Concise Synthesis and Fertility-Promoting Activity of Thiamidol
2023-10-29ZHURouyu朱柔钰GUOLina郭利娜ZHUJunDIAOHuaSHAOZhiyu邵志宇
ZHU Rouyu(朱柔钰), GUO Lina(郭利娜), ZHU Jun(朱 俊), DIAO Hua(刁 华)*, SHAO Zhiyu(邵志宇)*
1 College of Chemistry and Chemical Engineering, Donghua University, Shanghai 201620, China 2 NHC Key Laboratory of Reproduction Regulation, Shanghai Institute for Biomedical and Pharmaceutical Technologies, Shanghai 200032, China 3 National Rodent and Leporid Laboratory Animal Resource Center, Shanghai 200031, China
Abstract:In order to make the synthetic route of Thiamidol more efficient and easier to scale up, a new method was developed. The compound 2,4-dihydroxyacetophenone was dissolved in ethyl acetate and brominated with cupric bromide. After bromination, the mixture was filtered to remove cuprous bromide. The obtained ethyl acetate solution containing 2-bromo-1-(2,4-dihydroxyphenyl)ethanone was directly reacted with (2-methylpropanoyl)thiourea to obtain the precipitate of Thiamidol hydrobromide. The precipitate was neutralized by deacidification and recrystallized to afford Thiamidol with high purity. In addition, in the screening of fertility-promoting substances, Thiamidol was found to be effective in protecting human sperm from motility loss in vitro. This suggests that it may have potential application in fertility protection and promotion.
Key words:synthetic route; Thiamidol; sperm motility; fertility
Introduction
Thiamidol (isobutylamido thiazolyl resorcinol, CAS No.1428450-95-6), a resorcinol derivative, is currently the most efficient tyrosinase inhibitor for humans[1-4]. It can directly inhibit the formation of melanin[5-6]and will not be transformed into substances that may lead to re-blackening, irritation or other adverse reactions in the process of action[7-9]. Clinical studies have confirmed that it provides visible effects on pigmentation and hyperpigmentation when the effective mass fraction of Thiamidol in the formulas used by patients reaches 0.1%-0.2%[1-2,10-11].
Thiamidol was originally researched and developed by Beiersdorf AG (Hamburg, Germany) with the following synthetic route[12](seen in scheme A1). Firstly, isobutyryl chloride reacted with thiourea in toluene to form (2-methylpropanoyl)thiourea (intermediate, I). Then, the hydroxyl groups of 2, 4-dihydroxyacetophenone were protected by methyl chloroformate (other chloroformate esters could also be used instead). Finally, after halogenation reaction and cyclization reaction with I, the protective group was removed under the action of alkali to afford the final product Thiamidol (Ⅳ).
Ruishinuo Pharmaceutical Technology Co., Ltd. (Taizhou, China) improved the synthetic route by using resorcinol and bromoacetic acid as raw materials[13](seen in scheme A2). Friedel-Crafts reaction was catalyzed by boron trifluoride-ethyl ether to obtain 2-bromo-1-(2,4-dihydroxyphenyl)ethanone (intermediate, II), which then underwent cyclization reaction with I under the action of alkali to obtain the product Thiamidol (Ⅳ).
In the new synthetic route proposed by Beiersdorf AG in 2021[14](seen in scheme A3), first the Friedel-Crafts reaction of resorcinol and chloroacetonitrile was carried out at room temperature catalyzed by hydrogen chloride-dioxane and anhydrous zinc chloride. Then, 2-chloro-1-(2,4-dihydroxyphenyl)ethenone (intermediate, III) was obtained by hydrolysis reaction. The product Thiamidol hydrochloride could be prepared by cyclization reaction of Ⅲ and I. The structures of compounds I to IV are shown in Fig.1.
