Quality Control of Zhuang Medicine Xiaoyan Zhiyang Lotion
2023-10-31XianPENGZhenyingFUJiangcunWEIYinghongHUANGXianyiSHIWeiTIANWenZHONGGuodongHUANG
Xian PENG, Zhenying FU, Jiangcun WEI, Yinghong HUANG, Xianyi SHI, Wei TIAN, Wen ZHONG, Guodong HUANG
Guangxi International Zhuang Medicine Hospital, Nanning 530201, China
Abstract [Objectives] To establish the TLC identification methods of Llicis Rotundae Cortex and Mahoniae Caulis and the determination method of syringin content in Zhuang Medicine Xiaoyan Zhiyang Lotion. [Methods] Thin layer chromatography (TLC) was used to qualitatively identify Llicis Rotundae Cortex and Mahoniae Caulis in Zhuang Medicine Xiaoyan Zhiyang Lotion. The quantitative analysis of syringin in Zhuang Medicine Xiaoyan Zhiyang Lotion was carried out by high-performance liquid chromatography (HPLC). Chromatographic conditions are as follows: Luna 5 μm C18 (2) 100 A (250 mm×4.6 mm, 5 μm) column was adopted; acetonitrile (10%) -water (90%) was as the mobile phase, and the flow rate was 1.0 mL/min; the detection wavelength was 265 nm; the sample size was 5 μL; the column temperature was 30 ℃. [Results] Syringin had a good linear relationship with its peak area in the range of 0.190-2.856 μg (R2=0.999 5). The RSD values of precision, stability and repeatability experiments were all lower than 2.5%, and the average sampling recovery rate (n=6) was 100.37% (RSD=0.88%). [Conclusions] The method had the advantages of simple operation, strong specificity, good repeatability and stability, and can be used for the quality control of Zhuang Medicine Xiaoyan Zhiyang Lotion.
Key words Zhuang Medicine Xiaoyan Zhiyang Lotion, Thin layer chromatography (TLC), High-performance liquid chromatography (HPLC), Quality control
1 Introduction
At present, Western medicine is mostly used to treat skin diseases according to symptoms, the first-line drug glucocorticoid produces an instant effect, but many patients have pigmentation and other adverse reactions, and the disease is easy to repeat or aggravate, limiting clinical use. Because Guangxi is located in the subtropical zone, and has a hot and humid climate, wet toxicity, wind toxicity, insect toxicity and heat toxicity are easy to invade human body and lead to the frequent occurrence of skin diseases[1]. Zhuang medicine in the treatment of skin has the characteristics of attaching importance to external treatment, focusing on detoxification, early treatment and prevention of diseases, and so on[2]. In addition, Guangxi has rich Zhuang and Yao medicine resources, and it is rich in cold medicines. They have outstanding effects of clearing heat, detoxifying, removing dampness by diuresis and unique curative effects in the treatment of skin diseases, so they are favored by the majority of patients[3-5]. The prescription of Zhuang Medicine Xiaoyan Zhiyang Lotion is a Zhuang medicine test prescription obtained by Professor Li Fangmei of Dermatology Department of Guangxi International Zhuang Hospital based on years of clinical experience. This prescription has been used in Guangxi International Zhuang Medicine Hospital and Mingxiu Branch for many years in the form of agreement prescription. It is mainly composed of Scutellariae Radix, Sanguisorbae Radix, Portulacae Herba, Mahoniae Caulis, Llicis Rotundae Cortex, Chinese Knotweed Herb,etc.In the prescription, Llicis Rotundae Cortex, which has the effects of clearing heat, removing toxicity, and clearing damp, is the principle drug; Mahoniae Caulis, Chinese Knotweed Herb, and Portulacae Herba, which have the effects of clearing heat, detoxifying, and removing dampness, are as ministerial drugs; Scutellariae Radix and Sanguisorbae Radix, which have the effects of clearing heat, eliminating dampness, and regenerating tissue to heal wond, are as adjuvant drugs. The combination of the above drugs has the effects of clearing heat, removing dampness, cooling blood, detoxification, and regenerating tissue to heal wond, and has obvious effect and good safety for treating acute dermatitis eczema, contact dermatitis, hidden-wing dermatitis, burn and scald and other skin diseases. In order to more effectively control the quality of the preparation and ensure the efficacy and safety, the TLC identification method of Mahoniae Caulis and Llicis Rotundae Cortex and the content determination method of syringin in Zhuang Medicine Xiaoyan Zhiyang Lotion were proposed to provide theoretical and technical support for the popularization and use of Zhuang pharmaceutical preparations.
