Highly Sensitive Electrochemical Detection of Nitrite Based on Cationic Surfactant Modification of Conductive Carbon Black
2023-10-29HUJianmei胡建梅ZHANGXuan
HU Jianmei(胡建梅), ZHANG Xuan(张 煊)
Key Laboratory of Science and Technology of EcoTextiles, Ministry of Education, College of Chemistry and Chemical Engineering, Donghua University, Shanghai 201620, China
Abstract:Nitrite is a commonly used additive in cured foods and its sensitive detection is important to human health. In this work, a simple but sensitive electrochemical sensor for nitrite was developed. Conductive carbon black (VXC-72R) functionalized with a cationic surfactant cetyltrimethylammonium bromide (CTAB) was used as an electrode material, and was coated on a glassy carbon electrode (GCE) to fabricate the electrochemical sensor (CTAB/VXC-72R/GCE) for nitrite. Zeta potential characterization and a series of electrochemical tests were carried out on several materials. It was found that the present sensor showed an enhanced sensitivity towards nitrite detection due to the enhanced surface positive charge revealed by the Zeta potential. Under optimal conditions, the ranges of good linear relationship between the peak current and the nitrite concentration were obtained to be 0.5-5.0 μmol/L and 5.0-1 087.0 μmol/L with a lower detection limit of 0.30 μmol/L. It was also successfully used for the determination of nitrite in cured food samples with excellent reproducibility, stability and selectivity.
Key words:electrochemical sensor; nitrite; conductive carbon black; surfactant
0 Introduction
Cured foods, such as ham, salted fish, and pickled vegetables are popular in the diet because of their special taste. Usually, nitrite is used as a key additive in these cured foods to preserve the original flavor and the fresh color. The extensive consumption of nitrite-containing foods has posed a threat to public health, because the excessive amount of nitrite in body could interfere with oxygen transport and cause hypotension[1-2]. More importantly, it has been recognized that nitrite could react with secondary amines in acidic conditions to produce carcinogenic nitrosamines[3-5]. As a consequence, the World Health Organization (WHO) has recommended an upper limit of 3 mg/L for nitrite in drinking water, and the maximum limit of nitrite in cured foods is no more than 20 mg/kg in China[6-7]. Therefore, the development of a sensitive and simple method for rapid detection of nitrite in cured foods is highly desired for the routine analysis.
Many analytical methods have been proposed for nitrite determination, such as spectrophotometric, chromatographic and capillary electrophoresis methods[8-11]. However, neither their complex derivative reaction nor time-consuming operation could meet the requirements of rapidity and simplicity for the routine analysis. Recently, electrochemical sensors have received much attention in analytical science due to their high sensitivity, rapidity and simple operation. Based on various electrodes, electrochemical sensors for nitrite detection have been proposed. For example, Ahmadetal.[12]reported an electrochemical sensor for nitrite based on Fe2O3nanoparticles coated ZnO nanorods materials and applied in real water samples. Zhangetal.[13]decorated Ag-Ag2O nanoparticles on composite of multiwall carbon nanotube/NiCoAl-hydrotalcite to improve analytical performance for nitrite quantification. In general, carbon nanotubes, graphene, fullerene and Mxene materials are excellent electrode materials and are extensively employed for electrochemical sensing, but they are expensive[14-19]. It could be noticed that most of these nitrite sensors need precious metals and expensive electrode materials.
Conductive carbon black is one class of cheap carbon materials with a large specific surface area and a low resistance, and used as a common catalyst support[20-22]. The carbon black alone is not commonly used as an electrode material for sensors due to low sensing performance possibly resulting from poor dispersity in general solvents. Recently, surfactant, such as cetyltrimethylammonium bromide (CTAB), has been used to modify carbon black and other electrode materials in order to improve the electrochemical sensing performance, which promotes dispersion and enrichment of the target by electrostatic attraction or hydrophobic interaction[23-24]. For example, CTAB has been used to improve the detection performance of carbon black towards bisphenol A, and the hydrophobicity of the CTAB long-chain alkyl end can enhance the enrichment ability of the target[23]. In this work, conductive carbon black VXC-72R is functionalized by CTAB, and coated on a glassy carbon electrode (GCE) to construct an electrochemical sensor (CTAB/VXC-72R/GCE) for the detection of nitrite. Cationic charge on the electrode surface could efficiently enrich nitrite anions and accelerate electrochemical reactions.