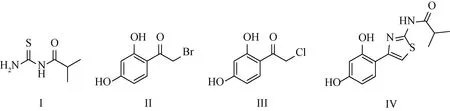
Fig.1 Structures of compounds I to Ⅳ
In order to solve the disadvantages of the existing synthetic routes such as toxicity, corrosiveness and environmental hazards, a more concise and efficient method for the synthesis of Thiamidol is developed in this paper, which is expected to be carried out easily and afford economic advantages as a result of a short synthesis route with high yields. It is more suitable for industrial-scale production than previous routes. In subsequent studies of compound activity, Thiamidol is unexpectedly found to be effective in the protection of sperm motilityinvitro, suggesting potential fertility-promoting effects.
1 Experiments
1.1 Reagents and instruments
Thiourea, isobutyryl chloride, sodium hydroxide, sodium bicarbonate, cupric bromide and 2,4-dihydroxyacetophenone were purchased from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. Dimethyl sulfoxide (DMSO) and tetrahydrofuran (THF) was purchased from Sigma-Aldrich Company, USA. Toluene, ethyl acetate(EA), deuterated chloroform (CDCl3) and deuterated DMSO (DMSO-d6) were purchased from Titan Technology Co., Ltd., Shanghai, China. All commercially available materials and solvents were analytically pure reagents and used as received without further purification.
Sperm BWW culture medium and computer-aided sperm analysis (CASA) slides were purchased from Leja®, the Netherlands.
1H nuclear magnetic resonance (NMR) and13C NMR spectra were measured on a Bruker AVANCE Ⅲ HD 600 MHz NMR spectrometer (Bruker, Switzerland) in CDCl3or DMSO-d6. Tetramethyl silane (TMS) was used as an internal standard and the chemical shift multiplied by 106was represented byδin this paper. Elemental analysis was measured on an Elementar Vario EL Ⅲ elemental analyzer (Elementar Analysensysteme GmbH, Germany). Sperm motility parameters were recorded in a Hamilton Thorne IVOS II fully-automated sperm analyzer (Hamilton Thorne Ltd., USA).
1.2 Synthesis
1.2.1(2-methylpropanoyl)thiourea(I)
The synthetic route of (2-methylpropanoyl)thiourea(I) is shown in Scheme 1.
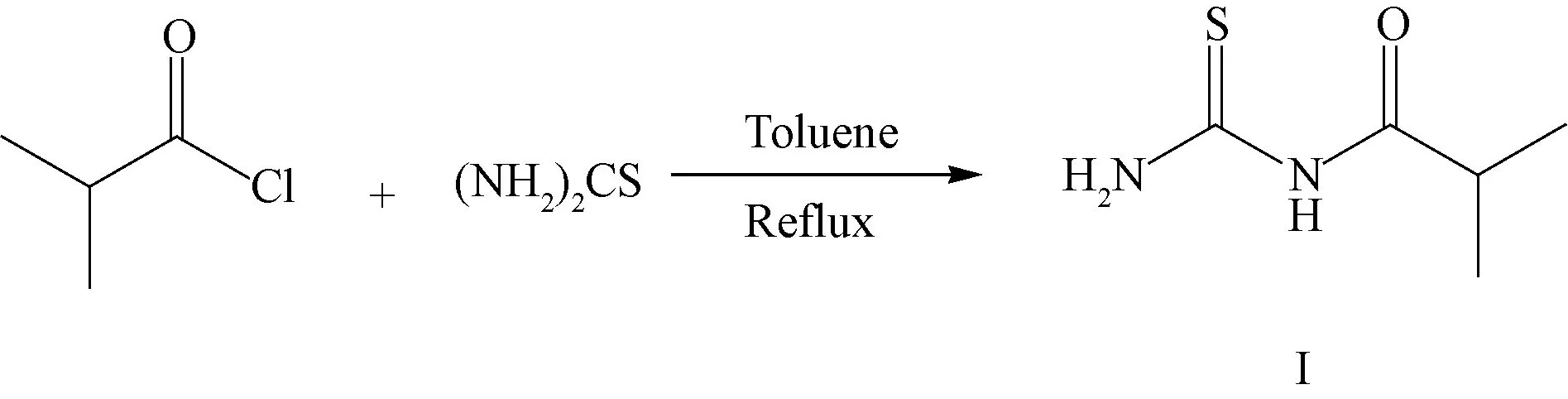
Scheme 1 Synthetic route of (2-methylpropanoyl)thiourea (I)
To a stirred mixture of thiourea (95 g, 1.25 mol) in toluene (300 mL), iso-butyryl chloride (106 g, 1 mol) was added dropwise. The reaction mixture was refluxed at 115 ℃ for 3 h. The exhaust gas was absorbed by a sodium hydroxide solution. After the reaction was finished, the mixture was cooled to room temperature, and water (200 mL) was poured into it. Toluene was removed in vacuum.
