ZIF-8/改性聚丙烯腈电纺纳米纤维的制备及其高效去除水中孔雀石绿
2023-10-19石伟山王钊贵
徐 鹏 戴 伟 石伟山 邢 刚 王钊贵
王莎莎1 李 群1 游超群1 郝德君*,3
(1南京林业大学化学工程学院,南京 210037)
(2江苏艾津作物科技集团有限公司,南京 210000)
(3南京林业大学林学院,南京 210037)
0 Introduction
Nowadays,as the industry is leaping forward,organic dyes have become one of several major water pollutants,and the removal of the organic contaminants before discharged into the environment takes on a critical significance[1-4].Among several methods (e.g.,electrochemistry,photochemistry,biodegradation,and adsorption)[5-8],adsorption is one of the most attractive methods for its simplicity and efficiency[9-10].Numerous traditional adsorbents (e.g.,activated carbon,zeolites,clay,cellulosic materials,and graphene composites)have been extensively used to remove organic dyes from industrial wastewater over the past few years[11-13].However,some of the above adsorbents face some challenges (e.g.,low adsorption capacity,poor selectivity,low surface areas,and poor stability).Thus,to enhance the adsorption performance,efficient and high-capacity adsorbents of dye wastewater treatment should be explored in depth.
Metal-organic frameworks (MOFs) have widely served as adsorbents for plentiful pollutants from wastewater for their good micropore structure and sufficiently high physical stability[14-18].Typical water - stable MOF(e.g.,UiO-66,MIL-125,ZIF-7,and ZIF-8)exhibits excellent dye adsorption properties[19-23].However,some defects such as powdered morphology and fragility restrict their practical applications.To overcome the above defects,the integration of MOF powder into a functional support substrate is a promising approach,which makes it easy to recycle and reuse in dye adsorption[24-27].
The electrospun nanofibers have recently acted as outstanding substrates for loading MOF crystals for their high surface area,high porosity,and large surface area to volume ratio[28-31].Moreover,the surface of electrospun nanofibers can be easily modified by functional groups,thus assisting the initial coordination of metal irons to the surface of the substrate and facilitating the uniform growth of MOF particles[32-34].
Based on the above discussion,zeolitic imidazolate framework-8 (ZIF-8) crystals were loaded onto the carboxymethylated polyacrylonitrile electrospun nanofibers(PAN-COOH NFs)using anin-situgrowth method.To examine the adsorption performance of ZIF-8/PANCOOH NFs,malachite green (MG) was selected as the model organic dye for the batch adsorption experiments.Furthermore,the kinetic and isothermal models were studied by the experimental data,and the reusability of the ZIF-8/PAN-COOH NFs was evaluated through the cycling test.
1 Experimental
1.1 Material
Polyacrylonitrile (PAN,Mw=150 000) andN,Ndimethylformamide (DMF) were offered by Sigma-Aldrich Co.,Ltd.(Shanghai,China).2-Methylimidazole(2-MIM) was purchased from Shanghai Macklin Co.,Ltd.(Shanghai,China).Zinc nitrate hexahydrate(Zn(NO3)2·6H2O) was provided by Nanjing Chemical Reagent Co.,Ltd.(Nanjing,China).Malachite green(MG) was supplied by Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).
1.2 Synthesis of PAN-COOH NFs
1.5 g of PAN was dissolved in 13.5 g of DMF to prepare the electrospinning solution.Subsequently,the electrospinning solution was added to a syringe with a 21G needle at an applied voltage of 20 kV.The distance between the needle tip and the collector was 15 cm.After electrospinning,the obtained nanofibers were dried in a vacuum oven at 60 ℃for 12 h.The obtained PAN NFs were hydrolyzed in a 1.0 mol·L-1NaOH solution at 50 ℃for 1 h to convert surface nitrile groups into carboxylic groups,and marked as PAN-COOH NFs.The obtained PAN-COOH NFs were dried in a vacuum oven at 55 ℃for 12 h.