2 Experimental materials
2.1 InstrumentsMain instruments included Agilent 1260 Infinity II HPLC instrument; Luna 5 μm C18(2) 100 Å chromatographic column (Agilent Corporation, USA, specification 250 mm×4.6 mm, 5 μm), ML204 Mettler analytical balance, Xiangyi H1650 desktop high speed centrifuge, KQ-500DE ultrasonic cleaner, YOKO-ZS ultraviolet analysis camera (Wuhan Pharmaceutical New Technology Development Co., Ltd.), HWS-26 electric thermostatic water bath, and Milli-Q ultrapure water integrated system.
2.2 Reagents and reference substancesLlicis Rotundae Cortex control medicine (batch No.:121076-201303), Mahoniae Caulis control medicine (batch No.:121461-201503), syringin control medicine (batch No.:111574-201605; purity: 95.2%; 20 mg each) were provided by National Institute of Food and Drug Control. Reagents mainly included acetonitrile (chromatographically pure, Thermo Fisher Science and Technology Co., Ltd.), methanol (AR, Merck Joint Stock Company), phosphoric acid (chromatography pure, Shanghai Zhanyun Chemical Co., Ltd.), ether (AR, Chengdu Kelong Chemical Reagent Co., Ltd.), NaHCO3(AR, Guangdong Guanghua Reagent Co., Ltd.), Na2CO3(AR, Guangdong Guanghua Reagent Co., Ltd.), hydrochloric acid (AR, Guangdong Guanghua Reagent Co., Ltd.), ethyl acetate (AR, Chengdu Kelong Chemical Reagent Co., Ltd.), petroleum ether (AR, Chengdu Kelong Chemical Reagent Co., Ltd.), formic acid (AR, Tianjin Fuyu Fine Chemical Co., Ltd.), methanol (AR, Chengdu Kelong Chemical Reagent Co., Ltd.), aluminum trichloride (AR, Tianjin Damao Chemical Reagent Co., Ltd.), sulfuric acid (AR, Guangdong Guanghua Reagent Co., Ltd.), and vanillin (AR, Guangdong Guanghua Reagent Co., Ltd.).
2.3 Medicinal materials and samplesThe medicinal materials used were purchased from Guangxi Xianzhu Chinese Medicine Technology Co., Ltd., and they were qualified products detected according to the requirements ofChinesePharmacopoeia. Zhuang Medicine Xiaoyan Zhiyang Lotion was prepared by Zhuang and Yao Pharmaceutical Preparation Center of Guangxi International Zhuang Medicine Hospital, with the batch number of 210601, 210602, and 210603.
3 Methods and results
3.1 TLC identification of Llicis Rotundae Cortex
3.1.1Preparation of reference solution. Firstly, 0.5 g of the control herbal powder of Llicis Rotundae Cortex was taken, to which 30 mL of methanol was added to. Afterwards, it was treated with ultrasound for 30 min and dried by distillation. 30 mL of water-saturated n-butanol was added to the residue to dissolve it, and it was washed with 20 mL of ammonia test solution twice. Finally, the washed n-butanol solution was dried by distillation, and 2 mL of methanol was added to the residue to dissolve it, making a control medicinal material solution.
3.1.2Preparation of test product and negative solution. At first, 20 mL of Zhuang Medicine Xiaoyan Zhiyang Lotion was taken, and 30 mL of water-saturated n-butanol was added to it. After the mixture was shaken fully, the n-butanol layer was taken, and washed with 20 mL of ammonia test solution twice. The washed n-butanol solution was dried by distillation, and 2 mL of methanol was added to the residue to dissolve it. The supernatant was taken as the test solution. The negative control solution without Llicis Rotundae Cortex was prepared by the same method.