A large amount of solid was obtained by cooling crystallization. The crude product was collected by suction filtration, washed with water, and dried in vacuum to obtain (2-methylpropanoyl)thiourea (I) as a light yellow solid (102 g, yield: 70%).1H NMR (CDCl3, 600 MHz)δ: 10.00 (1 H, s), 9.56 (1 H, s), 7.71 (1 H, s), 2.55 (1 H, m), 1.19 (6 H, d,J=7.2 Hz).
1.2.2Thiamidol(Ⅳ)
The synthetic route of Thiamidol (Ⅳ) is shown in Scheme 2.
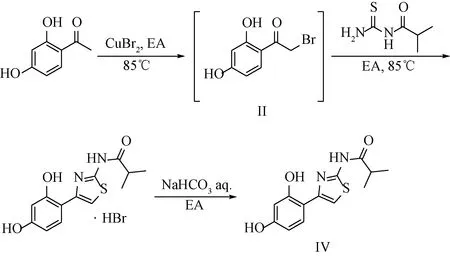
aq.— aqueous solution.Scheme 2 Synthetic route of Thiamidol (Ⅳ)
To a stirred solution of 2,4-dihydroxyacetophenone (3.05 g, 20 mmol) in EA(40 mL), cupric bromide (8.98 g, 40 mmol) was added. The reaction mixture was refluxed at 85 ℃ for 3 h. The exhaust gas was absorbed by the sodium hydroxide solution. After the reaction was finished, the mixture was cooled to room temperature, and cuprous bromide was removed by filtration. The filtrate containing II could be put into the next reaction without any purification.
The above filtrate was transferred to a 100 mL round bottom flask, and I (2.92 g, 20 mmol) was put into it. The reaction mixture was refluxed at 85℃ for 30 min, and solid precipitation was observed during the reaction. After the reaction was finished, the mixture was cooled to room temperature, and a large amount of solid was precipitated. The precipitation was filtered to obtain a white solid product, which was analyzed as Thiamidol hydrobromide (5.25 g, yield: 73%).1H NMR (DMSO-D6, 600 MHz)δ: 12.19 (1 H, bs), 7.66(1 H, d,J=8.4 Hz), 7.42 (1 H, s), 6.33 (1 H, d,J=1.8 Hz), 6.31 (1 H, dd,J=8.4, 1.8 Hz), 2.76 (1 H, m), 1.14 (6 H, d,J=6.6 Hz). According to elemental analysis, the mass fractions of C, H and N were 42.04%, 4.47% and 7.48%, respectively, which correspond to C13H14N2O3S·HBr·0.5H2O.
Thiamidol hydrobromide (10 g, 27.8 mmol) was added to the mixed system of the saturated sodium bicarbonate solution (30 mL) and EA(60 mL), shaken well and left for phase separation. EA was removed in vacuum to give the product Thiamidol (Ⅳ) as a white solid (7.04 g, yield: 91%). The total yield calculated from the starting material 2,4-dihydroxyacetophenone was 66%.1H NMR (DMSO-D6, 600 MHz)δ: 12.19 (1 H, bs), 10.92 (1 H, bs), 9.51 (1 H, bs), 7.67 (1 H, d,J=8.4 Hz), 7.41 (1 H, s), 6.33 (1 H, d,J=1.8 Hz), 6.32 (1 H, dd,J=8.4, 1.8 Hz), 2.76 (1 H, m), 1.15 (6 H, d,J=6.6 Hz).13C NMR (DMSO-D6, 150 MHz)δ: 175.7, 158.8, 157.5, 156.9, 147.0, 128.7, 111.2, 107.6, 105.8, 103.5, 34.4, 19.6. According to elemental analysis, the mass fractions of C, H and N were 56.18%, 5.17% and 9.98%, respectively, which corresponded to C13H14N2O3S.