1 Experiment
1.1 Reagents and materials
VXC-72R (Cabot Corp., Boston, USA) and CTAB (Macklin, Shanghai, China) were commercially available. Sodium nitrite, sodium dodecyl sulfate (SDS), potassium ferricyanide (K3[Fe(CN)6]), and chitosan were obtained from Sinopharm Chemical Reagent Corp. (Shanghai, China). Pickled cabbage and ham samples were purchased from the local supermarket in Shanghai, China.
1.2 Instruments
All electrochemical measurements were acquired on CHI-760E electrochemical workstation (Chenhua, Shanghai, China) with the GCE (3 mm diameter) as the working electrode, the Pt wire as the counter electrode, and the saturated calomel electrode (SCE) as the reference electrode. The Zeta potentials of the materials were obtained on the nanoparticle size and Zeta potential analyzer (Zetasizer Nano ZS, UK). The catalyst solution was sonicated in the ultrasonic cleaner (KQ3200, Kunshan, China). The sample solution was centrifuged on a high-speed centrifugal mixer (Optima MAX-TL, Beckman, USA). The pH value of the solution was determined by a pH meter (FE28-Standard, Swiss).
1.3 Preparation of composite
Firstly, the chitosan solution with a mass fraction of 0.1% was obtained by dissolving chitosan in 10 g/L acetic acid aqueous solution. CTAB/VXC-72R composite was then prepared by mixing different proportions of CTAB and VXC-72R in the chitosan solution under sonication to gain a series of 2 mg/mL catalyst inks. VXC-72R and SDS/VXC-72R catalyst inks were obtained in a similar manner.
1.4 Preparation of working electrode
GCE was polished with 0.05 μm alumina powder by the polishing cloth and ultrasonically cleaned in diluted nitric acid, ethanol and deionized water, respectively. Then, 9 μL CTAB/VXC-72R ink solution (2 mg/mL) was dropped onto the electrode and dried at 50 ℃. For comparison, VXC-72R/GCE and SDS/VXC-72R/GCE electrodes were also prepared in a similar way by using the corresponding catalyst solution.
1.5 Processes of sample treatment and electrochemical detection of nitrite
Pickled cabbage and ham were firstly cut to pieces. Then, 2 g sample was put into 80 mL deionized water in a 150 mL conical flask and sonicated for 30 min with shaking every five minutes. The solution was then heated at 75 ℃ for 5 min and filtrated to remove the solid residue. Finally, the solution was further centrifugated at 10 000 r/min for 15 min, and the supernatant was transferred into a 100 mL volumetric flask and diluted to the marked line for the following analysis.
The oxidation behaviors of nitrite were characterized by cyclic voltammetry (CV) and differential pulse voltammetry (DPV) from 0.4 to 1.0 V versus the SCE(vs.SCE) at the scan rate of 50 mV/s in 0.1 mol/L phosphate buffered solution (pH=4), withEvs.SCEandIstanding for the potential vs.SCE and the current, respectively. The conductivity of the electrode was evaluated by electrochemical impedance spectroscopy (EIS) in 0.1 mol/L KCl solution containing 5 mmol/L K3[Fe(CN)6], withZ′ andZ″ standing for the real part and the imaginary part of the impedance, respectively.
