Gastroprotective Effect of Alpinia zerumbet (Pers.) Burttet Smith on Ethanol-induced Gastric Ulcers in vivo and vitro
2023-10-31QingWEIShanshanLIANGYuechunYANGJingZHANG
Qing WEI, Shanshan LIANG, Yuechun YANG, Jing ZHANG
1. College of Pharmacy, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, China; 2. College of Basic Medicine, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, China; 3. Institute of Laboratory Animal Science, Guizhou University of Traditional Chinese Medicine, Guiyang 550025, China
Abstract [Objectives] To explore the protective effect of Alpinia zerumbet (Pers.) Burttet Smith ethanol extraction (YSJ) on gastric ulcers. [Methods] The ethanol-induced gastric ulcer model was generated by male Kunming mice. The mice were divided into six groups, including control, model (GU), positive omeprazole enteric-coated capsules (18 mg/kg), YSJ low (1.17 g/kg), medium (2.34 g/kg) and high (4.68 g/kg) dose groups. To observe the state of gastric tissues, hematoxylin and eosin (H&E) staining was applied and immunohistochemistry (IHC) was used to evaluate the expression of nuclear factor kappa-B p65 (NF-κB p65), interleukin 6 (IL-6), tumor necrosis factor-α (TNF-α), and Cyclooxygenase-2 (COX-2) in gastric tissues. The ulcer index, biochemical parameters, and inflammatory proteins were evaluated. In vitro, GES-1 cells were induced by anhydrous ethanol to found gastric ulcer model. The groups were the same as in vivo experiment. Cytotoxicity was tested by MTT and IL-1β, TNF-α, and IL-6 were detected by Elisa assays. [Results] The injuries of gastric tissue in the model group were unambiguously observed and improved after YSJ treatment. The levels of interleukin 1 beta (IL-1β), interleukin 6 (IL-6), interleukin 8 (IL-8), and TNF-α in serum and tissues were decreased (P<0.05 or 0.01), whereas interleukin 4 (IL-4) and prostaglandin E2 (PGE2) were increased after treatment with YSJ (P<0.05 or 0.01). The levels of IL-1β, IL-6, TNF-α, and IL-8 were decreased in YSJ groups, while IL-4 and PGE2 presented the opposite trend. The protein expression of p-NF-κB, NF-κB, p-IκBα, IκBα, and TNF-α was inhibited after treatment with YSJ (P<0.05 or 0.01). [Conclusions] These results demonstrate that YSJ alleviates the occurrence and development of gastric ulcers via inhibiting inflammation.
Key words Alpinia zerumbet (Pers.) Burttet Smith, Gastric ulcer, Inflammatory factors
1 Introduction
Gastric ulcer (GU) is a common chronic digestive system disease with high incidence and serious harm to human health. At present, nearly 5%-10% of the world population is affected by GU to varying degrees[1]. Clinically, the most common symptoms mainly include but are not limited to epigastric pain, abdominal distension, belching, and acid regurgitation. Under normal circumstances, the stomach can bear high concentration of hydrochloric acid, bile salts, alcohol and foods with different temperatures and osmotic pressures. However, when the level of gastric acid or pepsin in human body increases and the defense ability of mucosa decreases, it will cause damage to local tissues of gastric mucosa until ulcers are formed[2-3]. There are many factors that promote the occurrence of ulcers. The infection of non-steroidal anti-inflammatory drugs (NSAIDs) such as aspirin and celecoxib andHelicobacterpylori(Hp) are the two most important factors that destroy mucosal resistance[4]. In addition, factors such as environment (cigarettes, alcohol use and infectious pathogens) and emotional stress can also induce ulcers to some extent. In the stress-induced gastric ulcer, it is found that the surface of epithelial cells is seriously damaged, sometimes reaching the submucosa. Moreover, the activity of antioxidant enzymes in gastric mucosa is weakened, which indirectly leads to the increase of histamine and pepsin, breaks the balance in the stomach and causes ulcers. The occurrence of gastric ulcer is a complicated pathological process[5].