1.3 Preparation of ZIF-8/PAN-COOH NFs and ZIF-8/PAN NFs
0.02 g of PAN-COOH NFs was mixed with 0.587 g Zn(NO3)2·6H2O in 40 mL methanol for 24 h.Subsequently,2-MIM methanol solution (1.298 g,40 mL-1)was slowly added,and the mixture was oscillated at ambient temperature for 24 h.The samples obtained were washed with methanol for several times and then dried at 50 ℃for 6 h.Afterward,the above procedure was repeated twice,and the nanofibers were termed ZIF-8/PAN-COOH NFs.ZIF-8/PAN NFs were prepared using the same method,by substituting PAN NFs for PANCOOH NFs.
1.4 Batch adsorption experiment
The batch adsorption experiments were performed with 20 mL of MG solution by mixing 5 mg of PANCOOH NFs and ZIF-8/PAN-COOH NFs,respectively.The pH of the original MG solution was adjusted with 0.1 mol·L-1NaOH solution and 0.1 mol·L-1HCl solution.The batch adsorption experiments were performed to investigate the factors for the initial pH of MG solution (2-6),contact time (10-420 min),and initial MG concentration(50-1 500 mg·L-1)in terms of the adsorption of MG onto the ZIF-8/PAN-COOH NFs.All the batch adsorption experiments were performed at 298 K.The residual concentration of MG in the solutions was confirmed with a UV-Vis spectrophotometer atλmax=616 nm.The adsorption capacity at equilibrium(qe,mg·g-1),the adsorption capacity at each time (qt,mg·g-1),and the removal efficiency (R) of the MG were determined using the formula below[35]:
whereρ0,ρe,andρt(mg·L-1)represent initial concentration,concentration atttime,and equilibrium concentration of MG,respectively.Moreover,V(L) indicates the volume of the MG solution,andm(g) denotes the mass of the adsorbent.
1.5 Desorption and regeneration experiment
A certain quality of adsorbed ZIF-8/PAN-COOH NFs was immersed in a proportional amount of ethanol solution.Subsequently,the solution was placed in a shaker at 25 ℃for 12 h.The adsorbed dye (MG) was released into the ethanol solution.Afterward,the nanofibers were adequately washed with deionized water and then dried in the vacuum oven at 60 ℃.The adsorption-desorption recycling experiment was repeated nine times.
1.6 Characterization
The chemical structure of the samples was confirmed using Fourier transform infrared spectra (FT-IR,Nicolet,PerkinElmer,USA).The crystal structure of the obtained materials was characterized by X-ray diffractometer (XRD,Rigaku,Japan) equipped with CuKαradiation (λ=0.154 056 nm,45 kV,40 mA),and the scanning of XRD was running from 5° to 60° at the speed of 5 (°)·min-1.The surface morphologies and microstructure of the samples were observed under fieldemission scanning electron microscopy (SEM,Hitachi,Japan) with an acceleration voltage of 30 kV,and the elemental compositions of the composite nanofibers were examined using energy dispersive spectroscopy(EDS) apparatus based on the FE-SEM.The specific surface area was measured using an N2-sorption instrument (Quantachrome Autosorb-iQASIQ).Furthermore,the specific surface area was measured based on the Brunauer-Emmett-Teller(BET)method.
2 Result and discussion
2.1 Micro-structure of ZIF-8/PAN-COOH NFs
The morphology and structure of the fabricated nanofibers were examined using SEM.As depicted in Fig.1a and 1d,PAN NFs were intact,smooth,and uniform with an average diameter of about 200 nm.After alkaline hydrolysis,the surface roughness of PAN -COOH NFs slightly increased (Fig.1b and 1e).Fig.1c and 1f illustrate the morphology of ZIF-8/PAN-COOH NFs.As depicted in these figures,the ZIF-8 particles with rhombic dodecahedral shapes successfully grew on the surface of PAN-COOH NFs.The EDS mapping was further examined (Fig.2a-2c),thus indicating that ZIF-8/PAN-COOH NFs had a high content of Zn elements (mass fraction of 9.1%) and distributed evenly on the surfaces of the nanofibers.The above results confirmed that the ZIF-8 particles were successfully loaded onto the nanofiber surfaces.
