed with ethanol according to the same procedure.Then,10 µL of catalyst is dropped onto the surface of cleaned GC-RDE.The RDE is settled on an inverted pine rod,rotating at 400 r/min to form a uniform catalyst layer during the drying process in the air.Then,several cyclic voltammetric (CV) potential scaning from 0.05 V to 1.1 V,1.3 V,and 1.5 V at 50 mV/s are carried out to electrochemically clean the adsorbed oleylamine molecules until the catalysts display the best CO activity.After that,the CO oxidation polarization curves are recorded at 1600 r/min in a newly prepared 0.1 mol/L NaOH solution.
Pb-upd is carried out after the CO oxidation test has been completed.And Pb-upd is performed at 50 mV/s from 0.8 V to 0.25 V in 0.1 mol/L NaOH solution with 1 mmol/L Pb(NO3)2.All the electrochemistry measurements are conducted in the three-electrode system with a CHI-760E electrochemical workstation.The Au wire and an HgO/Hg electrode are used as the counter electrode (CE) and reference electrode (RE),respectively.Here,all potentials are calibrated with a Pt rotating disk electrode in 0.1 mol/L NaOH under saturated H2 at 1600 r/min and quoted to the reversible hydrogen electrode (RHE).The kinetic current density for CO oxidation is calculated by the Koutechy-Levich (K-L)equation.
The surface area of all the samples is assessed by Pb under potential deposition at a scanning rate of 50 mV/s in the potential range of 0.25-0.8 V.The charges for deposition of a monolayer Pb on the substrate are used to evaluate the active surface area of the Au nanoparticles.And the data for the whole surface,(111) facets and (110) facets of Au nanoparticles are 362,384,and,331 µC/cm2according to Refs.[37-39].The charges flow in the potential region of 0.354-0.493 V and 0.493-0.800 V are attributed to Pb-upd on(111) and (110) sites of the Au substrate,respectively.It must be pointed out that there is a very small stripping peak of 4H Au-1.3 at 0.45 V,which may originate from the Pb stripping from Au(100).We attribute it to(111) facets when integrating,since it is so small.
E.Physical characterization
Transmission electron microscopy (TEM) measurements are performed on HT-7700 at 100 kV and JEOL JEM-2100F microscope at 200 kV (University of Science and Technology of China).The SAED is carried out on JEOL JEM-2100F.
X-ray photoemission spectroscopy (XPS) measurements are done at a Thermo-VG Scientific Escalab 250 instrument equipped with an Al anode (Al Kα=1486.6 eV).The binding energy is normalized concerning the C 1s binding energy of 284.8 eV.The 4H and FCC Au samples are dropped on carbon paper after removing surface oleylamine molecules with acetic acid and electrochemical cleaning,then the XPS spectroscopy is collected before and after the CO oxidation experiment.
X-ray diffraction (XRD) spectra of the samples are recorded on an X-ray diffractometer (TTR-III,Rigaku Corp,Japan) using Cu Kα radiation (λ=1.54056 Å)withθ/2θmode.All the Au samples are mixed with XC-72R carbon and tested on a silicon wafer.Since the carbon powder (XC-72R) will only show a broad peak of low intensity at 2θ=23.2° in the diffraction pattern,while the diffraction peaks of both the 4H phase and FCC phase Au are higher than 30°,our sample-making method will not interfere with the crystalline phase analysis.
III.RESULTS AND DISCUSSION
A.Characterization of the phase structure of the as-prepared 4H Au NWs
The structure of the as-synthesized 4H Au NWs is carefully characterized by HR-TEM,SAED,and XRD,the results are shown inFIG.1.The low-magnification TEM image inFIG.1(a) shows the 4H Au NWs have a diameter of about 20-30 nm and a length of nearly 1 µm.The alternating light and dark stripes in the high-magnification TEM image (FIG.1(b) marked with a white rectangle) and HR-TEM image (FIG.1(c)) are the Moiré patterns arisng from the ‘ABCB’ close stacking sequence of Au atoms,which are the signature of the 4H phase [10,11,40].The SAED inserted inFIG.1(c) are taken from the single 4H Au NWs (marked with a red circle) inFIG.1(b),and the diffraction spots of (100)4H,(102)4H,and (004)4H facets provide strong evidence of the 4H phase in 4H Au NWs along the [10]4Hzone axis[10].In a word,the SAED pattern and the corresponding HR-TEM image of 4H Au NWs confirm the existence of the 4H phase structure in 4H Au NWs.

