Exploring the Mechanism of Blumea balsamifera (L.) DC in Preventing and Treating Alzheimer’s Disease Based on HPLC-ESI-HRMS and Network Pharmacology
2023-10-31DongLILingLIYujieHUJiangXiongMAWenlongZHANGChanyuanZHOUDongshengFANXiaojianGONG
Dong LI, Ling LI, Yujie HU, JiangXiong MA, Wenlong ZHANG, Chanyuan ZHOU, Dongsheng FAN, Xiaojian GONG*
1. Key Laboratory for Information System of Mountainous Areas and Protection of Ecological Environment, Guizhou Normal University, Guiyang 550001, China; 2. Department of Pharmacy, the First Affiliated Hospital of Guizhou University of Traditional Chinese Medicine, Guiyang 550001, China; 3. College of Chemistry and Materials Engineering, Guiyang University, Guiyang 550005, China
Abstract [Objectives] To expose the plausible mechanism of Blumea balsamifera (L.) DC. against Alzheimer’s disease via network pharmacology and HPLC-ESI-HRMS technology. [Methods] To begin with, HPLC-ESI-HRMS was employed to identify the components of B. balsamifera. Secondly, the potential targets of the components were identified and predicted based on chemical similarity and online databases. Thirdly, by way of topological analysis of a component-disease target interaction network, the primary candidate targets and potential active components were identified. Lastly, molecular docking analysis was used to confirm the interaction between active components and therapeutic targets. [Results] According to the final results, HPLC-ESI-HRMS identified 70 components. Out of these, 20 components were potentially biologically active, and most of them were sesquiterpenoids. According to the molecular docking results, the primary active components were appropriately coordinated with the core targets, indicating a high level of pharmacodynamic activity. Thus, the sesquiterpenes present in B. balsamifera are considered potential active ingredients having multi-target and multi-pathway effects for treating Alzheimer’s disease. [Conclusions] This research will provide a scientific reference for the future pharmacological activity and clinical application of B. balsamifera.
Key words HPLC-ESI-HRMS, Alzheimer’s disease, Blumea balsamifera (L.) DC., Network pharmacology, Molecular docking
1 Introduction
Alzheimer’s disease (AD) is a prevalent neurodegenerative disorder, and it leads to irreversible neuron loss and cognitive impairment[1]. Its pathological features typically comprise of senile plaques that are extracellular aggregations of amyloid Aβ and neurofibrillary tangles that are intracellular aggregations of hyperphosphorylated Tau protein[2-3]. The prevalence of AD is rising due to rapid global aging, bringing significant economic and medical challenges to society. Research shows that 15.07 million individuals aged 60 years and over in China have dementia, with 9.83 million having AD. Additionally, there are around 6.5 million US citizens aged 65 years and over who suffer from AD. Without a breakthrough in the prevention, mitigation, or cure of AD, the number of patients is projected to increase to 13.8 million by 2060[1,4]. The cause of AD has not yet been fully comprehended. The AChE inhibitors Tacrine, Galanthamine and Memantine Hydrochloride, which have clinical applications and can alleviate patients’ cognitive dysfunction, but they are unable to halt the progression of the disease. New drugs such as Sodium Oligomannate capsules and Aducanumab have recently received approval for use. Nevertheless, the clinical efficacy of these drugs needs to be further examined[5]. Therefore, it is imperative to identify drug candidates capable of reversing or terminating the pathological process of AD.
Blumeabalsamifera(L.) DC (Asteraceae) is a plant with perennial herb or subshrub characteristics long employed for medicinal reasons in the ethnicminority regions of Miao, Zhuang, Li and others[6]. The essential oil ofB.balsamiferacontains (-)-borneol. Borneol is a significant active component present inB.balsamifera, which is frequently used in traditional Chinese medicine for its properties of ‘resuscitation’. Borneol facilitates drug transportation across the blood-brain barrier and helps to enhance drug absorption in the brain[7-8]. Research indicates that (-)-borneol at a concentration of 100 μM protects SH-SY5 cells from Aβ-induced damage, reduces the production of reactive oxygen species, enhances the expression of HO-1 and nuclear translocation of Nrf2, has antioxidant properties, and increases the expression of Bcl-2 significantly. The inhibition of Aβ-induced apoptosis in SH-SY5Y cells was observed through a decrease in Bax expression[9]. The sesquiterpenoids and diterpenoids that have been extracted fromB.balsamiferacan substantially reduce the production of NO in microglia BV-2 cells and suppress neuroinflammation[10-11].
Based on the previous literature review[12], we analysed and identified the chemical components ofB.balsamiferausing HPLC-ESI-HRMS in this study. The active compounds fromB.balsamiferawith therapeutic properties for AD were screened by network pharmacology, and their mechanism of action was investigated and validated by molecular docking techniques. This research will offer a scientific reference for the future pharmacological activity and clinical application ofB.balsamifera.