The1H NMR spectra of Ⅰ, Ⅲ and Ⅳ are shown in Figs. A1, A2 and A3, respectively. The13C NMR spectrum of Ⅳ is shown in Fig. A4.
1.3 Biological activity of Thiamidol
A Thiamidol stock solution (50 mmol/L) was prepared in DMSO and stored at 4 ℃. All semen samples used in this paper were obtained from male volunteers aged 20-35 years at the Reproduction Center of Shanghai Jiao Tong University School of Medicine (Shanghai, China). The semen samples were collected by masturbation after 3-7 d of abstinence. The sperm BWW culture medium was pre-equilibrated in a 37 ℃ incubator for 30 min. Semen samples were incubated at 37 ℃ for 30 min to liquefy. CASA was performed to obtain sperm motility parameters.
Semen samples with all sperm parameters in the normal range[15]were mixed with an equal volume of pre-equilibrated sperm BWW culture medium, and the mixture of 300 g was centrifuged for 5 min. After centrifugation, the supernatant was removed from the samples and the sperm pellet was resuspended with a pre-equilibrated sperm BWW culture medium. The sperm concentration was adjusted to 10×106-20×106/mL. The samples were divided equally into the control group and the experimental group. The control group was named Sc, and the experimental groups with Thiamidol concentrations of 5, 25 and 50 μmol/L were named S5, S25and S50, respectively.
The Thiamidol stock solution was added to the experimental group and gently mixed, adjusted to designated concentrations, and then placed in an incubator at 37 ℃ with the control group for the designated periods. The concentration of DMSO in the control group was the same as that in the experimental group. Sperm motile motility, progressive motility, average path velocity (VAP), curvilinear velocity (VCL) and linear velocity (VSL) were measured by CASA. Ten different fields of view were recorded for each sample.
2 Results and Discussion
2.1 Effects of reaction conditions on yield of Thiamidol hydrobromide
Parallel experiments were used to explore the effect of different reaction conditions on the yield of Thiamidol hydrobromide, as shown in Table 1 and Table 2. The amount of Thiamidol hydrobromide was calculated directly from the precipitate produced in the reaction system. The yield was calculated from the starting material 2,4-dihydroxyacetophenone.
All reactions were monitored by thin-layer chromatography. For Ⅰ, the volume ratio of petroleum ether(PE) to EAVPE∶VEAis 80∶20, and the ratio of the distance moved from the origin by component to the distance moved from the origin by solvent (Rf) is 0.6. For Ⅱ,VPE∶VEA= 85∶15, andRf= 0.5. For Thiamidol (Ⅳ),VPE∶VEA=70∶30, andRf=0.3.
As shown in Table 1, when ethanol and methanol were used as solvents, although the product was formed, it could not be precipitated from the system and separated directly. When acetone was used as a solvent, only a small amount of precipitation was produced. A large amount of product was easily precipitated in EA. Therefore, EA was the preferred solvent.