FIG.1 TEM images of 4H Au nanowires with (a) low-magnification and (b) high-magnification,the Moiré pattern is highlighted by a white rectangle;(c) HR-TEM image of 4H Au NWs with SAED image inserted (taken from the one marked with a red circle in (b);(d) the XRD pattern of 4H Au NWs,the diffraction peaks from 4H phase crystal are marked in red,while those from FCC phase are shown in black.
To further investigate the global phase of the 4H Au NWs,the XRD (powder X-ray diffraction) is carried out withθ/2θmode.The obtained XRD pattern is shown inFIG.1(d),three characteristic peaks located at 36.2°,37.4°,and 40.9° correspond to the (100)4H,(101)4H,and (102)4H facet diffraction,respectively [10].The diffraction peak at 64.8° is the sum of (110)4H and(220)FCC facets since they have the same lattice spacing at 1.43 Å [10].The other diffraction peaks at 38.2°,44.3°,and 77.6° result from (111)FCC,(200)FCC,and(220)FCCfacet diffraction of the FCC phase [10].The diffraction peaks of the FCC phase in the as-prepared 4H Au NWs may come from two sources,one is the FCC fraction in the 4H Au NWs,the other is the Au nanoparticles (NPs),which are the by-products formed in the synthesis of 4H Au NWs as seen fromFIG.1(a) and (b).
B.Removing adsorbed oleylamine molecules by chemical and electrochemical cleaning
To eliminate the influence of adsorbed OAs at the surface of 4H Au NWs on its electrocatalytic performance,a chemical method is firstly used to remove the adsorbed OAs.4H Au NWs are immersed in acetic acid(HAc,99.99%) at 70 ℃ for 2 h [28,30].The sample after HAc cleaning is denoted as 4H Au-H.According to previous studies with Fourier transform infrared spectroscopy under attenuated total reflectance configuration (ATR-FTIRS) [28],surfactants cannot be completely removed just with acetic acid cleaning,hencein situelectrochemical cleaning is applied subsequently to further remove the residual OAs adsorbed on the surface of the catalyst.In order to visualize the efficiency of the electrochemical cleaning process,CO oxidation has been chosen as a probe reaction in such a treatment.Ten cycles of cyclic voltammetric potential scans with different upper potential limits are carried out at the working electrode loaded with 4H Au NWs in CO saturated 0.1 mol/L NaOH solution (FIG.2).The sample after electrochemical cleaning is labeled as 4H Au-X,andXis the upper potential limit applied in the cyclic voltammetric cleaning.
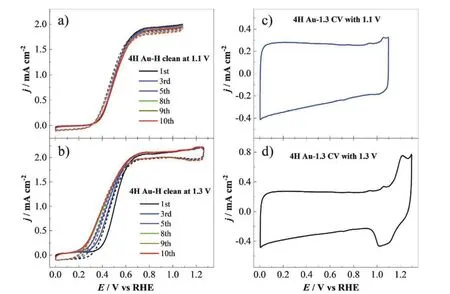
FIG.2 10 cycles of cyclic voltammetric potential scans recorded during the electrochemical cleaning process for the 4H Au-H (the 4H Au NWs after acetic acid cleaning) in CO saturated 0.1 mol/L NaOH solution,with the upper potential limits of (a) 1.1 V and (b) 1.3 V,scan rate 50 mV/s,electrode rotation speed,1600 r/min.The basic CVs of 4H Au-1.3 measured in Ar saturated 0.1 mol/L NaOH solutions with upper potential limits of (c) 1.1 V and (d) 1.3 V,the 4H Au-1.3 is the abbreviation of the 4H Au-H after electrochemical cleaning at 1.3 V.