2 Results and Discussion
2.1 Electrochemical characterization of various electrodes
The conductivity of various electrodes was firstly studied by EIS. As shown in Fig.1(a), there is a semicircle in a higher frequency range at the bare GCE, but the semicircle almost disappears at VXC-72R/GCE, SDS/VXC-72R/GCE and CTAB/VXC-72R/GCE, respectively. It could be attributed to better conductivity of conductive carbon black that promoted the electron transfer rate. In order to characterize the corresponding sensitivity of several prepared electrodes towards nitrite detection, the DPV response was recorded in a solution containing 400 μmol/L nitrite in the phosphate buffer solution (PBS, 0.1 mol/L, pH=4). It can be seen from Fig.1(b) that the oxidation peak potentialEpof nitrite is more negatively shifted about 60-70 mV on the modified GCE than that on the bare GCE. Furthermore, the highest oxidation peak currentIpis obtained on CTAB/VXC-72R/GCE (Figs.1(b) and 1(c)), which is 3.4 and 1.5 times of that on GCE and VXC-72R/GCE, respectively. These results indicate that the cationic surfactant can efficiently enhance the adsorption of the nitrite anion on the CTAB/VXC-72R/GCE surface and therefore significantly improve the sensitivity towards nitrite detection. As for comparison, the anionic surfactant could be unfavorable for nitrite anion enrichment, and it is confirmed by the observation of a lower peak current on SDS/VXC-72R/GCE than that on VXC-72R/GCE (Fig.1(c)). The Zeta potential can characterize the material surface charge, and then the Zeta potentials of various electrode materials are evaluated (Fig.1(d)). The Zeta potential of VXC-72R is 42.8 mV, but increases to 56.6 mV in CTAB/VXC-72R and sharply decreases to 7.62 mV in SDS/VXC-72R. The Zeta potential trend matches well with the electrochemical response sensitivity of nitrite on these electrode materials. This further confirms that cationic CTAB functionalization of VXC-72R brings more positive charges and facilitates the adsorption of the nitrite anion. In contrast, the existence of the anionic SDS reduces surface positive charges of VXC-72R and thus decreases the sensitivity towards the nitrite detection. Therefore, CTAB/VXC-72R/GCE could serve as a sensitive electrochemical sensor for nitrite.
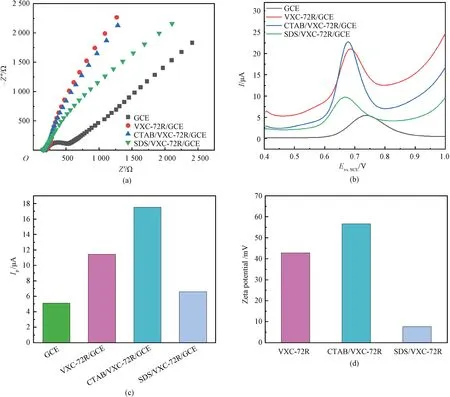
Fig.1 Electrochemical properties of various electrodes: (a) EIS plots in 0.1 mol/L KCl (with 5 mmol/L K3[Fe(CN)6]) solution; (b) DPV curves in PBS with 400 μmol/L nitrite; (c) currents; (d) Zeta potentials of electrode materials
2.2 Optimization of detection conditions
To obtain the optimal detection sensitivity of CTAB/VXC-72R/GCE towards nitrite, the determination conditions were optimized. Figure 2(a) shows the DPV curves of nitrite on CTAB/VXC-72R/GCE with different mass ratios of CTAB to VXC-72RmCTAB∶mVXC-72R. Obviously, the peak current significantly increases and the peak potential substantially negatively shifts from the ratio of 3∶1 to 2∶1, whereas the current slightly decreases from the mass ratio of 2∶1 to 1∶2. This phenomenon may be ascribed to that the cationic CTAB functionalization of VXC-72R would enrich the nitrite anion on the electrode surface and enhance the sensitivity, but the existence of excess CTAB could have a negative effect on the nitrite detection. Thus 2∶1 mass ratio of CTAB to VXC-72R is selected as the optimal mass ratio. Figure 2(c) shows the DPV curves of nitrite on CTAB/VXC-72R/GCE at different pH values. It can be seen that pH has little effect on peak potentials, but the oxidation peak current gradually decreases with pH increasing from 4.0 to 9.0 (Figs.2(c) and 2(d)). Thus pH of 4.0 is chosen for determination.
