Preparation and application of a novel monoclonal antibody specific for the heat shock protein 60 of Lawsonia intracellularis
2023-09-16XlAONingYunyunLlJiannanCHENChangfengLlNHuixingFANHongjie
XlAO Ning,LÜ Yun-yun,Ll Jian-nan,CHEN Chang-feng,LlN Hui-xing,FAN Hong-jie,
1 MOE Joint International Research Laboratory of Animal Health and Food Safety,College of Veterinary Medicine,Nanjing Agricultural University,Nanjing 210095,P.R.China
2 Jiangsu Co-innovation Center for the Prevention and Control of Important Animal Infectious Diseases and Zoonoses,Yangzhou University,Yangzhou 225009,P.R.China
Abstract
Porcine proliferative enteropathy (PPE),an important infectious disease in pig production caused by an obligate intracellular bacterium Lawsonia intracellularis,is commonly associated with diarrhea and reduced weight gain in growing pigs widespread.An accurate method for detecting L.intracellularis is particularly important for preventing and controlling PPE.Heat shock protein 60 (Hsp60) is an immunodominant bacterial antigen found in all eukaryotic and prokaryotic organisms.Thus,the purpose of the current investigation was to produce a novel L.intracellularis Hsp60 monoclonal antibody (mAb) useful for immunodiagnostics.Three hybridomas secreted anti-Hsp60 termed 3E5,4E2,and 9G6 were generated,and the titers of ascitic fluids of 3E5,4E2,9G6 were 1:1 024 000,1:2 048 000 and 1:2 048 000,respectively.The Western blotting analysis demonstrated that recombinant Hsp60 (rHsp60) was recognized by mAbs 3E5,4E2 and 9G6.Subsequently,analyses of specificity showed all the mAbs were highly specific to L.intracellularis while could not significantly react with other enteric bacteria commonly found in the ileum of pigs,such as Escherichia coli,Salmonella Choleraesuis,Salmonella Typhimurium,and Brachyspira hyodysenteriae.Furthermore,the mAbs were useful for detecting L.intracellularis in the infected monolayer cells and histological sections of the ileum from PPE-affected pigs.Our research will provide a foundation for the development of immunological diagnostic tests.
Keywords: porcine proliferative enteropathy,Lawsonia intracellularis,monoclonal antibody,immunodiagnostics,heat shock protein 60
1.lntroduction
Lawsoniaintracellularis,an obligate intracellular bacteria,is the causative agent of porcine proliferative enteropathies (PPE) in pigs (McOristetal.1993).The disease has a worldwide distribution and occurs commonly in all pig-raising regions and in all farm management systems,which generates serious economic losses in the swine industry worldwide by reducing the feed conversion efficiency by up to 50% and the average gain weight from 17 to 84% compared to uninfected pigs (Gogolewskietal.1991; McOrist 2005).The cost per infected pig is around 10 USD,soL.intracellularisis a huge problem for the swine industry worldwide (Jensen 2006; Jacobsonetal.2010).Clinical observation may include diarrhea,decreased weight gain,reduced feed efficiency,and increased mortality rates (Jacobsonetal.2010; Vannucci and Gebhart 2014).The two major forms of proliferative enteropathies (PE) in pigs are recognized: proliferative hemorrhagic enteropathy (PHE) and porcine intestinal adenomatosis (PIA).Bloody diarrhea and sudden death of finishing pigs or breeder pigs aged 4 to 12 months,with high morbidity and mortality,known as PHE,and chronic mild diarrhea and weight loss in grownfinishing pigs aged 8 to 20 weeks,known as PIA (Kimetal.2017; Obradovic and Wilson 2020).
In most veterinary diagnostic laboratories,the hematoxylin and eosin stain (H&E),warthin-starry (WS) silver stain,immunohistochemistry (IHC) and immunofluorescence (IF) are common evaluation methods for theL.intracellularisin the formalin-fixed samples of intestines (Guedesetal.2002; Huertaetal.2003; Jensenetal.2010).IF and IHC have been proven to be the most reliable methods for the postmortem diagnosis of PE (Huertaetal.2003; Jensenetal.2010).Previous studies indicated that the specificity and sensitivity of immunological staining using a monoclonal antibody (mAb) againstL.intracellulariswas superior to that of H&E and WS silver stains in pigs with histologic lesions of PPE (Guedesetal.2002; Guedes and Gebhart 2003c).However,the availability of specific antibodies is demanded in immunological tests.
