Extraction Process of Zhuang Medicine Fumigation Lotion
2023-10-31JiangcunWEIMeiyanQIUBingQINGXianyiSHIYinghongHUANGXianPENGWenZHONG
Jiangcun WEI, Meiyan QIU, Bing QING, Xianyi SHI, Yinghong HUANG, Xian PENG, Wen ZHONG
Guangxi International Zhuang Medical Hospital, Nanning 530201, China
Abstract [Objectives] To establish the extraction process and quality standard method of Zhuang medicine fumigation lotion. [Methods] The orthogonal design method was employed to optimize the water extraction process with the amount of water added, decocting time and extraction times as factors, and syringin content and dry extract yield as indexes. The content of syringin was determined by high performance liquid chromatography. [Results] The best water extraction process was: soaking in water for 1 h, decocting twice, added 10 times the amount of water each time, decocting for 1 h. The average content of syringin in 3 batches was 0.98 mg/g, and the average dry extract yield was 26.07%. [Conclusions] The project adopts water extraction method to prepare Zhuang medicine fumigation lotion, which has the characteristics of high efficiency and suitable for large-scale production. The quality control method is reliable, rapid and accurate, and can effectively control the quality of the lotion.
Key words Zhuang medicine fumigation lotion, Extraction process, Content determination
1 Introduction
Zhuang medicine fumigation lotion is an ethnic medicine prescription developed by the Dermatology Department of Guangxi International Zhuang Medical Hospital. The prescription is made of 14 kinds of traditional Chinese medicine, such as Cortex Ilicis Rotundae, Herba Violae, Herba Polygoni Perfoliati, Radix et Rhizoma Rhei, Radix Sangusorbae, Herba Portulacae,etc.More than 10 years of clinical application results show that the lotion has the effects of clearing heat and detoxification, dispersing knots and detumescence, treating various types of facial dermatitis, subacute eczema, folliculitis, skin furuncle and other skin diseases with erythema, papules, nodules and cysts as clinical manifestations, and it has obvious curative effect. In their long-term medical practice, the Chinese people have created a variety of methods to treat dermatosis, which is a general term for diseases occurring on the skin and its accessory organs[1-2].
Zhuang medicine fumigation lotion is made by decocting and boiling Chinese medicine, and the original medicine liquid is decocted in clinical application, which is extremely inconvenient to use. It is neither conducive to promptly controlling the disease, nor easy to carry and store. In order to overcome the above shortcomings, based on the compatibility of the original prescription, this study explored Zhuang medicine fumigation lotion according to the relevant requirements of medical institutions, which not only retains the effectiveness of the original prescription, but also conforms to the characteristics of the lotion, and is easy to carry and preserve, while improving the stability of the preparation. The pure traditional Chinese medicine preparation with good quality has a broad clinical application prospect and can produce significant social and economic benefits. In this project, the lotion was studied thoroughly and developed into a preparation. In order to further control the quality of this product and ensure its clinical efficacy, the extraction process and quality standard were studied.
2 Materials
2.1 InstrumentsAgilent 1260 high performance liquid chromatograph; ME155DU electronic balance (Mettler); Simplicity ultra-pure water system (Millipore China Co., Ltd.); TGL-16G high speed tabletop centrifuge (Shanghai Anting Scientific Instrument Factory); HWS-26 electric-heated thermostatic water bath (Shanghai Qixin Scientific Instrument Co., Ltd.).
2.2 MaterialsFourteen kinds of traditional Chinese medicine, such as Cortex Ilicis Rotundae, Herba Violae, Herba Polygoni Perfoliati, Radix et Rhizoma Rhei, Radix Sangusorbae, Herba Portulacae, Herba Taraxaci, Flos Chrysanthemi Indici,etc., were purchased from Guangxi Xianzhu Chinese Medicine Technology Co., Ltd. Syringin reference (batch No.: 111574-201605) and aesculetin reference (batch No.: 110741-202109) were purchased from China Institute for Food and Drug Control. Acetonitrile (batch No.: 170060) was a chromatographically pure produced by Thermo Fisher Scientific; phosphoric acid (batch No.: T200110324) was an analytical pure manufactured by Sinopharm Chemical Reagent Co., Ltd.