2 Material and methods
2.1 Materials
2.1.1Materials and instruments. Acetonitrile (Xilong Chemical Co., Ltd), formic acid (Tianjin Hengxing Chemical Reagent Manufacturing Co., Ltd), methanol (Chongqing Wansheng Chuandong Chemical Co., Ltd), and water (self-made ultra-pure water) were used in the experiments. All other reagents were of analytical grade.B.balsamiferawas collected from Ceheng County of Qianxinan Buyi and Miao Autonomous Prefecture, Guizhou Province. The medicinal material was identified as such by professor Zhang Wenlong at Guizhou University of Traditional Chinese Medicine.
Thermo Scientific Q exactive focus high resolution mass spectrometer, Dionex ultimate 3000 rslc liquid chromatograph, Electronic temperature control electric heating jacket 98-i-b (Tianjin Taist Instrument Co., Ltd.); Digital display thermostatic water bath hh-4 (Shanghai Lichen Bangxi Instrument Technology Co., Ltd.); Anheng electronic counter III ALM (Shenzhen Anheng Weighing Instrument Electronics Co., Ltd.); Pure water/ultrapure water system cleartm-d24uv (Merck Millipore); Ultrasonic cleaning machine kq-300de (Kunshan Ultrasonic Instrument Co., Ltd.); Electronic analytical balance xs10du [Mettler-Toledo Instrument (Shanghai) Co., Ltd.].
2.1.2Preparation of test solution. The collected medicinal materials were dried naturally and then mixed by a grinder. About 1 g of medicinal material powder was weighed accurately, placed in a 100 mL round bottom flask, and added with 100 mL of methanol accurately. After extracted by reflux for 2 h, the solution was allowed to cool at rest, and filtered into a 100 mL measuring flask. The filtrate was made up to the scale with methanol and shaken well. Finally, the filtrate was filtered through a 0.2 μm microporous membrane.
2.2 Methods
2.2.1Qualitative analysis by HPLC-ESI-HRMS. (i) Chromatographic conditions. Chromatographic column: Waters Acquity Uplc hss T3 (100 mm×2.1 mm, 1.8 μm); Gradient elution mobile phase: 0.1% formic acid aqueous solution (B), acetonitrile (0.1% formic acid)(A); Specific elution conditions: 95% B (0-2 min), 95%-5% B(2-42 min), 5% B (42-47 min), 5%-95% B (47-47.1 min), 95% B (47.1-50 min). The flow rate was 0.3 mL/min and the column temperature was set at 40 ℃. The injection volume was 5 μL.
(ii)Mass spectrometric conditions. The following MS setup was used: Electrospray ionisation (ESI) source in positive and negative ion mode; Full scan high-resolution accurate-mass acquisition mode over the mass range of 100-1 500m/z; Spectrum data type: Profile; Resolution: Full MS: 70 000, MS/MS: 17 500; Automatic gain control quantity: Full MS:1×106, MS/MS:2×105; Maximum IT: 100 ms (Full MS), 50 ms (MS/MS); Loop count: 3; MSX count: 1; Isolation width: 1.5 m/z; Step NCE: 20, 40, 60; Minimum AGC target: 8×103; Intensity threshold: 1.6×105; Spray voltage: positive ions 3.0 kV, negative ions 2.5 kV; Sheath gas flow rate: 35 psi; Auxgas flow rate:10 psi; Sweep gas flow rate: 0; Capillary temperature: 320 ℃.
2.2.2Data processing and component analysis. Based on the previous literature review, over 140 targets were collected and screened[12]. The HPLC-ESI-HRMS data underwent processing using Xcalibur 3.0. To allow comparison and analysis of the relative molecular weight, retention time (tR), fragment ion information, and fragmentation law of the target compound with the collected mass spectral data, Compound Discoverer 3.0 software was used. Abbreviations will be explained in the first instance of usage.
2.2.3Screening of active ingredient fromB.balsamiferaand target collection. The chemical constituents ofB.balsamifera, obtained by mass spectrometry and literature review, were screened for activity. Targets of the chemical constituents were obtained by entering the SMILES numbers of each constituent into the Swiss Target Prediction database, which predicts the targets of the constituents based on their 2D and 3D structures[13-14].
2.2.4AD target collection. AD targets were obtained from three databases: Swiss Target Prediction[15](http:∥ swisstargetprediction.ch/), OMIM (https:∥www.omim.org/), and GeneCards (https:∥www. genecards. org/). The targets were then canonically calibrated to Gene ID (primary) by UniProt (https:∥ www.uniprot.org/).
2.2.5Construction of "active ingredient target" network. The active ingredients and intersection targets ofB.balsamiferawere imported into Cytoscape 3.8.2 software, and the "active ingredient target" network was constructed according to the corresponding attribute relationship.
2.2.6Construction of protein-protein interaction network (PPI). The target information was obtained from STRING 11.5 (https:∥cn.string-db.org/) and imported into Cytoscape 3.8.2 software to construct the PPI network diagram.
2.2.7Go function and KEGG enrichment analysis. Metasacpe database (https://metascape.org/gp/index.html#/main/step1) was used for GO and KEGG enrichment analysis ofB.balsamifera-AD intersection targets. The collected data analysis results were visualized in the form of bubble maps.