For the case with the upper potential limit of 1.1 V(FIG.2(a)),we see that as the number of potential cycles increases to the 10th cycle,the potential of the same CO electro-oxidation current density remains almost unchanged.In contrast,when the upper potential limit is changed to 1.3 V,the onset potential of CO oxidation shows an obvious negative shift with the increase in the number of potential cycles up to the 8th cycle (FIG.2(b)).In the 8th to 10th cycles,the current wave for CO oxidation does not show obvious change.In addition,a pair of small redox peaks appear at ca.1.2 V which is superimposed with the diffusion limiting current for CO oxidation.The basic CVs of the 4H Au-1.3 sample after recording 10 cycles of CO oxidation with upper potential limits of 1.1 V and 1.3 V are shown inFIG.2(c) and (d),respectively.FromFIG.2(d) we see that the oxidation of the 4H Au-1.3 surface starts at the potential of about 1.1 V,and the 4H Au-H surface is already partially oxidized during the cyclic voltammetric scans with upper potentials of 1.3 V.This is confirmed by the appearance of the reduction current peak of AuOxobserved at 1.1 V during the reverse scan [41,42].We have also tried to further increase the upper potential limit for electrochemical cleaning to 1.5 V,and we found that the activity of CO electro-oxidation remains the same as that of 1.3 V (FIG.S1 in Supplementary material (SM)).The abrupt increase of CO oxidation activity after electrochemical cleaning with an upper potential limit of 1.3 V may probably be induced by the removal of OAs or the structure change of Au sample induced by electrochemical oxidation-reduction cycles with an upper potential limit higher than 1.1 V [43].In the following section,we will first check how the structure of 4H Au NW samples changes after the chemical and electrochemical cleaning.
C.Restructuring of 4H Au samples induced by chemical and electrochemical cleaning
To clarify what happens to the 4H Au samples after the treatment by chemical or chemical plus electrochemical cleaning,we have carried out a systematic structure characterization of the 4H Au samples.Firstly,to make sure whether the morphology and the crystalline phase structure of the 4H Au NWs change after acetic acid cleaning and electrochemical cleaning,the TEM and XRD are used to characterize these samples.FIG.3(a,c,e,g) and (b,d,f,h) are the TEM and the enlarged TEM images of 4H Au NWs,4H Au-H,4H Au-1.1,and 4H Au-1.3,respectively.The morphology of 4H Au NWs is destroyed after the adsorbed OAs have been partially removed by acetic acid,leading to the formation of large dumbbell-shaped nanoparticles with a diameter of about 200-400 nm as shown inFIG.3(c).And the large dumbbell-shaped nanoparticles remain unchanged after electrochemical cleaning with the upper potential of 1.1 V and 1.3 V as seen from the images given inFIG.3(e) and (g),respectively.FromFIG.3(d)we see that the characteristic stripes of the 4H phase remain,suggesting that some 4H phase still survives after acetic acid cleaning.This is further proved by the existence of (102)4H diffraction peak as shown inFIG.3(i).

FIG.3 The TEM image and XRD pattern of 4H Au NWs after different cleaning processes.The TEM images of 4H Au NWs as-synthesized (a,b);after HAc cleaning (c,d);after further electrochemical cleaning by cyclic voltammetry with an upper potential limit of 1.1 V (e,f) and 1.3 V(g,h).(i) The XRD pattern of 4H Au NWs after different cleaning processes;(j) the 4H phase content after different cleaning processes,the content is assessed by the intensity ratio of diffraction peaks(102)4H/(220)FCC as shown in FIG.3(i).
To illustrate the phase transition process during acetic acid and electrochemical cleaning,the relative changes in the intensity ratio of (102)4H/(220)FCC during the cleaning are plotted and shown inFIG.3(j).We see that the 4H phase content exhibits a huge drop after acetic acid cleaning,which suggests that the adsorbed OAs not only play a vital role in maintaining the morphology of the Au nanowires,but also in stabilizing the 4H crystalline phase.Moreover,it is interesting to find that the stripes exist after electrochemical cleaning at 1.1 V (FIG.3(f)) and vanish after electrochemical cleaning at 1.3 V (FIG.3(h)),which is consistent with the existence and disappearance of (102)4Hdiffraction peak of 4H Au-1.1 and 4H Au-1.3 (FIG.3(i)),respectively.Furthermore,the 4H Au-1.3 shows a typical FCC phase diffraction pattern (FIG.3(i)),which further implies that the metastable 4H phase transfers to the more stable FCC phase during electrochemical cleaning at 1.3 V.The 4H phase content of 4H Au-1.1 remains almost unchanged with 4H Au-H,but declines to 0 after electrochemical cleaning at 1.3 V,which further proves that the 4H phase can exist stably during electrochemical cleaning at 1.1 V,but not at 1.3 V.Eventually,the 4H phase fades away during the removal of adsorbed OAs with acetic acid and electrochemical cleaning.