To our knowledge,invitropropagation ofL.intracellularisis extremely difficult and remains a major research hurdle.Only few laboratories around the world could isolate and maintain their ownL.intracellularisstrain (Guedes and Gebhart 2003a).It is necessary to obtain specific antibodies againstL.intracellularisis as a diagnostic tool to evaluate infection in the infected tissues and monolayer cell culture to provide an accurate diagnostic method of PPE and obtain new bacterial isolates.Thus,the objective of the present study is to generate mAb againstL.intracellularisHsp60 that could confirm the organism directlyinvitromonolayer cell cultures and the intestine for diagnostic purposes.
2.Materials and methods
2.1.Bacterial strains,plasmids and cell lines
The live attenuated vaccine Enterisol®ileitis (L.intracellularisstrain B3903) used in this study was purchased from Boehringer Ingelheim Vetmedica,Germany.Escherichiacoli(CAU0751),SalmonellaCholeraesuis (S.Choleraesuis,C V C C 2 1 3 9 ),a n dSalmonellaTy p h i m u r i u m (S.Typhimurium,CVCC2220) were purchased from the China Veterinary Culture Collection Center (CVCC).Brachyspirahyodysenteriaewas courtesy of the Shanghai Animal Disease Prevention and Control Center,China.ISA206 adjuvant was courtesy of Jiangsu Academy of Agricultural Sciences,China.Myeloma cell line SP2/0 (stored in our laboratory) and mouse fibroblast cells (McCoy,ATCC CRL-1696) were grown in DMEM/high glucose (Gibco,USA) with 10% fetal bovine serum (FBS,Gibco,USA) in a humidified incubator in an atmosphere of 5% CO2and 95% air at 37°C.Growth ofL.intracellulariswas performed as described (Lawsonetal.1993).In brief,McCoy monolayers were co-cultured withL.intracellularis(the vaccine dissolved in sterile diluent as directed) at an MOI of 100 and then incubated in an atmosphere of 83.2% nitrogen (N2),8.8% carbon dioxide (CO2),and 8% oxygen (O2) for 7 days at 37°C.The number of avirulentL.intracellularisin the vaccine was calculated according to previous study (Guedes and Gebhart 2003b),that the number of theL.intracellularisin vaccine contains 5.3×105organisms mL–1.
2.2.Expression,purification and identification of Hsp60 protein
Heat shock protein 60 (Hsp60) gene (No.AB218756.1)was amplified from genomic DNA ofL.intracellularisby PCR using the specific primers F1 5´-CGGAATTCATGGC TTCTAAAGAAATC-3´ (EcoRI site underlined) and R1 5´-GCGTCGACCTAGTACATA CCGTCCAT-3´ (SalI site underlined).Subsequently,the PCR product was ligated into the prokaryotic expression vector pET-28a(+).The recombinant plasmids were verified by restriction enzyme digest and sequencing and then transformed intoE.coliBL21 (DE3) competent cells.Expression of the recombinant Hsp60 (rHsp60) protein was induced with 1 mmol L–1isopropyl-β-D-thiogalactopyranoside (IPTG,Sigma,USA) at 16°C for 18 h,and then,the rHsp60 protein was detected by SDS-PAGE after purification with a highaffinity Ni-NTA resin column (GE Healthcare Life Sciences,USA),and the concentration of proteins was calculated with the Bicinchoninic Acid Assay Kit (BCA Kit; Beyotime,China).Western blotting was used to detect the reactivity of pig anti-serum against the rHsp60 protein; purified rHsp60 protein and His-tag protein were subjected to SDS-PAGE,and transferred to a PVDF membrane.The membrane was blocked with 5% skim milk in TBST (20 mmol L–1Tris-HCl,150 mmol L–1NaCl,0.1% Tween-20,pH 7.4) for 2 h at 37°C then incubated with anti-L.intracellularispositive antibody (the antibody againstL.intracellularisobtained from SVANOVIR®L.intracellularis/Ileitis-Ab,Germany) overnight at 4°C.The membrane was washed three times with TBST then incubated with HRP-goat anti-pig IgG (1:5 000) for 1 h at 37°C.After three washes with TBST,the target protein band was visualized using ECL-Enhanced Chemiluminescence Reagents (ZOMANBIO,Beijing,China).