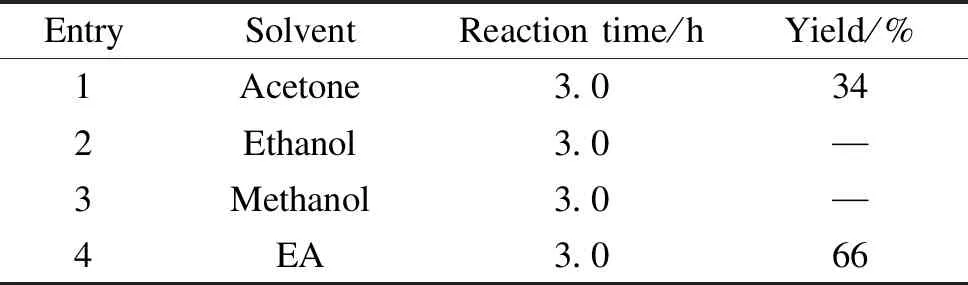
Table 1 Effect of solvent type on yield of Thiamidol hydrobromide
It was found that the yield of Thiamidol hydrobromide depended on the progress of the bromination reaction of 2,4-dihydroxyacetophenone and cupric bromide. As shown in Table 2, after the reaction proceeded for 3.0 h, the bromination progressed to the equilibrium point and the yield did not change significantly.
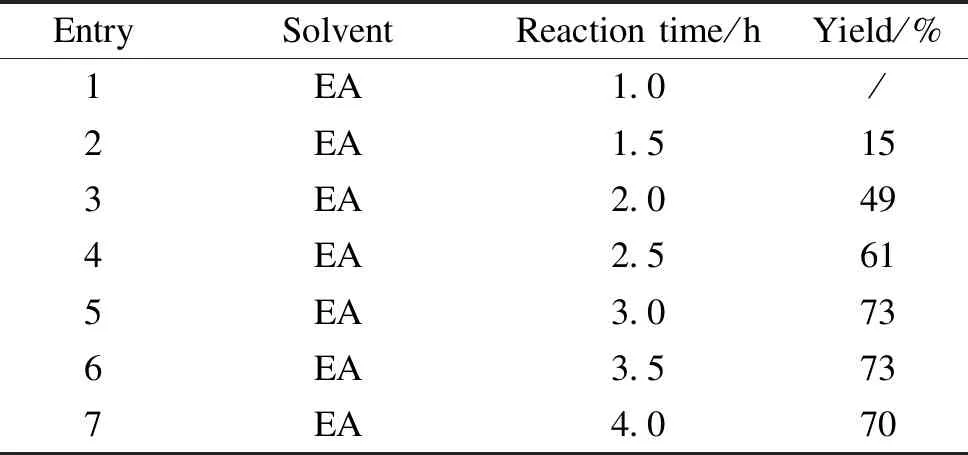
Table 2 Effect of reaction time on the yield of Thiamidol hydrobromide
2.2 Effects of Thiamidol on human sperm motility parameters
The sperm-motility-related activity of Thiamidol has been creatively investigated in this study. Figure 2 shows the effects of Thiamidol at a concentration of 50 μmol/L on human sperm motility parameters with eight typical independent experiment results. All results were statistically significant with the probabilityPless than 0.050. After 120 min incubation in an incubator at 37 ℃, S50showed significantly higher sperm motility including motile motility, progressive motility, VAP, VSL and VCL than Sc. This suggests that Thiamidol may have the potential to promote sperm motility and protect sperm from motility loss.
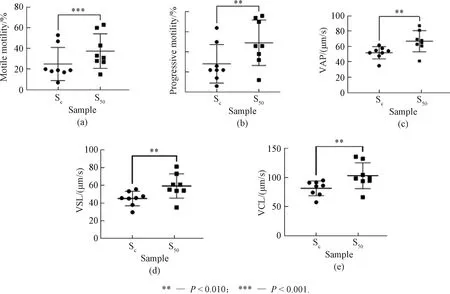
Fig. 2 Effects of Thiamidol on human sperm motility parameters: (a) motile motility; (b) progressive motility; (c) VAP; (d) VSL; (e) VCL
2.3 Effects of Thiamidol concentration and sperm incubation time on VAP, VSL and VCL
The effects of Thiamidol concentration and sperm incubation time on VAP, VSL and VCL are shown in Fig. 3. The results show that Thiamidol has a predominant effect on VAP, VSL and VCL. We observed that the prolonged sperm incubation time led to sample depletion, so we adjusted the sperm incubation time to 0-120 min and reran the experiment. With the sperm incubation time gradually increasing from 0 to 120 min (Fig. 4), the sperm motility parameters of the control group gradually decreased, and the sperm motility parameters of the experimental groups with different Thiamidol concentrations were higher than those of the control group. This indicates that Thiamidol may have a protective effect on sperm motility and may slow down sperm motility loss.