2.2.8Screening of key targets and active ingredients. The Analyze Network tool in Cytoscape 3.8.2 software was used for topological analysis, and the Cytohubba plug-in was used for degree calculation. The top 5 target points in the PPI network were selected as key genes. Ten drugs highly correlated withB.balsamiferain the treatment of AD were selected as key drugs in the "B.balsamiferadrug - target" network by MCC algorithm.
2.2.9Molecular docking validation. The active ingredient’s 2D structure was saved as a mol2 structure format. Then, the compound’s 2D structure was converted to the 3D format and saved as a PDB format file using Open Babel 3.1.1 software. Subsequently, AutoDock Tools 4.2.6 was employed to convert the ligand molecule’s ‘pdbqt’ format file. The crystal structure of the primary target was downloaded from the PDB database. Pre-processing of the receptor protein involved deleting water molecules, side chains, and ligands using PyMOL software. Subsequently, the receptor protein was transformed into the pdbqt format using Autodock Tools 4.2.6. Molecular docking was executed using AutoDock Vina 1.1.2, whereas the docking results were visualized by using PyMOL.
3 Results and analysis
3.1 Compound composition analysisSeventy compounds were identified by mass spectrometry, which were verified by comparison between the database (ChemSpider, MassBank, mzCloud) and references[16-29](Table 1). Total ion chromatogram diagram of positive and negative ion modes ofB.balsamiferasample is shown in Fig.1.
Fig.1 Total ion chromatograms (TIC) of Blumea balsamifera(L.) DC in positive (A) and negative (B) modes
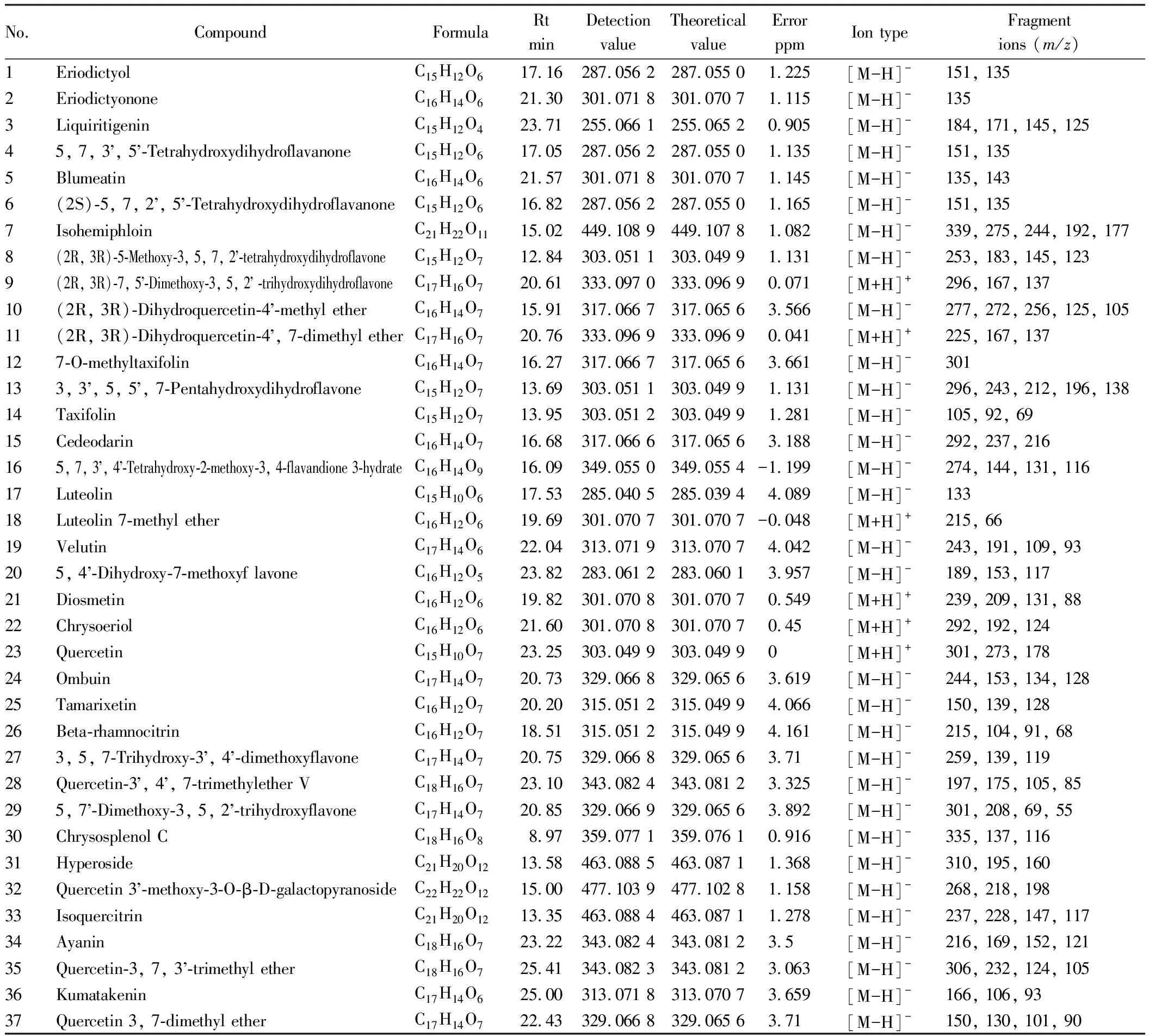
Table 1 Identification of chemical constituents in Blumea balsamifera (L.) DC. by HPLC-ESI-HRMS
(To be continued)
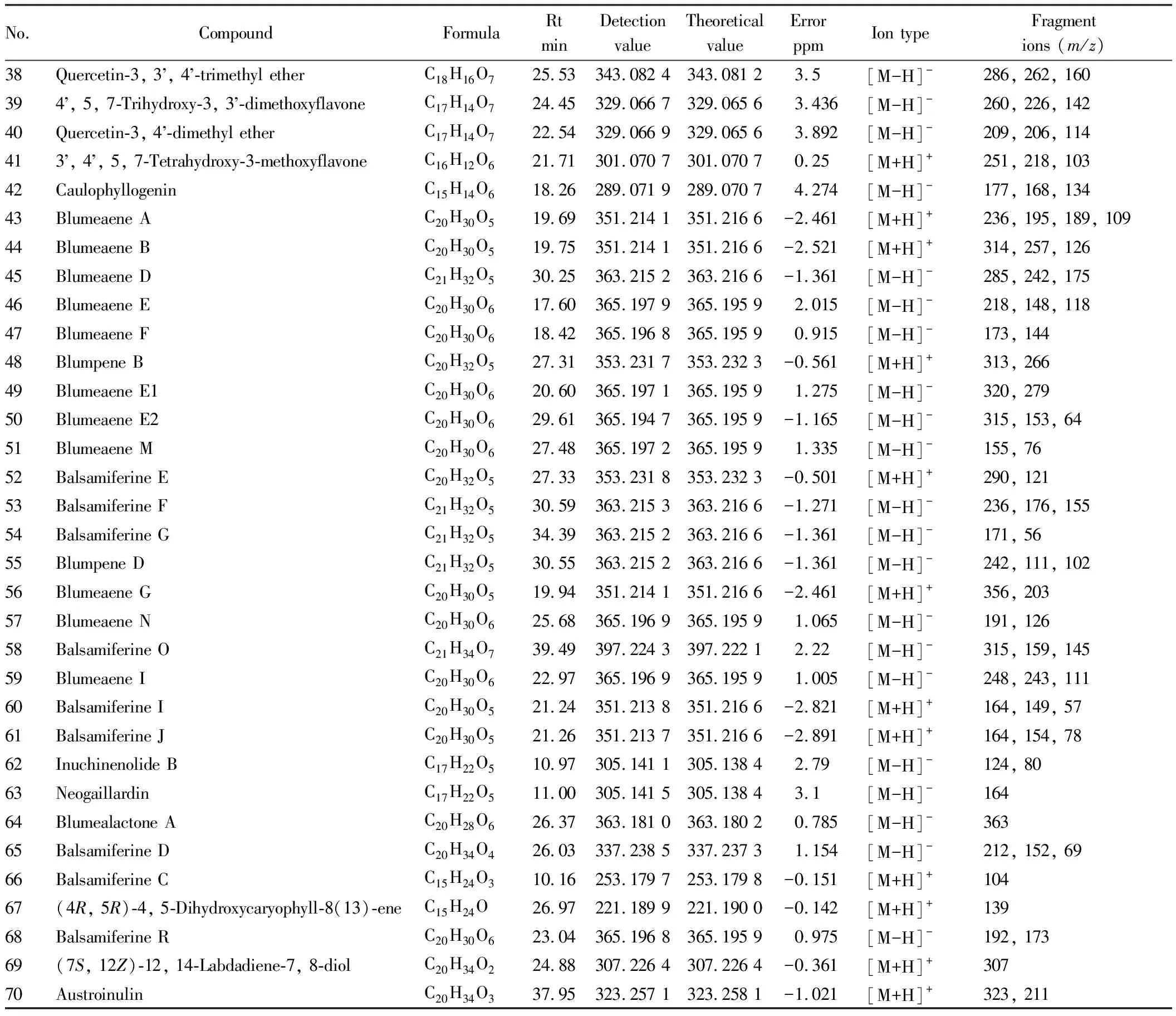
(Continued)
3.2 Screening of active ingredients ofB.balsamiferaTwenty active compounds were screened using the SwissADME online database under reference conditions of gastrointestinal absorption, druglikeness, and blood-brain barrier permeability (Table 2). These compounds, which include one flavonoid, 17 sesquiterpenoids, and 2 diterpenes, are presented in Fig.2.