3.1.3Thin layer chromatography. According to the thin layer chromatography in theChinesePharmacopoeia(2020 edition, No.40502), 1 μL of test product solution, 1 μL of negative test product solution, and 1 μL of control medicinal material solution were put on the same silica gel G thin layer plate, and the chloroform-methanol-formic acid (16:3:1) was used as the developing agent to develop and dry them, and then it was sprayed with 10% sulfuric acid ethanol solution. It was heated at 105 ℃ for 3-5 min, and then viewed under a fluorescent lamp. The results are shown in Fig.1.
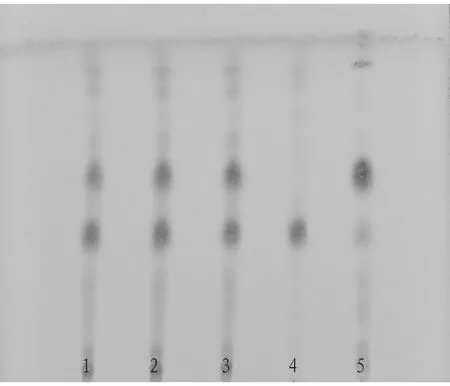
Note: 1-3. Test product solution (batch number: 210601, 210602, and 210603); 4. Negative solution 5. Control medicinal material.
3.2 TLC identification of Mahoniae Caulis
3.2.1Preparation of reference solution. At first, 0.1 g of the control herbal powder of Mahoniae Caulis was taken, to which 5 mL of methanol was added. Afterwards, it was treated with ultrasound for 30 min, and then the supernatant was taken as the control medicinal material solution.
3.2.2Preparation of test product and negative solution. Firstly, 20 mL of Zhuang Medicine Xiaoyan Zhiyang Lotion was taken, and 30 mL of water-saturated n-butanol was added to it. After the mixture was shaken fully, the n-butanol solution was taken, and washed with 20 mL of ammonia test solution twice. The washed n-butanol solution was dried by distillation, and 2 mL of methanol was added to the residue to dissolve it. The supernatant was taken as the test solution. The negative control solution without Mahoniae Caulis was prepared by the same method.
3.2.3Thin layer chromatography. According to the thin layer chromatography in theChinesePharmacopoeia(2020 edition, No.40502), 2 μL of test product solution, 2 μL of negative test product solution, and 2 μL of control medicinal material solution were put on the same silica gel G thin layer plate to form a strip, and the upper solution of n-butanol-glacial acetic acid-water (8:1:4) was used as the developing agent to develop and dry them, and then it was viewed under an ultraviolet lamp. The results are shown in Fig.2.
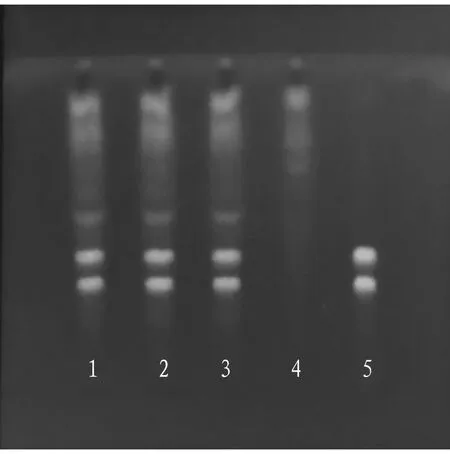
Note: 1-3. Test product solution (batch number: 210601, 210602, and 210603); 4. Negative solution 5. Control medicinal material.
3.4 Determination of syringin content
3.4.1Chromatographic conditions. Chromatographic column: Luna 5 μm C18(2) 100 Å(4.6 mm×250 mm, 5 μm); mobile phase: mobile phase A (acetonitrile) 10%, mobile phase B (water) 90%; the flow rate was 1.0 mL/min; the detection wavelength was 265 nm; the sample size was 5 μL; the column temperature was 30 ℃.
3.4.2Preparation of reference product solution. Firstly, 10 mg of of syringin reference product (batch No.:111574-201605; purity: 95.2%; being purchased from National Institute for Food and Drug Control) was weighed accurately, and put in a 100 mL volumetric bottle, to which 50% methanol was added until 100 mL to prepare the reference solution with a concentration of 0.095 2 mg/mL.
3.4.3Preparation of the test product solution. At first, 1 mL of Zhuang Medicine Xiaoyan Zhiyang Lotion was taken with a pipette, and a certain amount of water was added to a 10 mL volumetric flask until 10 mL. After the volumetric flask was shaken well, it was centrifuged for 10 min at a speed of 10 000 r/min, and the supernatant was taken through 0.22 μm microporous filter membrane to obtain the test product solution. The negative control solution without Llicis Rotundae Cortex was prepared by the same method.