3 Methods and results
In this test, the content of syringin, a component of Cortex Ilicis Rotundae, in the extracted medicinal liquid was determined by high performance liquid chromatography (HPLC) at a wavelength of 265 nm[3-4].
3.1 Preparation of reference solutionAppropriate amount of syringin reference was accurately weighed, dissolved in methanol and shaken well to prepare the reference solution with a concentration of 0.957 7 mg/mL.
3.2 Preparation of test solution and its negative reference solutionAccording to the proportion of the prescription, a dose of medicinal materials was weighed, and decocted twice. It was soaked in 10 times the amount of water for 1 h in the first time and was soaked in 8 times the amount of water for 1 h in the second time. The decoction was merged, and the total volume was accurately measured (mL). Appropriate amount of supernatant was filtered through 0.22 μm microfiltration membrane, and the solution obtained was the test solution.
According to the proportion of the prescription, a dose of Cortex Ilicis Rotundae prescription medicinal material was weighed, and the negative control solution was prepared by the same method.
3.3 Chromatographic conditionsChromatographic column: Gemini®-C18110Å (5 μm, 4.6 mm×250 mm); mobile phase: acetonitrile (A) -0.1% phosphoric acid aqueous solution (B); gradient elution (The elution schedule is shown in Table 1); flow rate: 1 mL/min; column temperature: 30 ℃; injection volume: 10 μL; detection wavelength of syringin: 265 nm; theoretical plate number not be less than 2 000 for the calculation of syringin peak. The syringin component in the prescription was well separated from other components, and other components in the sample did not interfere with the measured components (Fig.1).
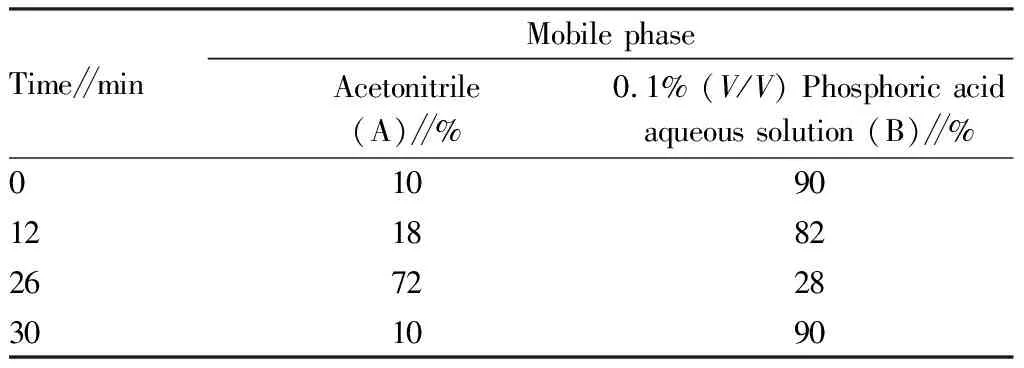
Table 1 Gradient elution schedule
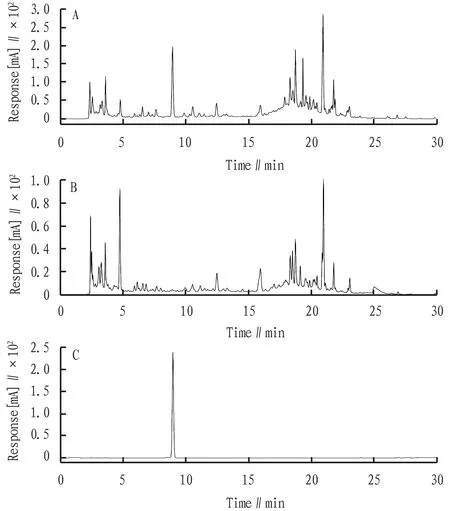
Fig.1 Chromatographic diagrams of sample solution (A), negative sample of Cortex Ilicis Rotundae (B), and syringin reference (C)
3.4 Methodology
3.4.1Linear relationship investigation. Certain amount of above reference solution was precisely absorbed, mixed in a brown volumetric bottle, and then set to a constant volume with methanol. Similarly, 7 different concentrations of Dachengqi decoction mixed reference solution were prepared. The injection volume was 10 μL. The samples were respectively injected and detected by liquid chromatograph. The peak area was measured. The standard curve was plotted with the concentration (mg/mL) as the abscissa and the peak area as the ordinate. The regression equation was calculated as follows:Y=26 306X-42.370,R2=0.999 9, showing a good linear relationship in the concentration range of 0.038 308-0.143 655 mg/mL.