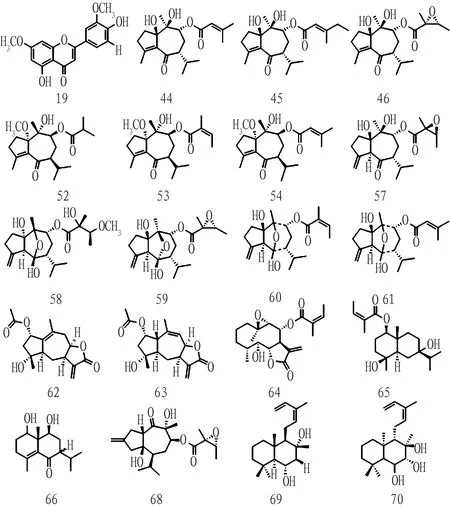
Fig.2 Structure of active ingredients
3.3 Collection of active ingredients and AD targets ofB.balsamiferaThe possible targets of the active ingredients were acquired, resulting in 239 targets. A total of 1 966 targets were related to AD. The Venny 2.1.0 Online Tool was used to find the intersection of the active ingredient targets and AD targets, leading to a total of 93 intersected targets (Fig.3).
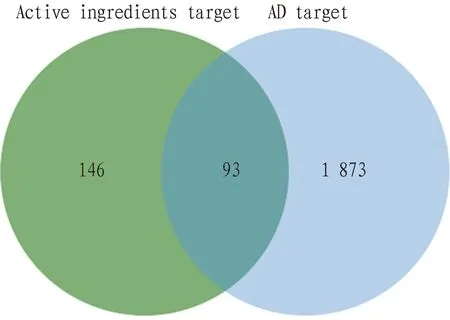
Fig.3 Venn diagram of active ingredients target and AD target
Table 2 Active ingredients
3.4 Construction of "active ingredient-target" network and screening of main active ingredientsTo create an "active ingredient intersection target" network diagram, which includes 268 interacting nodes and 555 edges (Fig.4), the active ingredients and intersection targets ofB.balsamiferawere imported into the Cytoscape 3.8.2 software. The network underwent topology analysis using the Analyze Network tool. By using the Cytohubba plug-in for MCC algorithm, 10 primary active ingredients, including 8 sesquiterpenes and 2 diterpenoids, were identified.
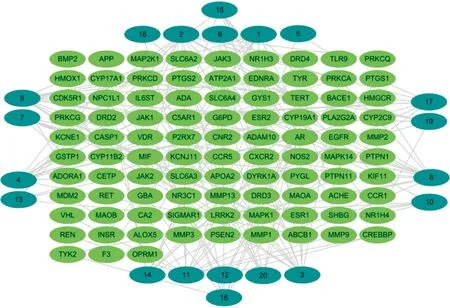
Fig.4 Network diagram of "active ingredient-target"
3.5 Construction of PPI and screening of key targetsNinety-three intersection targets that had been screened were entered into the String 11.5 database to create the network diagram. Then, the TSV file exported was imported into Cytoscape 3.8.2 software to build the PPI network diagram (Fig.5). Out of these, Cytohubba plug-in calculated and screened 5 significant gene targets with the following degrees: EGFR (degree=86), PTGS2 (degree=74), ESR1 (degree=68), MMP9 (degree=62), and MAPK1 (degree=58). All of these values significantly exceeded the median degree of 20. These significant gene targets exhibited strong interactions not only with other targets but also with 20 active ingredients, thus highlighting their importance and potential clinical significance.
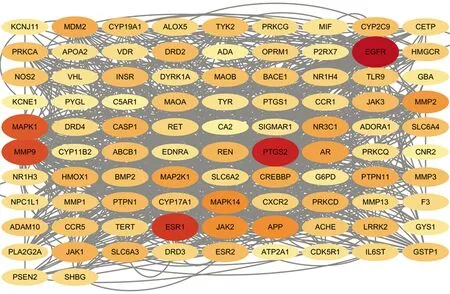
Fig.5 PPI network diagram
3.6 Go function and KEGG enrichment analysisThe Meta-scape database was used to perform GO enrichment analysis (P<0.05), resulting in a total of 466 entries for biological processes. The top 10 analysis results withPvalues were displayed (Fig.6). Of these, 244 biological processes were enriched (accounting for 52.36% of the total items), mainly related to the response of cells to nitrogen compounds, hormones, inflammation, inorganic substances, as well as the regulation of MAPK cascade, defense response, system process, and other biological processes. A total of 126 molecular functions were enriched, representing 27.04% of the total items. These functions were mainly related to the activity of protein serine/threonine/tyrosine kinase, non-membrane spanning protein tyrosine kinase, endopeptidase, and MAP kinase, as well as the regulation of steroid binding and phosphatase binding. A total of 96 cellular components were enriched, accounting for 20.60% of the total items. Most targets were distributed in membrane rafts, neuronal cell bodies, perinuclear cytoplasm, membrane side, neuromuscular junctions, and other regions. This shows that the active components ofB.balsamiferatreat AD through multiple GO functions.