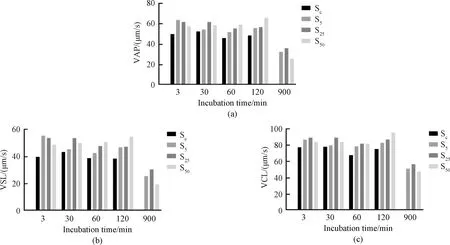
Fig.3 Effects of Thiamidol concentrations and sperm incubation time within 900 min on sperm motility: (a) VAP; (b) VSL; (c) VCL
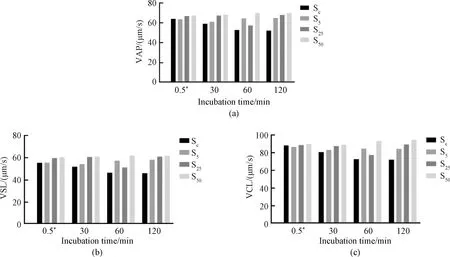
*—Timing more precise with a stopwatch. Fig.4 Effects of sperm incubation time within 120 min on sperm motility: (a) VAP; (b) VSL; (c) VCL
Since human sperm cannot remain active in sperm BWW culture medium for a long time under normal circumstances, 120 min was chosen as the final experimental sperm incubation time to facilitate the observation of the potential protective effect of Thiamidol. It turned out that Thiamidol could enhance sperm motility (reflected by multiple parameters) in a wide range of concentrations from 5 μmol/L to 50 μmol/L, suggesting that Thiamidol as a non-toxic reagent to sperm had potential application in male fertility protection and promotion.
3 Conclusions
The synthetic route of Thiamidol is improved. The core problem in the synthetic route of Thiamidol is to prepare 2-bromo-1-(2,4-dihydroxyphenyl)ethanone (Ⅱ). We skipped the purification step of the products of bromination of 2,4-dihydroxyacetophenone and made the products react with (2-methylpropanoyl)thiourea (Ⅰ) directly to obtain Thiamidol hydrobromide.
Thiamidol hydrobromide was insoluble in EA, so it could be directly isolated and then reacted to give the subsequent product. Through1H NMR,13C NMR and elemental analysis, the structure of the synthesized product was determined to be Thiamidol with high purity. The synthetic route developed in this paper is efficient, easier to operate, more environmentally friendly, and improves the purity and the yield of the product, which has great advantages in industrial-scale production.
The potential of Thiamidol to protect sperm motility is found. Thiamidol has the activity of protecting human sperm from motility lossinvitro, indicating its potential application for male fertility protection and promotion.