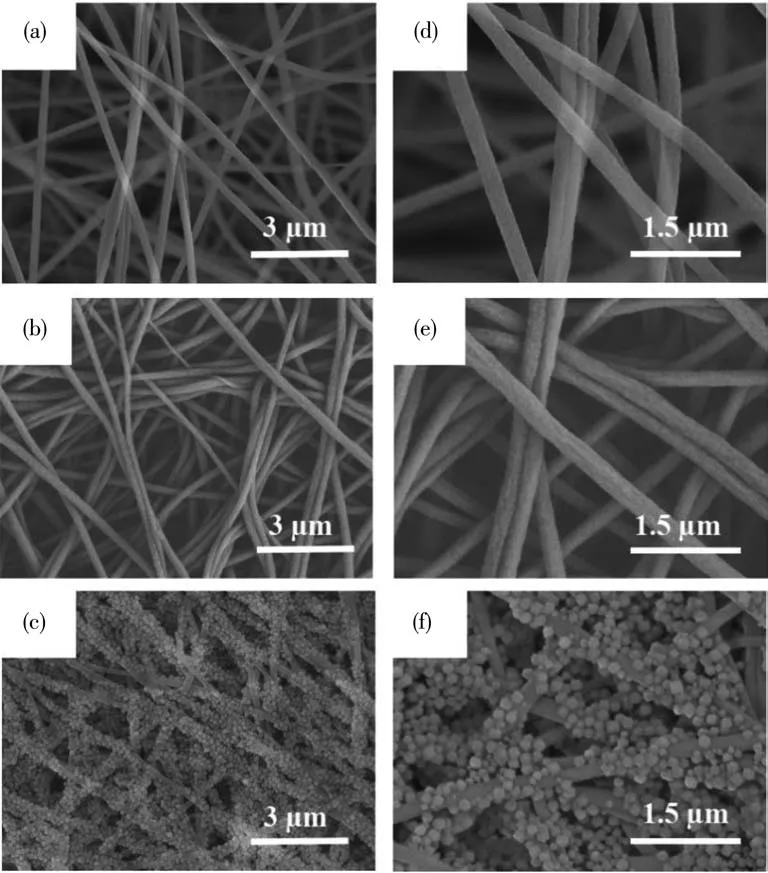
Fig.1 SEM images of the nanofibers:PAN NFs(a,d);PAN-COOH NFs(b,e);ZIF-8/PAN NFs(c,f)
N2adsorption-desorption measurements were performed to characterize the pore structures of the samples.Fig.3a illustrates N2adsorption-desorption isotherms of PAN NFs and ZIF-8/PAN-COOH NFs.The specific surfaces of them were calculated using the BET method.The specific surface area of PAN NFs was measured as 42 m2·g-1,while the specific surface area of the nanofibers coated with the ZIF-8 particles was obtained as 256 m2·g-1(Fig.3b).The significantly increased specific area of ZIF- 8/PAN-COOH NFs would create more active sites for adsorption.
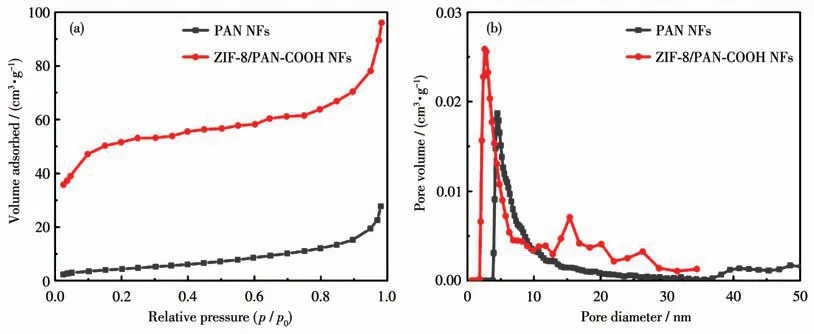
Fig.3 N2 adsorption isotherms(a)and pore size distributions(b)of PAN NFs and ZIF-8/PAN-COOH NFs
2.2 Material properties analysis
The crystalline phases of the synthesized nanofibers were examined through XRD (Fig.4).PAN NFs and PAN-COOH NFs indicated a strong diffraction peak at 16.9°,corresponding to PAN crystals.The XRD patterns of ZIF-8 and ZIF-8/PAN-COOH NFs showed intense diffraction peaks at 7.12° ,10.22° ,12.56°,14,54°,15.18°,16.28°,and 17.86°,respectively,corresponding to the (011),(002),(112),(022),(013),and (222) planes of the ZIF-8,which revealed the growth of ZIF-8 particles in the ZIF-8/PAN-COOH NFs.