3.4.2Precision test. The same syringin reference solution (0.15 mL of 0.957 7 mg/mL mother liquor is loaded into a 2 mL volumetric bottle and added with methanol to the scale, and the concentration is 0.071 827 5 mg/mL). The samples were injected for consecutive 6 times, and the peak area of syringin in Zhuang medicine fumigation lotion was determined by liquid chromatography described in Section3.3. The results showed that theRSDof the peak area of syringin in the lotion was 0.10%, and allRSDvalues were less than 3.00%, indicating good precision of the instrument.
3.4.3Stability test. According to the proportion of the prescription, a copy of Zhuang medicine fumigation lotion was prepared into test solution according to the method described in Section3.2, and the sample was injected at 0, 2, 4, 8, 12 and 24 h after the preparation of the test solution. The peak area of syringin in the test solution was determined according to the method described in Section3.3. The results showed that theRSDof the peak area of syringin was 1.37%, and allRSDvalues were less than 3.0%, indicating that the test solution was stable within 24 h.
3.4.4Repeatability test. According to the prescription ratio, 6 copies of Zhuang medicine fumigation lotion were weighed and prepared into test solutions according to the method described in Section3.2. According to the chromatographic conditions described in Section3.3, the peak area of syringin in each test solution of Zhuang medicine fumigation lotion was determined, and the average mass concentration of syringin and theRSDof mass concentration in each extract solution were calculated. The average mass concentration of syringin in the samples was 0.925 8 mg/mL, and theRSDwas 2.15%, indicating good repeatability.
3.4.5Recovery test. Six copies of Zhuang medicine fumigation lotion (batch No.: 210601) with known content were precisely measured, 5 mL each copy, were placed in 10 mL volumetric bottles. Afterwards, appropriate amount of syringin was added precisely, and pure water was added to the scale, shaken, and mixed well. Appropriate amount of sample solution was centrifuged at 13 000 r for 10 min, and the supernatant was filtered by 0.22 μm microfiltration membrane. The peak area of syringin was determined according to the chromatographic conditions described in Section3.3, and the content of syringin in this product was calculated by external standard one-point method. The calculated recovery rates ranged from 97.10% to 102.20%, and the average recovery rate was 99.62%. TheRSDwas 1.89% (n=6), indicating good accuracy of the method (Table 2).
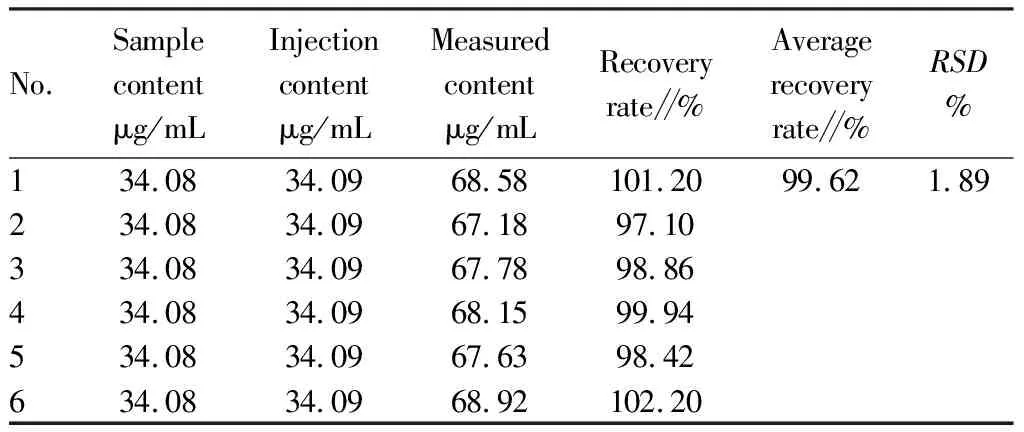
Table 2 Experimental results of recovery rate of Zhuang medicine fumigation lotion (n=6)
3.5 Orthogonal testThe Chinese medicine was extracted by water boiling method. The extraction efficiency can be affected by the amount of water added, decocting time, soaking time, extraction times and particle size of Chinese medicine. Considering the actual large-scale production, working efficiency and economic cost, the extraction effect was greatly affected by the amount of water added, decocting time and extraction times. Therefore, the amount of water added, decocting time and extraction times were selected for orthogonal test, and the content of syringin and dry extract yield were used as evaluation indexes to optimize the extraction process.