It is interesting to find that the crystalline phase transition and the gold surface oxidation take place after electrochemical cleaning by cyclic voltammetry with an upper potential limit of 1.3 V (FIG.2(d)).In order to avoid such phase transition,we have also tried an even milder method to remove OAs adsorbed at the 4H Au samples.Briefly,the adsorbed OAs are replaced by the diethylamine (DEA) in ethanol solution at room temperature,then the adsorbed DEA is cleaned with acetic acid [44].However,the 4H phase has also been found to be transferred to the FCC phase after the substitution as evidenced by the disappearance of (102)4Hdiffraction peak (FIG.3(i)).In a word,although different methods are tried to remove adsorbed OAs,the results are the same,namely,the 4H phase would transfer to a more stable FCC phase.One possible reason is that the 4H phase is a metastable phase with high energy [18,19,40,45] and the adsorbed OAs are helpful to decrease the surface energy [2,46],which helps to stabilize the 4H phase in 4H Au NWs.Upon the removal of adsorbed OAs,the surface energy increases,and the system spontaneously reduces energy by transferring to a more stable FCC phase and reducing the surface area.So,whatever method is used for removing adsorbed OAs,the phase transition to the FCC phase and the formation of dumbbell-shaped nanoparticles take place during/after the cleaning process.
D.Electrocatalytic CO oxidation performance of 4H Au samples before and after cleaning
To demonstrate how the electrocatalytic behavior of 4H Au samples changes before and after the cleaning procedure,the CO electro-oxidation in CO saturated alkaline (0.1 mol/L NaOH) solution is used as a probe reaction under a rotating disk electrode (RDE) configuration.The polarization curves of CO oxidation at the 4H Au samples after three different cleaning procedures are shown inFIG.4(a).The 4H Au-H and 4H Au-1.1 display similar CO electro-oxidation activity,while the 4H Au-1.3 exhibits a huge increase of activity as evidenced by an obvious shift of the current wave for CO oxidation to the lower potentials.On the other hand,the Tafel slopes for CO oxidation at the three 4H Au samples (insert inFIG.4(a)) are close.To quantitatively evaluate the CO oxidation activity,the kinetic current density (jk) is calculated with the Koutechy-Levich equation and the results are shown inFIG.4(b).Thejkfor CO oxidation at 4H Au-1.3 is 0.283 mA/cm2at 0.35 Vvs.RHE,which is about 8.1 and 7.4 times higher than that at 4H Au-1.1 and 4H Au-H (ca.0.035 mA/cm2and 0.038 mA/cm2at 0.35 Vvs.RHE).
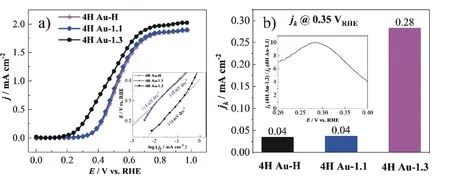
FIG.4 (a) Polarization curves for CO electro-oxidation on 4H Au-H (magenta line+star),4H Au-1.1 (blue line+rhombus),and 4H Au-1.3 (black line+circle) recorded in the positive-going scan in 0.1 mol/L NaOH solution,scan rate 20 mV/s,1600 r/min,the inset is the Tafel plots of corresponding to kinetic current density (jk) as a function of applied potential;(b) the comparison of the kinetic current density (jk) for CO oxidation at the three samples at 0.35 V,the inset is the jk ratio of 4H Au-1.3 and 4H Au-1.1 in the range of 0.2-0.4 V.