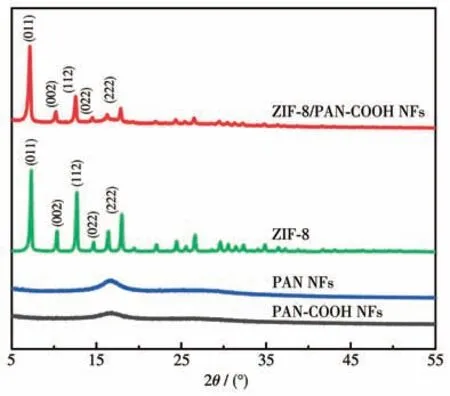
Fig.4 XRD patterns of PAN NFs,PAN-COOH NFs,and ZIF-8/PAN-COOH NFs
The interaction between ZIF-8 particles and PANCOOH NFs was characterized through the FTIR analysis.As depicted in Fig.5,the comparison of the spectrum of original PAN NFs revealed that PAN-COOH NFs exhibited two different absorption peaks at 1 650 and 3 420 cm-1,corresponding to the stretching vibration of C=O and O—H in carboxyl functional groups.The peaks at 954,1 175,and 1 309 cm-1belong to the stretching vibration of the imidazole ring.The typical peaks at 693 and 759 cm-1belong to the Zn—N and Zn—O stretching vibration,respectively.The band at 1 453 cm-1belong to CH2rocking and bending vibrations.The adsorption band at 2 243 cm-1belong to the —CN vibration.As revealed by the emergence of the new characteristic bands of imidazole ring and ZIF-8 in the spectra of the ZIF-8/PAN-COOH NFs,the above substances existed in the sample.
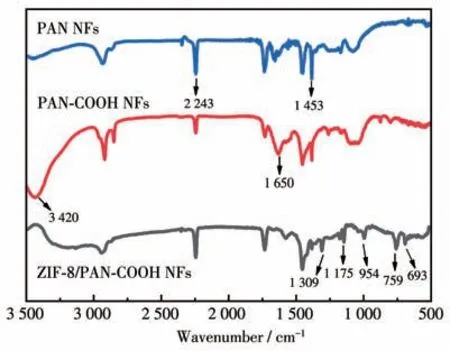
Fig.5 FTIR spectra for PAN NFs,PAN-COOH NFs,and ZIF-8/PAN-COOH NFs
2.3 Adsorption evaluation for ZIF-8/PAN-COOH NFs
The cationic dye (MG) was employed as the adsorption object to optimize the adsorption capacity of ZIF-8/PAN-COOH NFs.Batch adsorption experiments were conducted to assess the adsorption behavior.
2.3.1 Effect of pH
The pH of the solution is an essential parameter influencing the adsorption process of dye molecules.Since the color of the MG solution changed under the alkaline condition,the pH values tested in the experiment varied in the range of 2-6.As depicted in Fig.6,the removal efficiency of MG by ZIF-8/PAN-COOH NFs increased with the increase of pH from 2 to 4 and leveled off in the scope of 5-6.Under high acidic conditions,ZIF-8 on the surface of ZIF-8/PAN-COOH NFs was dissolved,thus resulting in low removal efficiency.With the increase in pH,the surface charge of ZIF-8/PAN-COOH NFs became more negative,and the electrostatic interaction between MG and the nanofibers also became stronger[36].As revealed by the above results,the optimal pH value of ZIF-8/PAN-COOH NFs for MG adsorption was examined as 5.