3.5.1Factor level design. According to the single factor test results and actual production conditions, the factors affecting the extraction process were: the amount of water added, decocting time and soaking time. Combined with the actual production, the amount of water added (A), decocting time (B) and extraction times (C) were selected as the investigation factors in the orthogonal test, and the content of syringin and dry extract yield were taken as evaluation indexes. L9(34) orthogonal design was used to optimize the extraction process of Zhuang medicine fumigation lotion, and the level of factors is shown in Table 3.
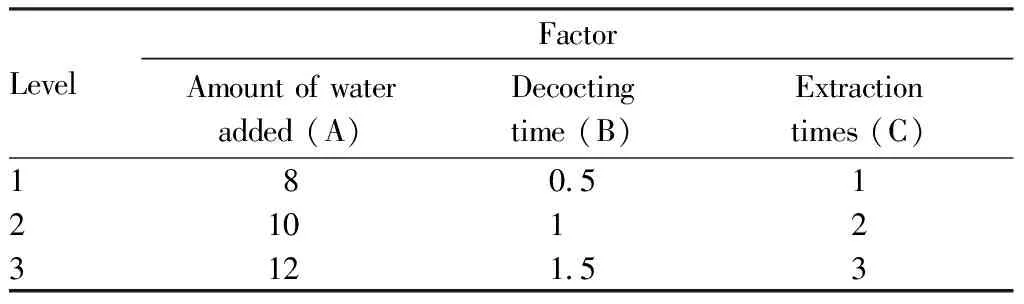
Table 3 Factor level of L9 (34) orthogonal design for the extraction process of Zhuang medicine fumigation lotion
3.5.2Orthogonal test. According to the factors and levels of orthogonal test designed in Table 3, the medicinal materials were weighed according to the proportion of THE prescription, extracted following the conditions in Table 4, and filtered for later use.
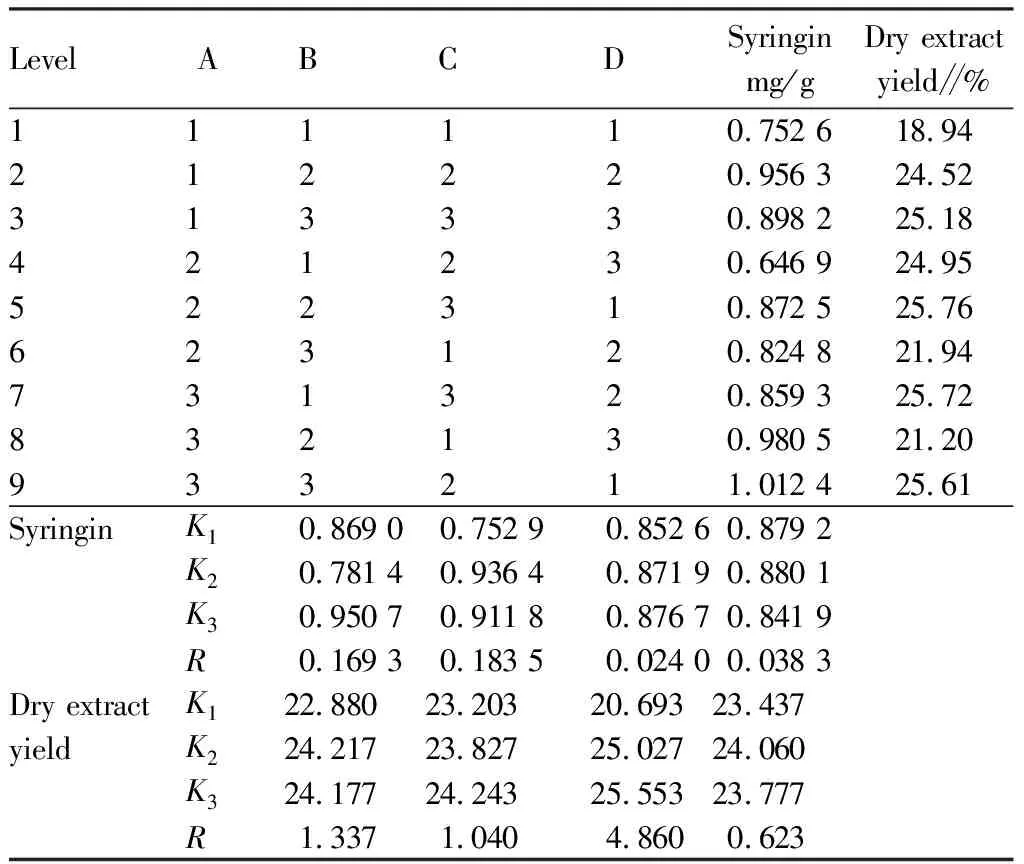