In order to unravel the reason for the vast difference in CO oxidation activity,the electronic and geometric properties of the three samples have been characterized by X-ray photoelectron spectroscopy (XPS) and Pbupd.The XPS result in FIG.S2 in SM shows that the binding energies of Au 4f in 4H Au-H,4H Au-1.1,and 4H Au-1.3 are the same.This implies the surface Au atoms in the three samples have the same chemical valence.The geometric structure of the three Au samples surfaces is characterized by Pb-upd [38].The 4H Au-H and 4H Au-1.1 show almost the samej-Ecurves for Pbupd (FIG.5(c) and (d)),which is similar to the 4H Au-1.3.This suggests that the three samples have similar surface structures.The 4H Au-1.3 is the FCC phase during CO oxidation since the 4H phase has completely transferred to the FCC phase after electrochemical cleaning at 1.3 V (FIG.3(i)).According to the Pb-upd on FCC single crystal electrode [38,47] (FIG.5(b)),the deposition/the stripping peaks at 0.52 V/0.56 V and 0.38 V/0.41 V in thej-Ecurves of 4H Au-1.3 (FIG.5(a))are attributed to the deposition of Pb2+/the oxidative removal of Pb on the (110) and (111) facets on Au surface,respectively.Moreover,the new stripping peak at 0.45 V can be assigned to Au (100) facets (FIG.5(a)).The Pb-upd current in the potential range of 0.25-0.35 V is attributed to the Pb stripping from the defect sits (steps and terraces) [38,47].Similarly,the stripping peak at 0.56 V and 0.41 V in the Pb-upd curves of 4H Au-H and 4H Au-1.1 can be attributed to the (110)and (111) facets,as they display nearly the same cur-rent waves for Pb-upd in the same potential regime,even though both samples have some fraction of the 4H phase.
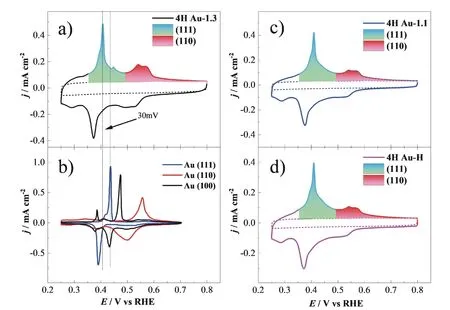
FIG.5 The j-E curves of Pb-upd on 4H Au-1.3 (a),4H Au-1.1 (c),and 4H Au-H (d) recorded in 0.1 mol/L NaOH +1 mmol/L Pb(NO3)2,scan rate: 50 mV/s,the stripping peaks of (111) facets and (110) facets are filled with blue and red,respectively;(b) the j-E curves of Pb-upd on the single-crystal electrode of Au(111) (blue),Au(110) (red) and Au(100) (black) in 0.1 mol/L NaOH+1 mmol/L Pb(NO3)2 solutions,scan rate: 50 mV/s [38].
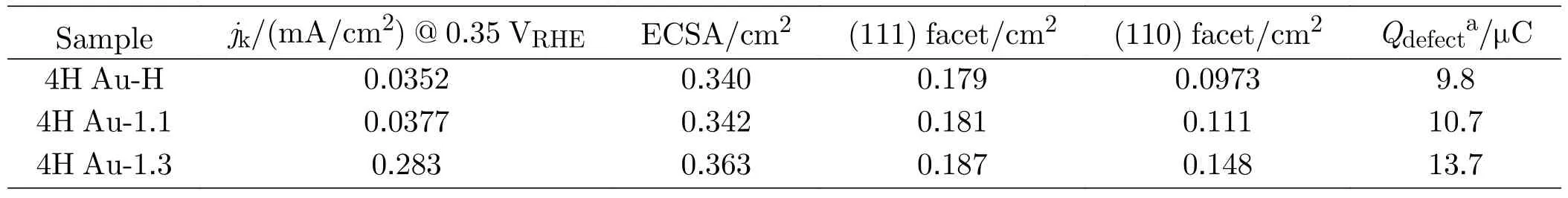
TABLE I The electrochemical surface area (ECSA) of 4H Au samples and other characteristics.