Appendix
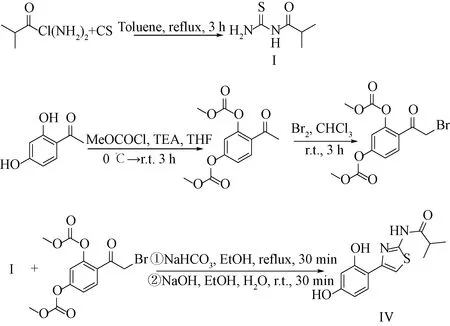
r.t.—room temperature.Sheme A1 Synthetic route of Thiamidol by Beiersdorf AG[12]
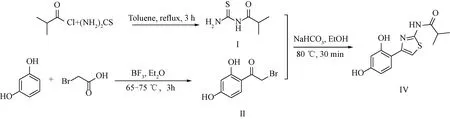
Sheme A2 Synthetic route of Thiamidol by Ruishinuo Pharmaceutical Technology Co., Ltd.[13]
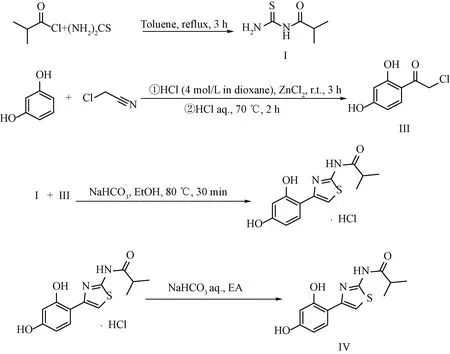
Sheme A3 Synthetic route of Thiamidol by Beiersdorf AG[14]
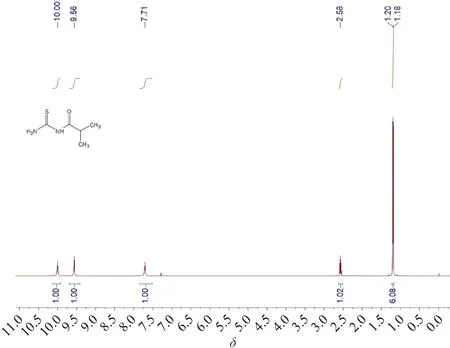
Fig.A1 1H NMR spectrum of (2-methylpropanoyl)thiourea (I)
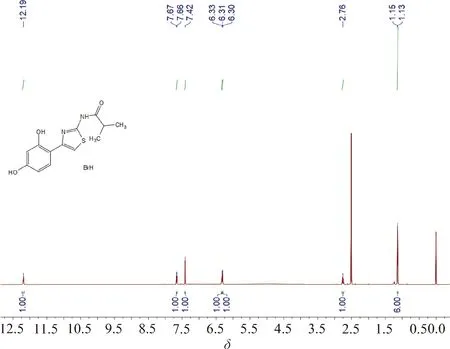
Fig.A2 1H NMR spectrum of Thiamidol hydrobromide
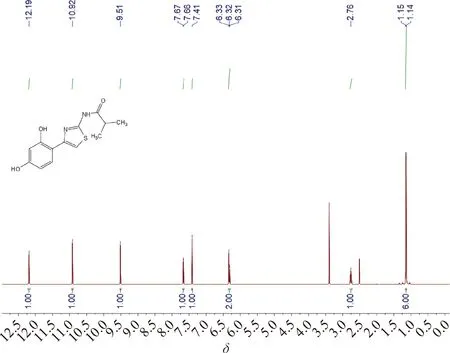
Fig.A3 1H NMR spectrum of Thiamidol (Ⅳ)
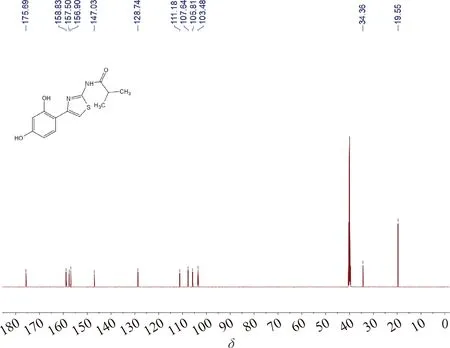
Fig.A4 13C NMR spectrum of Thiamidol (Ⅳ)
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Preparation of Cellulose Acetate Butyrate Porous Micro/Nanofibrous Membranes and Their Properties
- Highly Sensitive Electrochemical Detection of Nitrite Based on Cationic Surfactant Modification of Conductive Carbon Black
- Calculation and Analysis of Acoustic Characteristics of Straight-Through Perforated Pipe Muffler Based on Multilayer Sound Absorbing Material
- Design and Structure Optimization of Plenum Chamber with Airfoil Baffle to Improve Its Outlet Velocity Uniformity in Heat Setting Machines
- Modeling of Micropores Drilling Force for Printed Circuit Board Micro-holes Based on Energy Method
- Image Retrieval Based on Vision Transformer and Masked Learning