Table 4 Arrangement and results of L9 (34) orthogonal design for the extraction process of Zhuang medicine fumigation lotion
The ANOVA results of syringin extraction (Table 5) showed that factor B had significant influence on the extraction process (P<0.05), and the main influencing factors were B>A>C, that is, the decocting time had the greatest influence, followed by amount of water added and extraction times, and the optimal combination was A3B2C3.
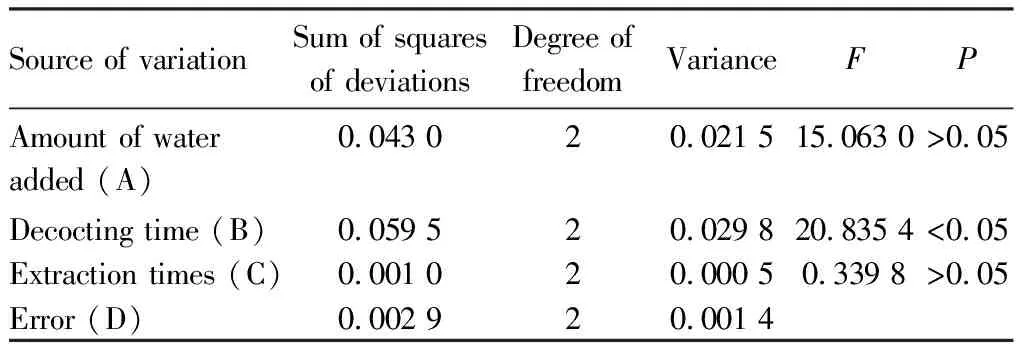
Table 5 Analysis of variance table for syringin extraction
The ANOVA results for the extraction of dry extract yield (Table 6) showed that the amount of water added (A) and decocting time (B) had no significant influence on the extraction process (P>0.05), while the factor of extraction times (C) had significant influence on the extraction process, and the influencing factors successively were: C>A>B, that is, the extraction times had the greatest influence. Combined with the intuitive analysis results, it can be seen that the optimal extraction process combination was: A2B3C3.