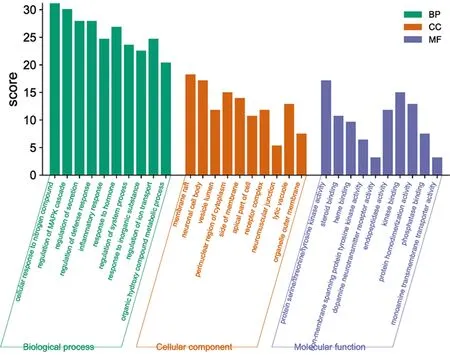
Fig.6 GO function enrichment analysis
The KEGG pathway enrichment analysis (P<0.05) revealed that 158 pathways were enriched. The top 20 analysis results withPvalues were chosen for display in the bubble diagram (Fig.7). The involved pathways consist of the cancer pathway, 5-serotonergic synaptic pathway, HIF-1 signalling pathway, hepatitis B pathway, chemokine signalling pathway, cMAP signalling pathway, dopaminergic synaptic pathway,etc.Multiple diseases involving neurodegenerative pathways and receptor interactions of neuroactive ligands suggest that the active ingredient ofB.balsamiferais a treatment involving multiple pathways for AD.
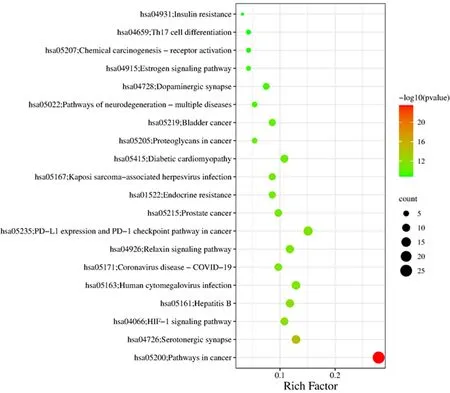
Fig.7 KEGG pathway enrichment analysis
3.7 Molecular docking verification resultsThe 10 primary active ingredients (Table 3) chosen based on Section3.4were subjected to docking with their respective key targets (Table 4). The prominent active ingredient was defined as a small molecule ligand, and the key target was identified as a receptor protein. Docking verification was done using AutoDock Vina 1.1.2. The docking results are shown in Table 5.
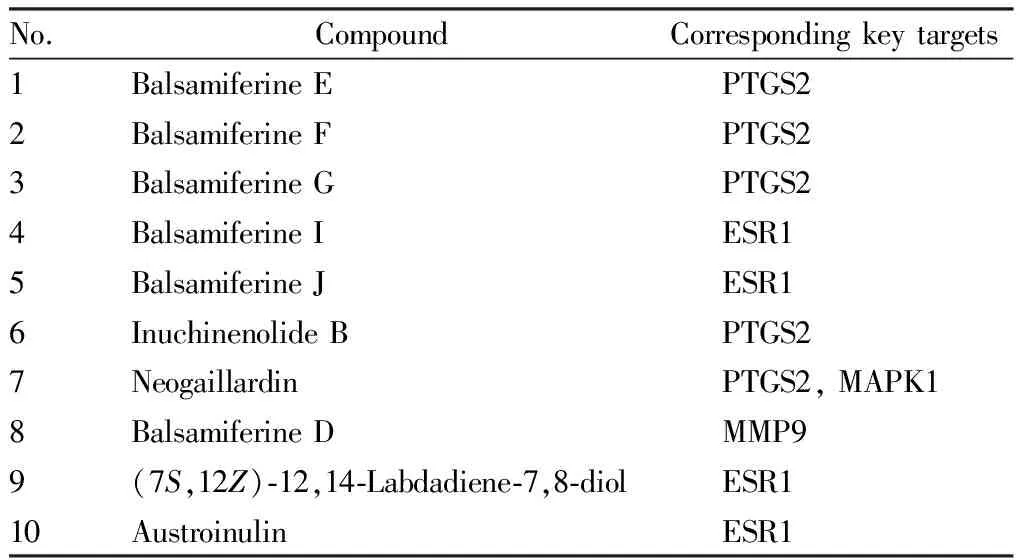
Table 3 Main active ingredients
The results showed that the binding energies of the main active ingredients to the key targets ranged from -6.3 to -8.8 kcal/mol. In order to compare the accuracy of structure prediction, we used the distance metric RMSD between the experimental and predicted structures. The RMSD value is based on the symmetry, partial symmetry and approximate symmetry in a simple heuristic way. In short, the RMSD value is the structural difference between two molecules (or between two states of the same molecule). The smaller the value, the greater the docking reliability. When it is less than 2 Å, the docking accuracy is higher[32-35]. In order to determine the binding activity and binding sites of components and target proteins, six docking results with the lowest docking energy were selected, and the RMSD values and hydrogen bonds within the docking range of the six results were calculated by PyMOL software (Table 6) and visualized (Fig.8).