The electrochemical surface area (ECSA) assessed by Pb-upd are 0.340,0.342 and 0.363 cm2for 4H Au-H,4H Au-1.1 and 4H Au-1.3 (Table I).Therefore,the similar Pb-upd curves and ECSA suggest that surface oxidation during the electrochemical cleaning at 1.3 V does not drastically roughen the surface structures [48].Comparing to the Pb stripping peak of (111) and (110)facets on the single-crystal Au surface [38] (FIG.5(b)),it is apparent that the Pb stripping peaks of the three 4H Au samples are broader and negatively shifted for ca.30 mV.A similar phenomenon is also observed for the Pb-upd at Au(775) [6(111)-(110)] electrode single crystal facet,which is a stepped surface consisting of unit structure with six Au atoms on the (111) terraces and a(110) step [38].Previous studies revealed that the little domains with large amounts of grain boundaries and defects cause sluggish deposition and stripping of Pb,which results in the broadening and negative shift of the Pb stripping peak [38,47].Based on these facts,we think the broader and negative shift peaks for Pd-upd can serve as an indication that there are lots of little crystal domaining on 4H Au samples.This is also supported by the appearance of the shoulder peak of Pb stripping on (110) facets at about 0.56 V.
The surface area of (111) and (110) facets are evaluated by integrating the charge of the respective stripping peaks located around 0.41 V and 0.56 V(FIG.5(c-e)),respectively.Comparing to the 4H Au-1.1,the area of (111) facets increases a little (ca.3%)while the area of (110) facets increase for ca.33% after the electrochemical cleaning process at 1.3 V (Table I).There seem to be two reasons for the increase of (110)facets,the one is the re-exposure of the exposed active site poisoned by residual OAs [43,49],as evidenced by the emergence of Au(100) facets at 0.45 V (FIG.5(a));the other possible reason is that more defects and boundaries are created during the multi-cycle oxidation/reduction potential scans (Table I).This is also supported by the slight increase of the current for Pbupd in the potential range of 0.25-0.35 V (Table I)[50-52].Unfortunately,The two processes seem to be synergistic and occur simultaneously,therefore it is difficult to distinguish them.
On the other hand,the kinetic current density of CO electro-oxidation at 0.35 V increases about 7 times,while the increase of fraction of (110) and (111) facets on the sample surface only amounts to ca.33% and 3.3%,respectively.Previous studies by Markovićet al.found that the three basic single-crystalline Au surfaces show poor CO oxidation activity,and the CO oxidation activity decreases in the order of Au(110)>Au(100)>Au(111) (Table II) [53].In contrast,Koperet al.reported that the CO oxidation activity of the three basic single-crystalline Au surfaces decreases in the order of Au(111)>Au(100)>Au(110) (Table II).Furthermore,Koperet al.found that at 0.35 V,CO oxidation activity at Au(111) isca.5.5 times higher than that on Au(110) (Table II) [41,53].The activity of CO oxidation at Au(111),Au(110) and Au(100) obtained by Koperet al.isca.650,10 and 10 times higher than that reported by Markovićet al.[41].Koperet al.suggested that the better CO oxidation activity of the (111) facet originates from the self-promotion of adsorption of oxidizing species (such as hydroxyl) at the nearest site after CO adsorption as supported by DFT calculation,which only occurs at Au(111) facet [41,54-56].Koperet al.speculated that the poor CO oxidation activity observed by Markovićet al.is probably due to that the single less ordered crystalline electrode and the presence of surface impurities,as evidenced by the blank voltammograms [41,53].The poor specific CO oxidation activity at OAs poisoned 4H Au-H and 4H-Au-1.1 obtained in this work is just ca.2 times smaller than that at Au(110) (comparable to that at Au(100)) as re-ported by Markovićet al.As inferred from the Pb-upd results,the ECSA of 4H-Au-1.1 is nearly the same as that of 4H-Au-H,even after electrochemical cleaning with upper potential limit of 1.3 V,the overall ECSA only increases 6%.Considering that 4H-Au-H and 4HAu-1.1 still has about 60% of the 4H-phase (FIG.3) and their poor CO oxidation performance (Table I) compared to that at three basel single crystalline Au surfaces as reported by Koperet al.(Table II),we conclude that the 4H-phase probably does not have beneficial effect toward CO oxidation.

TABLE II The jk of single crystal electrode @ 0.35 VRHE.