The catalyst is an important factor in sensitivity. Thus the optimal amount of CTAB/VXC-72R coated on GCE was examined. As shown in Figs.2(e) and 2(f), the peak current gradually increases with the increase of the catalyst ink volumeV, and almost reaches constant above 9 μL. Therefore, 9 μL catalyst ink (2 mg/mL) is used as the optimal amount in the following detection. Figures 2(g) and 2(h) show the effect of the accumulation timetto the DPV response of nitrite on CTAB/VXC-72R/GCE. It is shown that the peak current has little change from 30 s to 120 s, which indicates a fast accumulation rate. The accumulation time is chosen to be 30 s in this work.
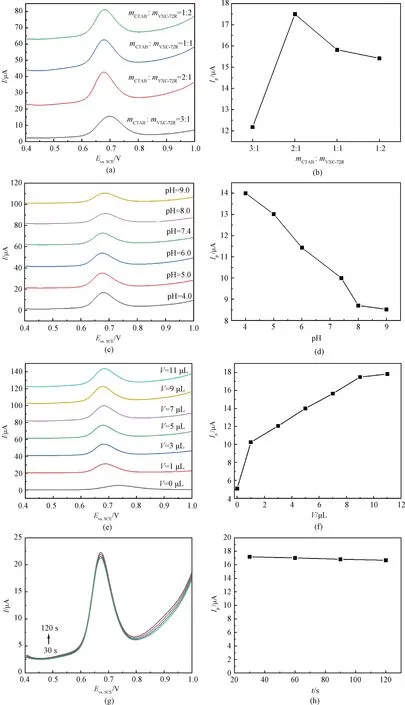
Fig.2 DPV curves of nitrite (400 μmol/L) measured on CTAB/VXC-72R/GCE and the corresponding relationship curves between peak currents and various experimental factors: (a) and (b) various mass ratios of CTAB to VXC-72R; (c) and (d) different pH values; (e) and (f) amounts of catalyst ink volume; (g) and (h) accumulation time
2.3 Dynamic investigation of nitrite on CTAB/VXC-72R/GCE
As shown in Fig.3(a), the oxidation peak current of nitrite gradually increases with the increasing scan rate (20-200 mV/s), accompanied by a positive-shift of the oxidation peak potential. The oxidation peak potentialEpis found to be linearly correlated to the logarithm of the scan rate lnv(Fig.3(b)), with the linear equation ofEp=0.020 49lnv+ 0.658 2 (R2=0.993 4).R2represents the correlation coefficient. Moreover, there is a linear relationship between the oxidation peak currentIpand the square root of the scan ratev1/2(Fig.3(c)), with the linear equation ofIp=7.416 4v1/2-15.334 7 (R2=0.997 3). This suggests that the oxidation reaction of the nitrite anion on CTAB/VXC-72R/GCE is a diffusion-controlled process[25].
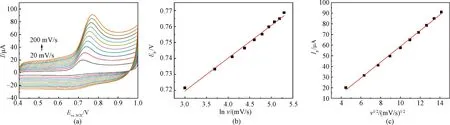
Fig.3 CV curves at different scan rates and their linear relationships: (a) CV curves of nitrite (400 μmol/L) measured on CTAB/VXC-72R/GCE at various scan rates ranging from 20-200 mV/s; (b) linear relationship between Ep and ln v; (c) linear relationship between Ip and v1/2
2.4 Linear titration of nitrite
Under the optimal detection conditions, electrochemical titration of nitrite was carried out by DPV. As shown in Fig.4(a), the oxidation peak current gradually increases with the increasing concentration of nitrite, and there is a good linear relationship between the oxidation peak currentIpand the concentration of nitriteCin the range of 0.5 to 5.0 μmol/L and 5.0 to 1 087.0 μmol/L (Fig.4(b)), with the linear equation ofIp=0.113 9C- 0.038 8 (R2=0.992 3) andIp= 0.039 4C + 0.606 4 (R2=0.998 6), respectively. The concerntration limit of detection(LOD) is estimated to be 0.30 μmol/L, revealing that the present CTAB/VXC-72R/GCE exhibits a comparable and even better sensitivity with cheaper electrode materials (Table 1). Therefore, the facile fabrication and the excellent sensing performance make CTAB/VXC-72R/GCE a promising electrochemical sensor for the nitrite detection in real samples.