Alpiniazerumbet(Pers.) Burttet Smith is the dry root ofA.zerumbet(Pers.) Burttet Smith, it is warm in nature and tastes acrid. The root ofAlpiniazerumbet(Pers.) Burttet Smith also has the functions of warming middle energizer, drying wetness, promoting qi, relieving pain, and reducing malaria. It is mainly used to treat chest psychalgia, chest and abdominal fullness, indigestion, vomiting and diarrhea. As a characteristic national medicinal in Guizhou province China,A.zerumbet(Pers.) Burttet Smith is a commonly used medicine among ethnic minorities, and also commonly used by the Shui nationality to treat gastric ulcers with good efficacy[6-8]. Studies onA.zerumbet(Pers.) Burttet Smith ethanol extraction (YSJ) has mostly concentrated on the pharmacological properties and chemical components of the leaves or flowers[9-10]. Domestic studies have primarily concentrated on its anti-myocardial ischemic, anti-atherosclerotic, anti-inflammatory, analgesic, and anti-hypertensive effects[10-13], but less research has been conducted on its traditional efficacy against gastric ulcers.
Based on the traditional efficacy ofA.zerumbet(Pers.) Burttet Smith, this study established a mouse model of acute gastric ulcer with anhydrous ethanol. The protective effect of YSJ on acute gastric ulcersinvivoandvitrowas assessed by using interleukin-1 beta (IL-1β), interleukin-4 (IL-4), interleukin-6 (IL-6), interleukin-8 (IL-8), tumor necrosis factor (TNF-α), cyclooxygenase 2 (COX-2), prostaglandin E2(PGE2), and other indicators. This evaluation provides an experimental basis for the development and utilization of YSJ in the clinic.
2 Materials and methods
2.1 Materials
2.1.1Reagents and apparatus.Reagents and apparatuses included 0.9% NaCl solution (Shimen Pharmaceutical Co., Ltd., Henan, China), BCA protein assay kit (Beyotime Biotechnology Co., Ltd., Shanghai, China), mice IL-1β, IL-4, IL-6, IL-8, PGE2, TNF-α and GAS ELISA kits (Hualianke Bio., Wuhan, China), specific antibodies against GAPDH, p-NF-κB, NF-κB, p-IκBα, IκBα, and TNF-α (Wuhan Sanying Biotechnology Co., Ltd., Wuhan, China).
2.1.2Plant material and preparation of the extract.A.zerumbet(Pers.) Burttet Smith collected from Guizhou Province were identified as the roots ofA.zerumbet(Pers.) Burttet Smith by researcher Qingwen Sun, a plant taxonomist from Guizhou University of Traditional Chinese Medicine. The dry roots ofA.zerumbet(Pers.) Burttet Smith were preserved in the College of Pharmacy, Guizhou University of Traditional Chinese Medicine (GY20221005001). Briefly, the root powder (100 g) was refluxed and extracted with 95% ethanol (1 000 mL) for 3 times, 2 h each time. The three extracts were mixed, and concentrated by rotary evaporation to obtain 18.69 g extracts.
2.1.3Experimental animals. Forty-eight 8-week-old KM mice with body masses 18-22 g were acquired from the Institute of Laboratory Animal Science, Guizhou University of Traditional Chinese Medicine (Guiyang, China), license number SCXK (Qian) 2021-0005. The experimental animals were reared in a setting with a relative humidity of 60% to 70% and a temperature of 24 to 26 ℃. Prior to the experiment, they were given a week of free access to regular food and water to help them become used to their surroundings. The experimental techniques were authorized, and the feeding circumstances, food, and padding all meet health requirements (Institute of Laboratory Animal Science, Guizhou University of Traditional Chinese Medicine, China). The ethics approval for the use of animals in this study is approved by Guizhou University of Traditional Chinese Medicine Ethical Committee for Animal Review (No.:20210104).
2.2 Methods
2.2.1Animals treatment. All animals were habituated for one week before being randomly assigned to one of six groups, each of which had eight mice: the control group, the model (GU) group, the positive omeprazole enteric-coated capsules (18 mg/kg), and the YSJ low (1.17 g/kg), medium (2.34 g/kg), and high (4.68 g/kg) dose groups. For seven consecutive days, the mice in the three dosing groups received YSJ through gavage in the appropriate quantity. The mice in both the normal group and the model group received distilled water daily for 7 days in a row before being strictly forbidden from eating for 24 h. Except for the control group, all mice were gavaged with 0.1 mL/10 g of anhydrous ethanol on the eighth day, 1 hour after the last intragastric injection, and blood was drawn from their eyes an additional hour later. Blood was kept in a -80 ℃ environment after being centrifuged at 12 000×g for 15 min. After that, the mice’s gastric tissues were quickly sliced away from the cardia along the larger curve to the pylorus. After being cleaned with ordinary saline, the gastric tissue was split into three pieces, and stored at -80 ℃.