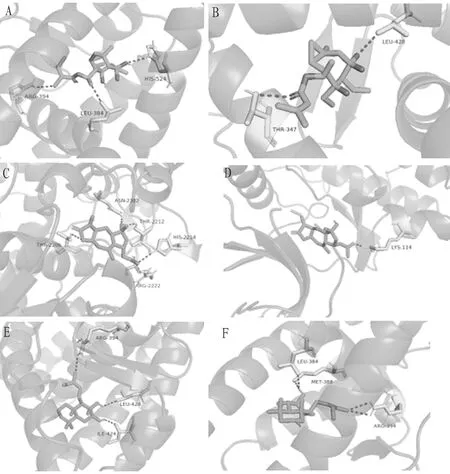
Fig.8 Docking diagram
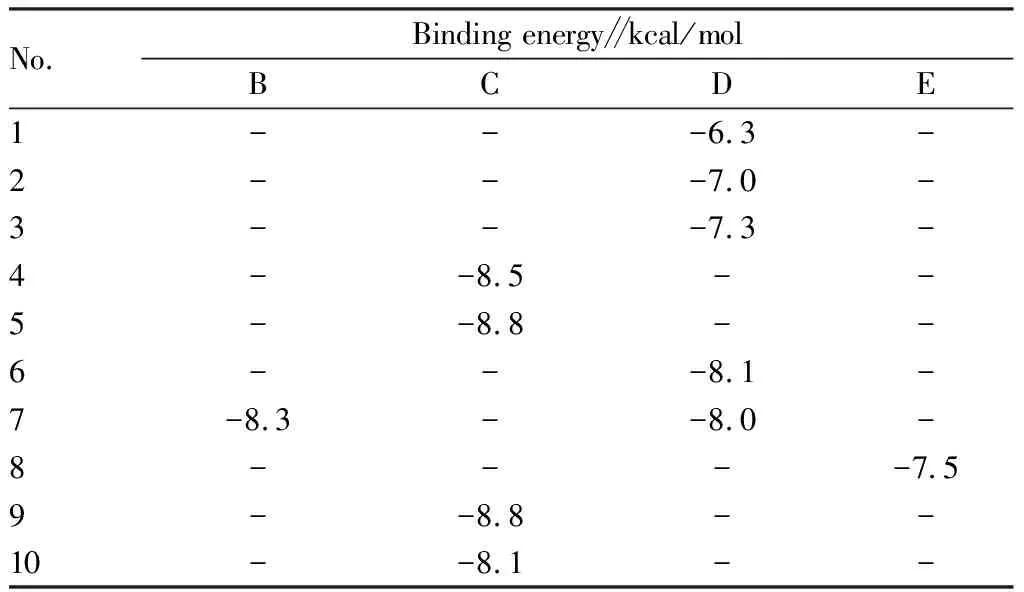
Table 5 Docking results
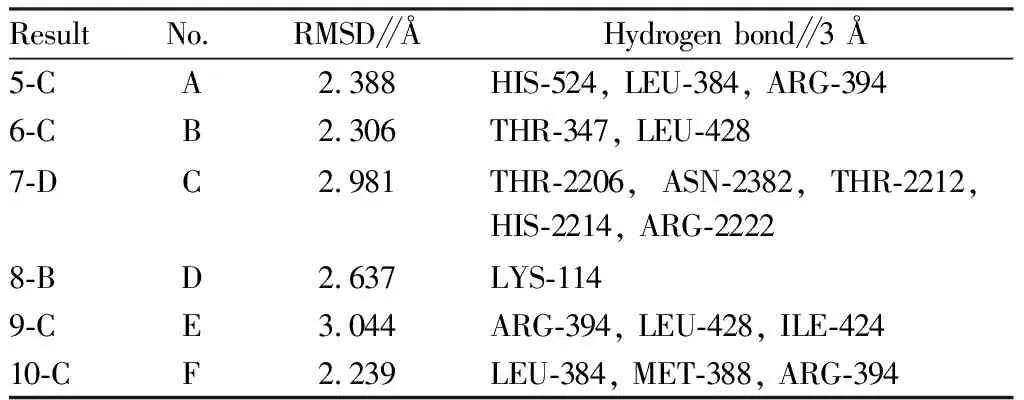
Table 6 RMSD and hydrogen bond
4 Discussion
Recently, the rapid advancement of computer simulation technologies, including network pharmacology, molecular docking, and pharmacophore modelling, has introduced novel approaches to unveil the material foundation and operation mechanism of traditional Chinese medicine and compounding. It has also brought new techniques for compound activity screening whilst considerably reducing the time and cost associated with discovering active compounds[36]. This study screened 29 active components, mostly terpenoids and mainly sesquiterpenes, fromB.balsamiferausing HPLC-ESI-HRMS combined with network pharmacology. Previous studies have reported that sesquiterpenes, which were isolated fromB.balsamifera, can significantly inhibit the production of NO by BV-2 microglial cells induced by lipopolysaccharide. This inhibition can lead to the suppression of neuroinflammation[10-11]. The above findings suggest that sesquiterpenes may be the primary active component with anti-AD properties inB.balsamifera.
AD is a degenerative neurological condition with a complex origin. In recent decades, while significant progress has been made in the molecular and cellular studies concerning AD pathology, scientists are still endeavouring to develop innovative strategies for the treatment of the condition[37].