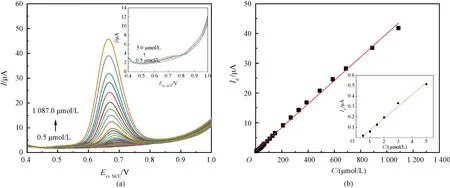
Fig.4 DPV plots for linear titrations and linear relationship plots: (a) DPV curves of nitrite (0.5-1087.0 μmol/L) on CTAB/VXC-72R/GCE in PBS (0.1 mol/L, pH=4); (b) linear relationship between Ip and C of nitrite (0.5-1087.0 μmol/L)
2.5 Stability, reproducibility and interference study
To evaluate the stability of CTAB/VXC-72R/GCE, the prepared electrode was continuously used for DPV detection of 400 μmol/L nitrite every five days for 30 d. After each test, the electrode surface was gently rinsed with deionized water, dried and exposed in air. As shown in Fig.5(a), the peak current value remains essentially constant after 30 d, indicating that CTAB/VXC-72R/GCE owns good stability.
In addition, five CTAB/VXC-72R/GCE electrodes were independently prepared by the same operation, and used for the nitrite detection to examine the reproducibility. It can be seen that the five different electrodes show almost reproducible DPV curves, with a relative standard deviation (RSD) of 2.6% (Fig.5(b)), demonstrating the high reproducibility of CTAB/VXC-72R/GCE.
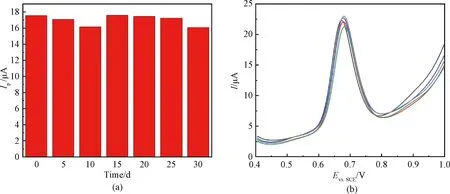
Fig.5 Stability and reproducibility of electrodes: (a) change of peak current at various storage time measured on the same CTAB/VXC-72R/GCE electrode; (b) DPV curves obtained from five CTAB/VXC-72R/GCE electrodes
To examine interference from potential coexisting species, the DPV detection of nitrite (400 μmol/L) was performed in the presence of 4 mmol/L NaNO3, CaCl2, KCl, MgSO4, NaCl, Na2SO4, ZnSO4, NH4Cl and glucose, and 0.5 mmol/L ascorbic acid (AA), uric acid (UA) and dopamine (DA), respectively. As shown in Fig.6, the change of peak currentIp/I0undergoes a small variation (<12%), indicating no significant interference from these potential coexisting substances.
2.6 Pickled cabbage and ham sample analysis
Pickled cabbage and ham samples were used to evaluate the practical application of the present CTAB/VXC-72R/GCE for the nitrite detection. The standard addition method was also performed to obtain the recovery rate. The analytical results are listed in Table 2. Nitrite is found to be present in these samples, with the original nitrite mass fraction of 11.80 mg/kg and 13.66 mg/kg in pickled cabbage and ham, respectively. Therefore, the mass fraction of nitrite in above cured foods meets the food safety requirement (< 20 mg/kg) for cured food in China. The recovery rates are varied in the range of 97.8% to 103.3%. This suggests that the present CTAB/VXC-72R/GCE could be used as a promising electrochemical sensor for nitrite detection in cured food analysis.