3.4.4Specific experiment. 5 μL of each of the above control solution, test solution and negative control solution was injected into the HPLC chromatograph for determination. The retention time of chromatographic peak of syringin in the tested product was consistent with that of the control product, and the negative control had no interference. The results are shown in Fig.3.
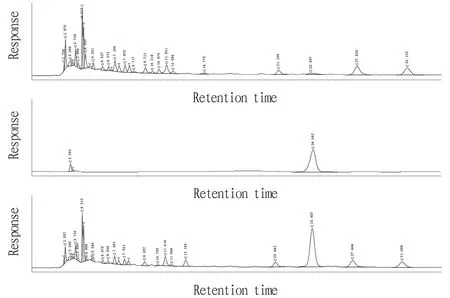
Fig.3 HPLC chromatograms of negative control product (A), syringin control product (B) and test product (C)
3.4.5Investigation of linear relationship. The control solution with syringin content of 0.095 2 mg/mL prepared in Section3.4.2was passed through 0.22 μm microporous filter membrane, and injected into Agilent high performance liquid chromatography for detection. The injection volume was 2, 5, 10, 15, 20, 25 and 30 μL, respectively, and the peak area of syringin was recorded. With the peak area of syringin (X) as the horizontal coordinate and syringin concentration (Y, μg) as the vertical coordinate, linear regression was performed by the least square method, and the regression equation of syringin was obtained as follows:y=0.000 4x-0.033 9,R2=0.999 5. The results showed that the linearity of syringin was good from 0.190 to 2.856 μg.
3.4.6Precision experiment. The control solution with syringin content of 0.095 2 mg/mL prepared in Section3.4.2was injected six times according to the method in Section3.4.1. The peak area of syringin was measured and recorded. TheRSDof the peak area of syringin was 2.57%, indicating that the precision of the instrument was good.
3.4.7Repeatability experiment. Six samples of Zhuang Medicine Xiaoyan Zhiyang Lotion of the same batch (batch No.:210602) were prepared according to the method in Section3.4.3, and were detected according to the method in Section3.4.1. TheRSDof the peak area of syringin was 2.11%, showing that the method had good repeatability.
3.4.8Stability experiment. The test solution was prepared according to the method in Section3.4.3, and the peak area of syringin was determined when it was left at room temperature for 0, 3, 6, 12 and 24 h, respectively. TheRSDof the peak area of syringin was 1.53%, indicating that the solution had good stability within 24 h.
3.4.9Sampling recovery experiment. Firstly, six samples of Zhuang Medicine Xiaoyan Zhiyang Lotion with known content were taken, and each was 0.5 mL in volume. Each of them was placed in a 10 mL volumetric flask. Afterwards, an appropriate amount of syringin reference substance was weighed to prepare 0.952 mg/mL reference solution, and 1 mL of the solution was accurately taken and added into each sample. A certain amount of water was added to the 10 mL volumetric flask until 10 mL. After the volumetric flask was shaken well, it was centrifuged for 10 min at a speed of 10 000 r/min, and the supernatant was taken through 0.22 μm microporous filter membrane. The peak area of syringin was measured and recorded. The average recovery rate was 100.37%, andRSDwas 0.88%. The experimental results are shown in Table 1.