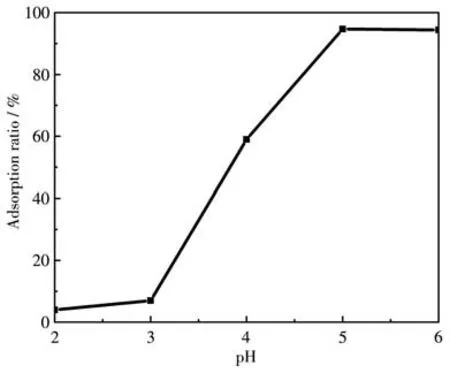
Fig.6 Effect of pH on the adsorption of MG onto ZIF-8/PAN-COOH NFs
2.3.2 Adsorption kinetics
The adsorption kinetics was studied to gain more insights into the adsorption mechanism and adsorption rate of PAN-COOH NFs and ZIF-8/PAN-COOH NFs for MG.Fig.7a and 7d illustrate the effect of contact time on the MG adsorption by PAN-COOH NFs and ZIF-8/PAN-COOH NFs,respectively.The adsorption capacity increased with the increase in the contact time.As revealed by the results,the adsorption capacity of PAN-COOH NFs reached equilibrium after 400 min,while that of ZIF-8/PAN-COOH NFs reached equilibrium after 300 min.
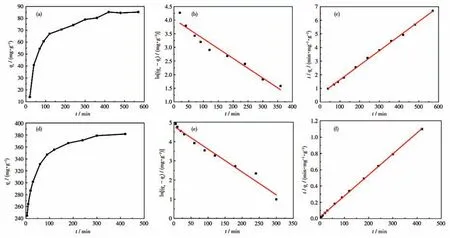
Fig.7 Adsorption kinetic curves of MG on PAN-COOH NFs and ZIF-8/PAN-COOH NFs(a,d),and corresponding pseudo-first-order kinetic model(b,e)and pseudo-second-order kinetic model(c,f)
To analyze the adsorption kinetics in-depth,the kinetic data were fitted by the pseudo-first-order and pseudo-second-order equations.The equations of the two dynamical models are generally expressed as[37-38]:
whereqt(mg·g-1) denotes the adsorption capacity of MG at any contact timet(min);qe(mg·g-1) represents the adsorption capacity of MG adsorbed at the equilibrium time;k1(min-1) andk2(g·mg-1·min-1) express the equilibrium rate constant of the pseudo-first-order and pseudo-second-order kinetic,respectively.Table 1 lists the adsorption kinetics parameters of MG dye molecules using PAN-COOH NFs and ZIF-8/PAN-COOH NFs,and Fig.7b,7c,7e,and 7f present the corresponding graphs.
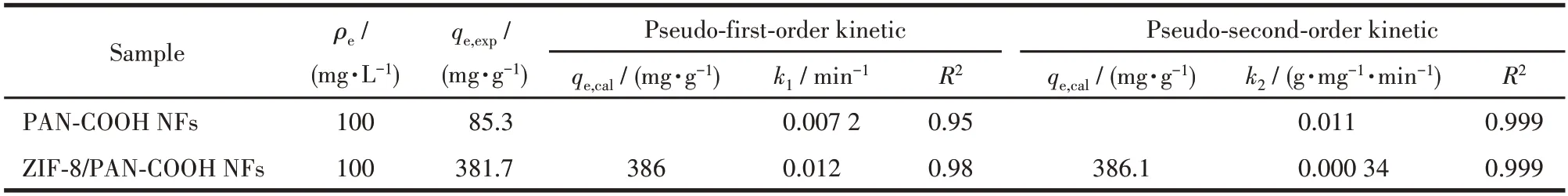
Table 1 Kinetic parameters for the adsorption of MG onto PAN-COOH NFs and ZIF-8/PAN-COOH NFs
The result suggested that the coefficients of correlation (R2) were found to be higher for the pseudo-second-order kinetics than those of the pseudo-first-order kinetic model,establishing that the adsorption to MG conformed to the pseudo-second-order kinetic equation.The above results confirmed that the adsorption process of PAN-COOH NFs and ZIF-8/PAN-COOH NFs arose from the chemical interactions between the polar functional groups on the surface of nanofibers and MG molecules.