At the rather clean 4H-Au-1.3 sample,CO oxidation activity (Table I) is also significantly smaller than that at the three basel Au single crystalline electrode surface reported by Koperet al.(Table II).This further supports that in addition to the amount of the (111) facets on the surface,the orderness of (111) facets is very important in delivering higher CO oxidation performance[41].Obviously,the 7 times enhancement of the CO oxidation activity at the 4H Au-1.3 after electrochemical cleaning with an upper potential limit of 1.3 V cannot be exclusively attributed to the increase of the fraction of (110) and (111) facets on the surface of Au samples at all.We think a significant enhancement of CO oxidation activity probably comes from the defects and grain boundaries created during the electrochemical cleaning process.This is in agreement with previous reports that such sites are more favorable for the adsorption of oxidizing species (such as hydroxyl group and oxygen atoms) [53],which leads to an increase in the catalytic activity of the whole surface of the catalyst.
IV.CONCLUSION
Metastable 4H phase Au nanowires are synthesized with OAs as the surfactant agent.The stability of the 4H crystalline phase,the surface structure and the catalytic behavior of 4H Au samples are characterized by HR-TEM,XRD under potential deposition of Pb as well as electrochemical oxidation of CO,respectively.The adsorbed OAs on 4H Au NWs are found to play an important role in maintaining the stability of the 4H phase and the morphology of the Au nanowires.Although great efforts and different methods have been tried to remove adsorbed OAs while maintaining the 4H crystalline phase,the crystalline phase of the 4H Au NWs transfers from the 4H phase to the FCC phase,and the morphology changes from nanowire to large dumbbell-shaped nanoparticles during such cleaning processes.One logical reason is that the adsorbed OAs reduce the surface energy of the metastable 4H Au NWs,maintaining the existence of the metastable 4H phase and the morphology in 4H Au NWs.Upon the removal of adsorbed OAs,the 4H Au NWs spontaneously reduce their energy by transferring to a more stable FCC phase and reducing the surface area with the formation of large dumbbell-shaped nanoparticles.
The Pb-upd results show that the 4H Au-H,4H Au-1.1,and 4H Au-1.3 have similar surface structures and the surface oxidation during cleaning at 1.3 V would not drastically change the surface structures and the roughness.The 4H phase Au sample does not show enhanced intrinsic CO oxidation activity compared to the Au samples with FCC phase.Compared to the 4H-Au samples just with HAc cleaning,there is slight increase in the amount of (110) (ca.33%) and (111) (3%) facets on the surface.This is probably due to the re-exposure of the poisoned areas via the removal of the residual OAs.The vast enhancement of CO electro-oxidation performance (ca.7 times) of the 4H Au-1.3 after its electrochemical cleaning probably originate from the defects and grain boundaries created during the cleaning process,which improve the adsorption of oxidizing species (such as hydroxyl groups),leading to the enhanced CO electro-oxidation across the whole surface of the nanoparticles.The fast changes in the morphology of 4H Au NWs and conversion of the crystalline phase from metastable 4H phase to the more stable FCC structure as revealed in this study suggest that more attention should be paid to characterizing the surface structure and crystalline phase transition during the reaction process when studying the ‘structure-function relationship’ in electrocatalysis.This is especially important for nanocatalysts with novel crystalline phase or structure which have high electrocatalytic activity but are intrinsically metastable in nature.
Supplementary materials:The electrochemical cleaning process and CO electro-oxidation activity for 4H Au samples with different upper potential limits,and the XPS pattern of 4H Au-H and 4H Au-1.3 are shown.
V.ACKNOWLEDGMENTS
We sincerely thank Chen Ye from Hua Zhang’s group for the direction of the synthesis of the 4H Au nanowire.This work was supported by the National Natural Science Foundation of China (No.22172151 and 21972131).
杂志排行
CHINESE JOURNAL OF CHEMICAL PHYSICS的其它文章
- Atomistic Modeling of Lithium Materials from Deep Learning Potential with Ab Initio Accuracy
- On-the-Fly Nonadiabatic Dynamics of Caffeic Acid Sunscreen Compound
- Minimum-Modified Debye-Hückel Theory for Size-Asymmetric Electrolyte Solutions with Moderate Concentrations
- Photothermal Catalytic Selective Oxidation of Isobutane to Methacrylic Acid over Keggin-Type Heteropolyacid
- Design Strategy of Infrared 4-Hydroxybenzylidene-imidazolinone-Type Chromophores based on Intramolecular Charge Transfer: a Theoretical Perspective
- Quantum Dynamics Calculations on Isotope Effects of Hydrogen Transfer Isomerization in Formic Acid Dimer