Research has shown the importance of estrogen in maintaining normal brain function. Moreover, estrogen receptors α and β (ESR1 and ESR2) have a strong correlation with the development of AD, and estrogen slows down the progression of AD[38]. ESR1 might protect memory from impairment by stimulating Aβ degradation and down-regulating amyloidosis as well as neurogenic inflammation in knockout ESR1 mouse model experiments[39]. The levels of ERRα and mRNA regions reduce with age in the APP/PS1 mouse model. Overexpression of ERR1 in HEK293 cells inhibits amyloidosis process in APP, reducing Aβ1-40/1-42 levels. Moreover, ERR1 overexpression in HEK/APP cells reverses APP and Tau phosphorylation alterations caused by hydrogen peroxide[40-41]. Therefore, ERR1 shows potential as a therapeutic target for AD.
The MAPK pathway plays a crucial role in transmitting signals from the outer membrane of a cell to the interior of the nucleus, ultimately regulating numerous cellular activities like proliferation, differentiation, apoptosis, survival, inflammation, and innate immunity[42]. In the adult brain, ERK is abundant and plays a significant role in regulating neuronal function. Additionally, the ERK pathway can serve multiple roles in activity-dependent regulation of neuronal function[43]. In a mouse model of LPS-induced memory damage, trans-cinnamaldehyde accelerates the destabilization of the mRNA for inducible nitric oxide synthase (iNOS) by disrupting the mitogen-activated protein kinase ERK1/2 pathway. This observation significantly reduces the production of nitric oxide (NO) in microglia and decreases inflammatory damage[44]. Additionally, the modulation of the RAS/MEK/ERK signalling pathway, by modulating the expression of MEK, p-MEK, ERK, and p-ERK, considerably improves cognitive performance and reduced pathological damage in AD mice[45].
PTGS2 is the gene that encodes for cyclooxygenase-2 (COX-2), an enzyme responsible for the conversion of arachidonic acid to prostaglandins (PG). It plays a crucial role in several inflammatory processes as well as normal functions in neurons. In the hippocampal and cortical neurons, COX-2 expression regulates neuroplasticity through PG production[46]. Research has indicated that pharmacological inhibition of COX-1/2 can aim at pathophysiological mechanisms that oppose AD’s progression by regulating processes upstream and downstream of Aβ and Tau[47]. Dysregulation of COX-2 causes abnormal cleavage of the β-amyloid precursor protein, and leads to the aggregation and deposition of Aβ as well as phosphorylated Tau tangles[48-50].
In the central nervous system, MMPs can be produced by all brain cells and are associated with neurogenesis, neuronal plasticity, and other physiological activities of the nervous system. Studies have shown that increased levels of MMP expression in AD may contribute to or interfere with the pathophysiological mechanisms of the disease[51]. Of these, MMP-9 is expressed in responsive astrocytes that encircle amyloid plaques. Its capability to break down soluble Aβ and amyloid plaques into non-toxic fragments supports neuron protection[52]. The cognitive impairment caused by Aβinvivoand neurotoxicityinvitrocan be significantly alleviated in MMP-9 pure knockout (KO) mice and by administering MMP inhibitors[53]. Moreover, inhibiting MMP9 can improve certain neurobehavioral deficits associated with AD, like anxiety and social recognition memory[53].
Molecular docking techniques were utilized to investigate the binding ability of the 10 primary active ingredients to their key targets. According to the results, the main active ingredients created a robust affinity with the ao acid residues of the key targets by hydrogen bonding, indicating a high level of potent activity. Nonetheless, the molecular docking technique possesses a few limitations because it does not reflect directly whether the binding action of the component to the target is inhibitory or activating[54].
In conclusion, this research has made preliminary conclusions regarding the active components and mode of action ofB.balsamiferain the treatment of AD through HPLC-ESI-HRMS and network pharmacology. This provides a theoretical foundation for studying the pharmacological mechanisms ofB.balsamifera. Nevertheless, the findings of this study are derived from the network pharmacology approach and are presently limited to theoretical analyses. To achieve a complete comprehension of its mechanism of action and to advance the research and development ofB.balsamiferaas an ethnomedicine, further substantiation via bothinvitroandinvivoinvestigations is necessary. This could further provide valuable insights and scientific references.
杂志排行
Medicinal Plant的其它文章
- Gastroprotective Effect of Alpinia zerumbet (Pers.) Burttet Smith on Ethanol-induced Gastric Ulcers in vivo and vitro
- Protective Effect and Mechanism of n-butanol Extract from Diploclisia glaucescens (B1.) Diels on Rats with Adjuvant Arthritis
- Effects of Early-stage Phased Rehabilitation Training on Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis
- Quality Control of Zhuang Medicine Xiaoyan Zhiyang Lotion
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Research Progress and Ideas on the Anti-liver Fibrosis Effect of Ethnic Medicine Plumbagin Based on microRNAs/TLR4/NF-κB and NLRP3 Inflammasome Activation