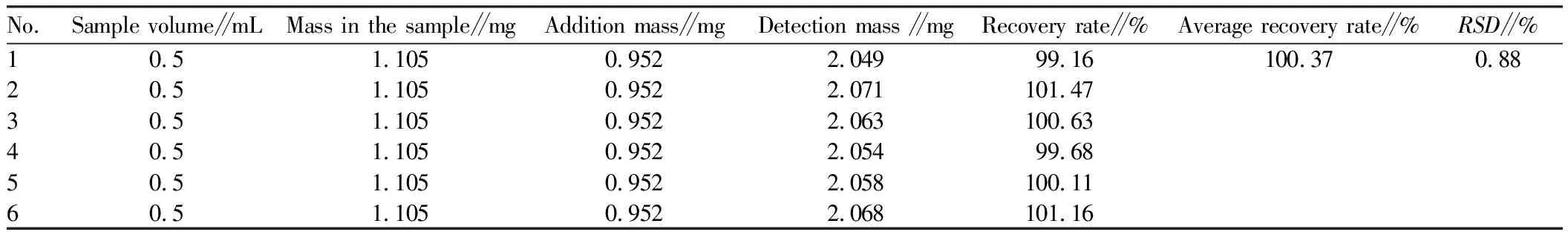
Table 1 Sampling recovery rate of syringin (n=6)
3.4.10Determination of syringin content in samples. At first, 1 mL of 3 batches of Zhuang Medicine Xiaoyan Zhiyang Lotion (batch No.: 210601, 210602, and 210603) was taken to prepare samples according to the preparation method of test product in Section3.4.3, and they were detected according to the chromatographic conditions in Section3.4.1. The content of syringin in Zhuang Medicine Xiaoyan Zhiyang Lotion samples was calculated, namely 0.225, 0.221 and 0.222 mg/mL, respectively.
4 Discussion and conclusions
4.1 Index component and selection of mobile phaseLlicis Rotundae Cortex, which is the principle drug of Zhuang Medicine Xiaoyan Zhiyang Lotion, contains saponins, terpenoids, organic acids and other chemical components, and its characteristic components are syringin and ilexin. Due to the small amount of ilexin content and the low detection limit of other components such as ilexsaponin A and Ilexsaponin B2, syringin was as the quality control index in this study[7-10]. The content of syringin is different in different harvesting periods. In addition, the large composition of the formula may affect the separation degree of the index component[11-14], so four different mobile phase systems of acetonitrile-water, acetonitrile-0.1% phosphoric acid aqueous solution, methanol-water, and methanol-0.1% phosphoric acid aqueous solution were investigated respectively for elution. The results showed that when acetonitrile-aqueous solution was used as the mobile phase, the separation effect of chromatographic peak was optimal, and the peak shape was better, so it was chosen as the mobile phase.
4.2 Selection of detection wavelengthIn this study, DAD detector was used to perform full-wavelength scanning of syringin at 190-800 nm. The results showed that the baseline was stable at 265 nm, and the chromatographic peak of syringin was good in shape. Moreover, there was no interference from impurities. Therefore, 265 nm was selected as the detection wavelength.
4.3 Selection of flow rate of mobile phase and column temperatureHere, the effects of three levels of flow rate (0.8, 1.0 and 1.2 mL/min) and column temperature (25, 30 and 35 ℃) on the chromatographic behavior of syringin were studied. The results revealed that when the flow rate was 1.0 mL/min and column temperature was 30 ℃, the baseline was stable, the separation and shape of the chromatographic peak were the best. Therefore, 1.0 mL/min was selected as the flow rate, and 30 ℃ was as the column temperature of the method.
In summary, the TLC identification methods of Llicis Rotundae Cortex and Mahoniae Caulis and the determination method of syringin content in Zhuang Medicine anti-inflammation and anti-itch lotion was established for the first time. The results showed that the thin layer chromatographic separation effect of Llicis Rotundae Cortex and Mahoniae Caulis was good, and the spots were neat and clear. The specific transfer value was moderate, and the reproducibility was good. There was no negative interference, and the specificity was strong. The HPLC method for the determination of syringin content had the advantages of simple operation, good repeatability, good stability, high precision and strong specificity, and it can be used for the quality control of Zhuang Medicine Xiaoyan Zhiyang Lotion, which provides a scientific basis for its later market production.
杂志排行
Medicinal Plant的其它文章
- Gastroprotective Effect of Alpinia zerumbet (Pers.) Burttet Smith on Ethanol-induced Gastric Ulcers in vivo and vitro
- Protective Effect and Mechanism of n-butanol Extract from Diploclisia glaucescens (B1.) Diels on Rats with Adjuvant Arthritis
- Effects of Early-stage Phased Rehabilitation Training on Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis
- Exploring the Mechanism of Blumea balsamifera (L.) DC in Preventing and Treating Alzheimer’s Disease Based on HPLC-ESI-HRMS and Network Pharmacology
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Research Progress and Ideas on the Anti-liver Fibrosis Effect of Ethnic Medicine Plumbagin Based on microRNAs/TLR4/NF-κB and NLRP3 Inflammasome Activation