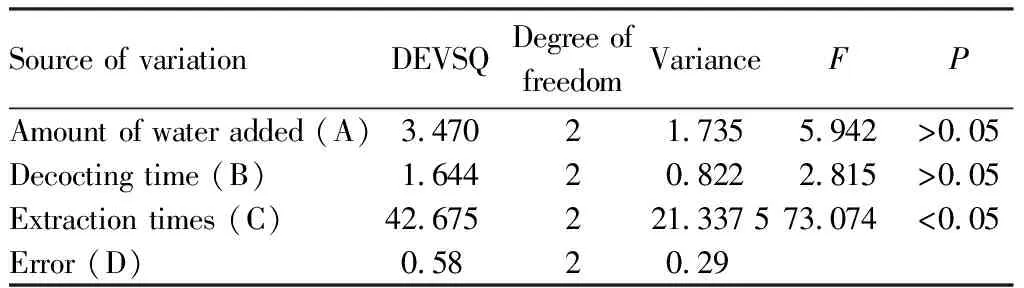
Table 6 Analysis of variance table for the extraction of dry extract yield
Considering the actual large-scale production and economic cost factors, and to adapt to industrial large-scale production,combined with the traditional decocting method, the extraction process of this product was determined as A2B2C2, that is, each flavor of medicinal materials was weighed according to the prescription, soaked in water for 1 h, extracted twice, added 10 times the amount of water each time, and decocted for 1 h.
3.5.3Extraction process verification. The process was verified according to the optimization results of above extraction process. The medicinal materials were weighed according to the prescription, soaked in water for 1 h, extracted twice, added 10 times the amount of water each time, and decocted for 1 h. The extraction solutions were merged. The test was repeated for 3 consecutive times to determine the syringin content and dry extract yield of this product, and the results are shown in Table 7.
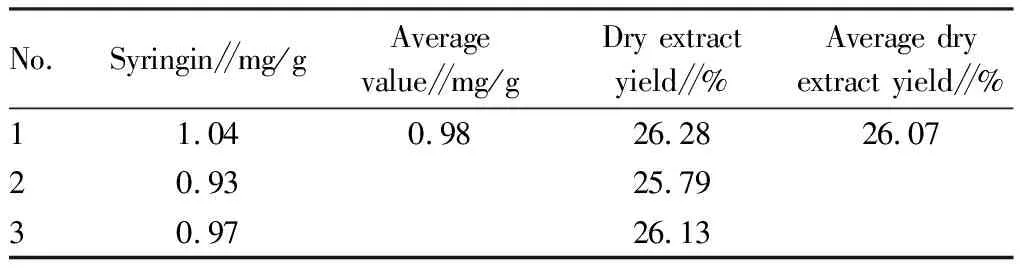
Table 7 Verification test results of the extraction process of Zhuang medicine fumigation lotion
The experimental results showed that the average content of syringin was 0.98 mg/g and the average dry extract yield was 26.07% according to the optimized process. The better results demonstrated that the process was reasonable and feasible.
4 Conclusions
In this study, 14 kinds of traditional Chinese medicine, such as Cortex Ilicis Rotundae, Herba Violae, Herba Polygoni Perfoliati, Radix et Rhizoma Rhei, Radix Sangusorbae, Herba Portulacae,etc., were used as raw materials to prepare Zhuang medicine fumigation lotion by water extraction process, and a quality standard was preliminarily established to evaluate and control the quality of Zhuang medicine fumigation lotion.
杂志排行
Medicinal Plant的其它文章
- Quality Control of Zhuang Medicine Xiaoyan Zhiyang Lotion
- Research Progress and Ideas on the Anti-liver Fibrosis Effect of Ethnic Medicine Plumbagin Based on microRNAs/TLR4/NF-κB and NLRP3 Inflammasome Activation
- Gastroprotective Effect of Alpinia zerumbet (Pers.) Burttet Smith on Ethanol-induced Gastric Ulcers in vivo and vitro
- Exploring the Mechanism of Blumea balsamifera (L.) DC in Preventing and Treating Alzheimer’s Disease Based on HPLC-ESI-HRMS and Network Pharmacology
- Observation on Therapeutic Effect of Erxian Decoction on Relieving Low Back Pain after PVP of PMOP-derived Vertebral Fracture
- Effects of Early-stage Phased Rehabilitation Training on Acute Respiratory Distress Syndrome: A Systematic Review and Meta-analysis