2.3.3 Adsorption isotherm
Adsorption isotherms take on great significance in describing the interaction between the MG molecules and ZIF-8/PAN-COOH NFs.The adsorption equilibrium isotherm was studied for the Langmuir isotherm and Freundlich isotherm models based on the above obtained experimental data.The Langmuir isotherm model was adopted to assume that the adsorption of adsorbate onto the adsorbent was monolayer adsorption.The Freundlich isotherm model was employed to express that the adsorption of the adsorbate onto the adsorbent was multilayer adsorption.The linear forms of Freundlich isotherm and Langmuir isotherm are written as[39-40]:
whereqm(mg·g-1) denotes the maximum adsorption capacity of MG;qe(mg·g-1) represents the adsorption capacity of MG at the equilibrium time;ρe(mg·L-1)expresses the concentration MG solution at equilibrium;KL(L·mg-1) represents the Langmuir constant,correlated with the adsorption bonding energy;KF(mg1-1/n·L1/n·g-1) is the Freundlich constant;nis the adsorption strength;1/nis the heterogeneity factor of the adsorbent.
Table 2 lists the isotherm obtained constants for the Langmuir isotherm model and Freundlich isotherm model fitting,and the corresponding curves are presented in Fig.8.As depicted in the figure,the linear correlation coefficient (R2) in the Langmuir isotherm model was higher than the Freundlich isotherm model,thus suggesting that it was more suitable for the Langmuir model.It can be inferred that the adsorption of MG by the ZIF-8/PAN-COOH NFs tend to be mono-layer adsorption.The maximum adsorption capacity of the ZIF-8/PAN-COOH NFs for the adsorption of MG was obtained as 3 604 mg·g-1for MG.Table 3 presents different adsorbents for removing dyes from aqueous media.
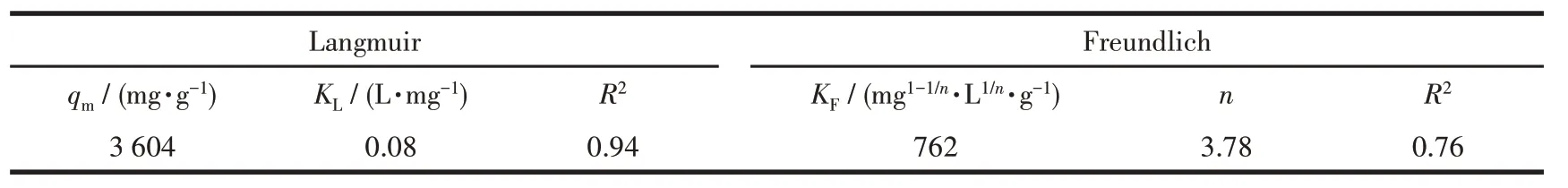
Table 2 Langmuir and Freundlich isotherm parameters using ZIF-8/PAN-COOH NFs for the adsorption of MG