2.2.2H&E staining and Immunohistochemical analysis. The following procedure was used for the histopathological analysis of the stomach tissue using hematoxylin-eosin (H&E) staining. Gastric tissue was cut into 5 μm pieces after being embedded in paraffin and treated with paraformaldehyde at a 4% concentration. After that, each segment was dewaxed, dyed, and magnified by 100 on a Nikon DS-U3 (Tokyo, Japan). The previously described method was used to score each stomach tissue’s pathological lesion[8].
To determine the levels of NF-κB p65, IL-6, TNF-α, and COX-2 protein expression in the cell nuclei of the gastric tissue, immunohistochemistry (IHC) was performed. Dewaxing, rehydrating, and incubating in bovine serum albumin (BSA, 5 percent) were all performed on a piece of stomach tissue. The tissue segment was next incubated with the primary antibody at 4 ℃ overnight. A secondary antibody was added after the sample had undergone three PBS washes. Each portion was then cleaned and scrutinized with the Nikon DS-U3 equipment.
2.2.3Cell culture.GES-1 cells (ATCC, Manassas, USA) were extracted from liquid nitrogen, added to RPMI-1640 culture solution with 10% FBS, and then cultivated at 37 ℃ in a cell incubator with 5% CO2. After the culture medium had been changed after 24 h, an appropriate volume of the cell suspension was then injected into a fresh culture flask for cell culture in a subsequent experiment.
2.2.4Cell viability assay. A 96-well plate containing 5×104logarithmic phase cells was seeded and grown at 37 ℃ with 5% CO2for 24 h. The concentrations in the YSJ groups were 15, 30, 60, and 120 μg/mL, with six parallels in each group. After 24 h, 20 μL of the MTT solution that had been previously made and preserved in the dark was added, and the wells were then put in an incubator set at 37 ℃ for culture. Then, 4 h later, the MTT solution and cell culture media were carefully removed from the culture plate wells, 150 μL of DMSO reagent was applied to each well. The microplate reader measured the absorbance (OD) at 490 nm (Thermo Scientific Multiskan FC, Waltham, USA). Furthermore, GES-1 cells were pretreated with YSJ (15, 30, and 60 g/mL) for 24 h prior to being exposed to anhydrous ethanol for an additional 12 h to test the cytotoxicity. Cell viability was assessed by MTT test.
2.2.5Detection of the levels of cytokines. IL-1β, IL-4, IL-6, IL-8, TNF-α, PGE2and GAS enzyme-linked immunosorbent assay (ELISA) kits were used to conduct the experiments. Thermo Scientific Multiskan FC microplate reader was used to measure the absorbance.
2.2.6Western blot assays. After being homogenized in RIPA lysis buffer, the gastric tissues were placed in an ice bath for ten minutes. The protein content of the sample was measured by using BCA protein assay kits for further determination of protein levels after the tissue homogenate were centrifuged at 12 000 g for 15 min. The manufacture of separating glue and concentration glue, electrophoresis, membrane transfer, primary antibody incubation (GAPDH, 1:20 000; p-NF-κB, p-IκBα, IκBα, 1:2 000; NF-κB, TNF-α, 1:1 000), secondary antibody incubation (1:2 000), and development were all parts of the procedure.
2.2.7Statistical analysis. Thettest was used to the experimental data, and statistical graphs were created by GraphPad Prism 9.0.1. One-way analysis of variance was employed to compare data from two or more independent experiments between groups. The means ± SDs, or means ± SEMs of all experimental data are displayed. Statistical significance was considered to be shown by a value ofP<0.05.
3 Results and analysis
3.1 Effect of YSJ on gastric mucosal pathology in miceIn the control group, there was no damage to the smooth surface of the gastric mucosa. The model group’s injuries were more serious, and there were very noticeable blood patches and bleeding regions. The degree to which the stomach mucosa bled was reduced in various concentration groups when compared to the model group was variable. Among these, only a tiny portion of the bleeding spots were visible in the positive group, which displayed clear signs of stomach mucosal bleeding. The frequency of bleeding patches and bleeding area value were attenuated in the positive group compared to the model group. Each administration group reduced its bleeding points and bleeding area to varying degrees, but there were much fewer bleeding points and almost no bleeding area in the YSJ-H group, which demonstrated that the YSJ group had a strong healing effect on mouse gastric mucosa damage (Fig.1). The bleeding sites and bleeding regions of the gastric mucosa were counted and quantified in accordance with the degree of gastric mucosal injury in each group of mice. The levels of gastrin (GAS) in the model group were higher than those of the control group (P<0.01), but the levels of GAS in the YSJ groups were lower (P<0.05 orP<0.01). Significant dosage dependence and clear drug dependence were seen in the dosing groups (Fig.2).