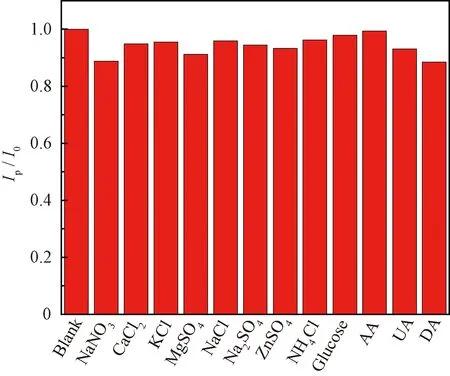
Fig.6 Change of peak current Ip/I0 of 400 μmol/L nitrite in absence and presence of various potential interfering species
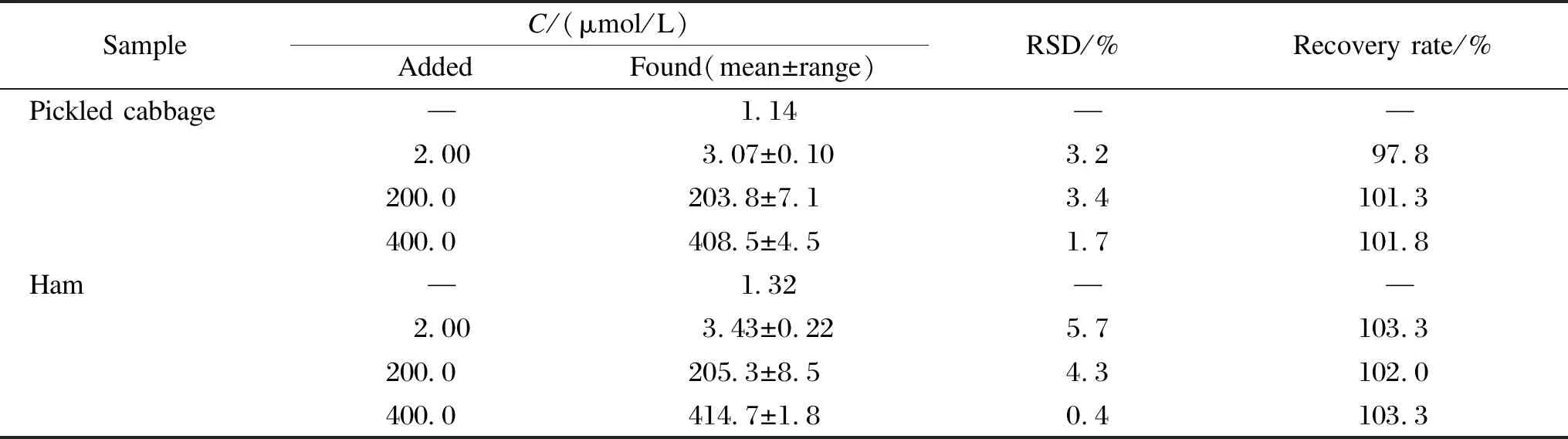
Table 2 Determination results in pickled cabbage and ham (n=3)
3 Conclusions
In conclusion, a highly sensitive electrochemical sensor for nitrite was facilely fabricated by employing carbon black VXC-72R functionalized with CTAB as a cheap electrode material. The positive charge on the electrode surface enhanced the enrichment of the nitrite anion and accelerated the electrochemical reaction, and therefore improved the sensitivity. The present CTAB/VXC-72R/GCE sensor owned good selectivity, reproducibility and stability, and the detection limit for nitrite was 0.30 μmol/L. Satisfactory recovery rates were obtained for the practical application in the cured food analysis.
杂志排行
Journal of Donghua University(English Edition)的其它文章
- Preparation of Cellulose Acetate Butyrate Porous Micro/Nanofibrous Membranes and Their Properties
- Concise Synthesis and Fertility-Promoting Activity of Thiamidol
- Calculation and Analysis of Acoustic Characteristics of Straight-Through Perforated Pipe Muffler Based on Multilayer Sound Absorbing Material
- Design and Structure Optimization of Plenum Chamber with Airfoil Baffle to Improve Its Outlet Velocity Uniformity in Heat Setting Machines
- Modeling of Micropores Drilling Force for Printed Circuit Board Micro-holes Based on Energy Method
- Image Retrieval Based on Vision Transformer and Masked Learning