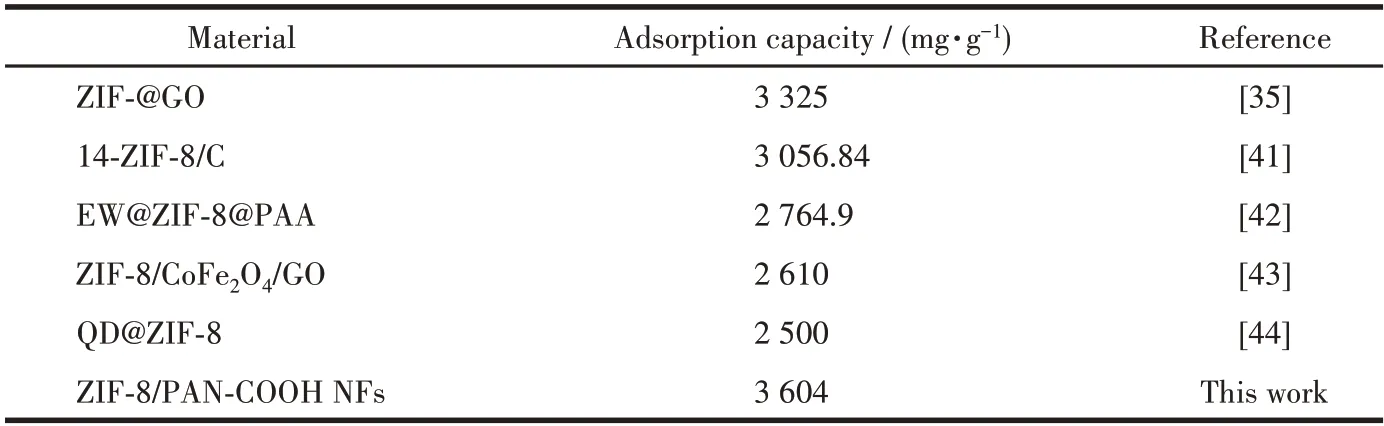
Table 3 Comparison of dye adsorption capacities of numerous ZIF composites as adsorbents
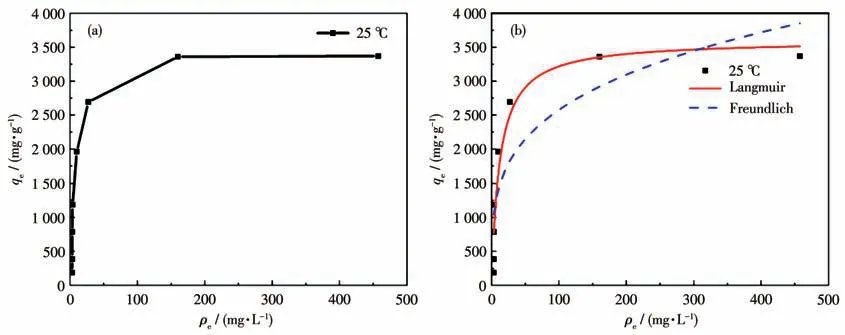
Fig.8 Adsorption isotherm of MG on ZIF-8/PAN-COOH NFs at 25 ℃(a);Corresponding Langmuir and Freundlich isotherm models(b)
2.3.4 Recyclability experiments
The recyclability of adsorbents has been found as the key to their practical application.Accordingly,the renewability of the ZIF-8/PAN-COOH NFs was investigated for MG adsorption.The ethanol washing method was employed to desorb MG for its simplicity and maneuverability.As depicted in Fig.9,after nine-cycle regeneration,ZIF-8/PAN-COOH NFs still exhibited 96%of the first adsorption capacity,thus revealing that ZIF-8/PAN-COOH NFs could be easily regenerated,and the adsorption capacity was nearly constant.
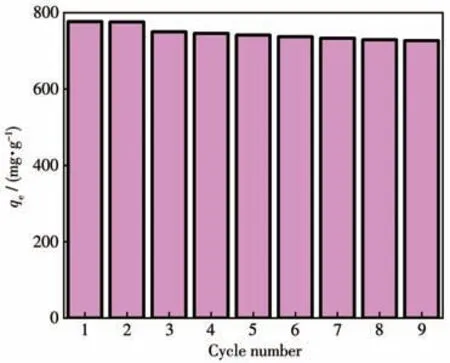
Fig.9 Recycling efficiency of ZIF-8/PAN-COOH NFs for MG removal
3 Conclusions
In this study,ZIF-8 particles were successfully immobilized on electrospun nanofibers compactly and uniformly using thein-situgrowth method.Furthermore,thein-situgrowth of ZIF-8 particles on the surface of the carboxymethylated PAN NFs led to an increase in the specific surface area of the original nanofibers.The ZIF-8/PAN-COOH NFs exhibited high adsorption capacity for MG (3 604 mg·g-1).As revealed by the results,the equilibrium data matched the Langmuir isothermal model,and the kinetic equilibrium data were consistent with the pseudo-second-order kinetic equation.Furthermore,the ZIF-8/PAN-COOH NFs exhibited high reusability.The above features enable the synthetic sample to be an effective and promising adsorbent in the removal of MG from wastewater.
Acknowledgements: This investigation was supported by the Jiangsu Province Natural Science Foundation (Grant No.BK20130969).