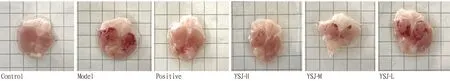
Fig.1 Macroscopic analysis of the mouse gastric mucosa
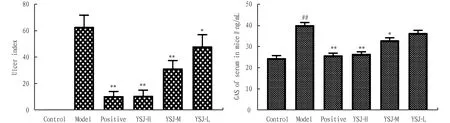
Note: Values are shown as the means±SEMs, n=8 for each group; compared with control group, ##P<0.01; compared with model group. *P<0.05, **P<0.01.
3.2 HE results of YSJ in treating gastric ulcerAs shown in Fig.3, the glands that make up the gastrointestinal mucosa were unharmed, no damage was noticed in the control group. The control group’s stomach tissue cells were neatly and evenly spaced out, and the tissue’s structure was likewise clear. While in the model group, chaotic submucosal inflammatory cells, disorganized gastric mucosa epithelial cells, and disorganized stomach glands were all visible. The stomach tissues and glands in the positive group were nicely organized in contrast to the model group, and mucosa damage and shedding were undetectable. The gastric tissues of the mice responded differently in each administration group. The morphology of the stomach tissue in the YSJ-L group was the same as that of the model group, despite the mucosa being more intact and less damaged. The gastric tissues were organized and little mucosal damage was shown in the YSJ-M and YSJ-H groups.
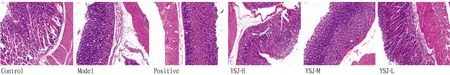
Note: Sections were observed under a light microscope (×200)
3.3 Effects of YSJ on inflammatory cytokines and related protein expression in ethanol-induced gastric ulcersThe levels of IL-1β, IL-6, TNF-α, and IL-8 in the gastric mucosa were increased, while IL-4 and PGE2were decreased in the model group than that in the control group (P<0.01). The levels of IL-1β, IL-6, TNF-α, and IL-8 were decreased in YSJ groups with a dose-dependent relationship. Nevertheless, IL-4 and PGE2presented the opposite trend. The levels of IL-1β, IL-6, TNF-α, and IL-8, IL-4 and PGE2in the serum have the same trend with those in the gastric mucosa (Fig.4).
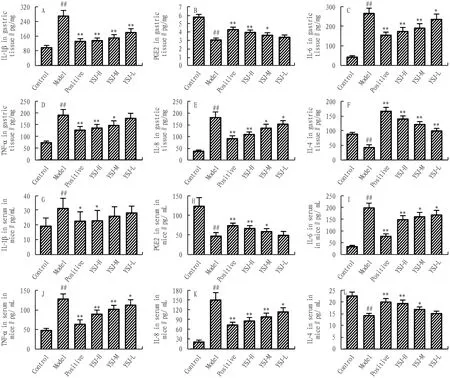
Note: Values are shown as the means±SEMs. Compared with control group, ##P<0.01, compared with model group, *P<0.05, **P<0.01 compared with model group.
More positive nuclear NF-κB p65, TNF-α, IL-6, and COX-2 staining was detected in gastric tissues of ethanol-treated mice than in those of control mice. Nevertheless, the levels of NF-κB p65, TNF-α, IL-6 and COX-2 positive cells were reduced by YSJ administration (Fig.5). In addition, the protein expressions of p-NF-κB/NF-κB, p-IκBα/IκBα, and TNF-α were markedly increased by administration of YSJ (Fig.6).
3.4 Effects of YSJ on inflammatory cytokines in ethanol-induced GES-1 cellsAs shown in Fig.7A, the results indicated that YSJ (15, 30, 60, 120 μg/mL) exhibited no significant toxicity in GES-1 cells. Additionally, pretreatment with YSJ (15, 30, 60 μg/mL) reduced cytotoxicity after exposure to ethanol (Fig.7B). Therefore, 60 μg/mL YSJ which has no obvious toxicity to cells and is highly effective, was selected for follow-up experiments. The levels of inflammatory cytokines IL-1β, IL-6, and TNF-α in model group were increased, whereas after YSJ treatment, the expressions of the three inflammatory cytokines were significantly suppressed (Fig.7C-E).
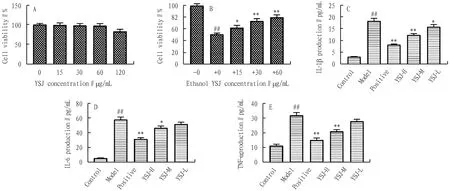
Note: (A). The cytotoxicity of YSJ to GES-1 cells for 24 h and then induced with ethanol for 12 h (B). The levels of IL-1β, TNF-α, and IL-6 in ethanol-induced GES-1 cells (C-E). Values shown are the means±SEMs of three independent tests. Compared with control group, #P<0.05, ##P<0.01, compared with model group, *P<0.05, **P<0.01.
4 Discussion
Gastric ulcer mainly refers to the tissue damage of the gastric mucosa beyond the muscularis mucosa caused by factors such as the digestion of gastric digestive juice itself[14]. The bad habits of modern people in terms of diet, drinking, smoking,etc.will cause physical or chemical damage to the gastric mucosa and its barrier and then destroy the mucosal barrier and cause gastric ulcers.A.zerumbet(Pers.) Burttet Smith is a national medicine commonly used by ethnic minorities in Guizhou Province and is often used in folk medicine to treat gastric ulcers. Therefore, in this study, the protective effect of YSJ on acute gastric ulcers was assessedinvivoandinvitro.
The results of this study showed that pretreatment with YSJ could effectively reduce the bleeding point, ulcer area and ulcer index in the gastric lining of mice. Mouse gastric mucosal hyperemia and submucosal edema alleviated the damage to gastric mucosal structures and the disappearance of glandular structures to varying degrees.
Ethanol-induced ulcers can also stimulate the immune system and cause an inflammatory response. PGE2, which is the primary COX-2 byproduct at inflammatory locations, can help the gastric mucosa defend itself by increasing blood flow, promoting the secretion of mucus and bicarbonate, and boosting the resistance of epithelial cells to stimulation[15-16]. COX-2 mediates the body’s inflammatory response and generally produces PGE2at low levels. Two NF-κB binding sites have been found in the COX-2 promoter region, which suggests that NF-κB, as COX-2 upstream signaling pathway, is crucial for the control and expression of COX-2 transcription[17]. TNF-α regulates IL-1, iNOS, IL-6, IL-2, IL-8, IL-12, and IL-1β as inflammatory cytokine that promotes the formation of neutrophils may cause the release of inflammatory mediators[18]. IL-4 can increase the activity of Th2 cells to inhibit the activity of Th1-cell subsets, and reduce the functional expression of macrophages. IL-4 can reflect the body’s anti-inflammatory level and immunosuppression[19]. Therefore, six indicators, including IL-1β, TNF-α, IL-4, PGE2, IL-6, and IL-8, were selected for detection in the experiment. The results showed that pretreatment with YSJ could reduce the levels of the proinflammatory factors IL-1β, TNF-α, IL-6, and IL-8, and increase the levels of PGE2and IL-4 in ulcer tissue and serum, which suggests that YSJ can promote ulcer healing by inhibiting the release of inflammatory factors, resist inflammatory damage, and protect gastric mucosa.
NF-κB is involved in the occurrence and development of gastric ulcers. According to studies, the phosphorylation, degradation, and activation of NF-κB are all directly impacted by the activity or inactivation of IKK[20]. IκB degradation and NF-κB activation can both be significantly increased by the activity of IKK. IKK-activated NF-κB may trigger a large number of cytokines and growth factors, and the rising levels and release of these inflammatory substances further activate NF-κB, exacerbating the inflammatory response[21-22]. The production and release of additional inflammatory cytokines (including IL-1β and TNF-α) are stimulated in the nucleus by the NF-κB protein, thereby promoting the development of ulcers[23-24]. In this study, YSJ treatment in IHC decreased the amounts of NF-κB p65, IL-6, COX-2, and TNF-α positive cells. Furthermore, the expression of p-NF-κB/NF-κB, p-IκBα/IκBα, and TNF-α was decreased after treatment with YSJ.
In conclusion, the YSJ has a protective effect on ethanol-induced acute gastric ulcersinvivoandvitro, and its mechanism may be the reduction of gastric mucosal injury by inhibiting the inflammatory response.
杂志排行
Medicinal Plant的其它文章
- Quality Control of Zhuang Medicine Xiaoyan Zhiyang Lotion
- Protective Effect and Mechanism of n-butanol Extract from Diploclisia glaucescens (B1.) Diels on Rats with Adjuvant Arthritis
- Effects of Early-stage Phased Rehabilitation Training on Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis
- Exploring the Mechanism of Blumea balsamifera (L.) DC in Preventing and Treating Alzheimer’s Disease Based on HPLC-ESI-HRMS and Network Pharmacology
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Research Progress and Ideas on the Anti-liver Fibrosis Effect of Ethnic Medicine Plumbagin Based on microRNAs/TLR4/NF-κB and NLRP3 Inflammasome Activation