2.3.Preparation and purification of mAbs against Hsp60
To prepare mAbs against Hsp60,purified rHsp60 was completely emulsified with an equal volume of ISA206 adjuvant three times to immunize 6-week-old BALB/c mice (Yangzhou University Comparative Medical Center,China).The mouse with higher titer was injected intraperitoneally as the further immunization with 20 μg rHsp60 fusion protein mixed with 100 μL PBS.Three days later,splenocytes were isolated from the immunized mouse and fused with SP2/0 murine myeloma cells at a ratio of 8:1 with the polyethylene glycol (PEG 4000,Sigma,USA),then the fusion cells were distributed into 96-well cell culture plates in which feeder cells had been added.These cells were cultured in DMEM with 20% FBS/HAT medium at 37°C in an incubator containing 5% CO2.Five days later,the 50% medium in cell culture plates was substituted with the fresh one.Ten days later,the positive hybridomas were identified by indirect enzyme-linked immunosorbent assay (iELISA),described in detail in the next section.The positive clones with high titer were subcloned three times by limiting dilution,stable hybridomas were injected into the abdominal cavity of paraffin-primed BALB/c mice to produce ascites.
2.4.Characterization of the anti-Hsp60 mAbs
The mAbs from ascetic fluid were purified using the combination of saturated ammonium sulfate precipitation and HiTrapTMProtein G HP Column (GE,USA).Determination of the mAbs isotype was identified according to the instruction of mouse Monoclonal Antibody Isotyping Kit (Thermo Scientific,USA).The titers of the ascites fluid were determined by iELISA.Briefly,the 96-well plate was coated with 5 μg/well of purified rHsp60 protein overnight at 4°C.The wells were blocked with 5% skim milk in PBS for 2 h at 37°C.Then,serial twofold dilutions (1:1 000 to 1:214×1 000) of ascites fluid were added to the wells in triplicate for 1 h at 37°C,respectively.Meanwhile,normal ascites (generated by SP2/0 cells) were used as a negative control.The plate was incubated with HRP-conjugated goat anti-mouse IgG (1:5 000; Abmart,China) for 1 h at 37°C,and wells were incubated with 3,3´,5,5´-tetramethylbenzidine (TMB) for 15 min at room temperature,color development was stopped with 2 mol L–1H2SO4.The OD450nmwas measured by an ELISA Plate Reader (Bio-Rad Benchmark Plus,USA).The cut-off value was confirmed when the OD450nmratio (OD450nmof monoclonal antibody/OD450nmof unimmunized mouse serum) was above 2.1.The titers of mAbs were defined as the highest dilution that yielded a net OD450nmvalue greater than the calculated cut-off.Subsequently,Western blotting was used to detect the reactivity of rHsp60 with mAbs.The reference described the same method but with some modifications (Fuetal.2020).Briefly,purified rHsp60 and His-tag protein were subjected to SDS-PAGE,and then the target bands were transferred to PVDF membranes.The membrane was blocked with 5% skim milk in TBST (20 mmol L–1Tris-HCl,150 mmol L–1NaCl,0.1% Tween-20,pH 7.4) for 2 h at 37°C then incubated with mAbs (1:500 diluted with PBS),polyclonal antibody (pAb,the mouse serum against Hsp60 protein,1:1 000 diluted with PBS) and normal ascites at 4°C overnight,respectively.The membrane was washed five times with TBST then incubated with HRP-goat antimouse IgG for 1 h at 37°C.After five washes with TBST,the target protein band was visualized using enhanced ECL chemiluminescence reagents (ZOMANBIO,Beijing,China).
2.5.Determination of specificity of mAbs
The specificity of mAbs against Hsp60 was determined by iELISA and Western blotting.Briefly,the bacterial pellets ofL.intracellularis,E.coli,S.Choleraesuis,S.Typhimurium andB.hyodysenteriaewere harvested and lysed immediately in RIPA buffer (Beyotime,China).Lysate bacterial protein quantification was performed with a BCA Kit.For Western blotting,an equal amount of each sample (L.intracellularis,E.coli,S.Choleraesuis,S.Typhimurium andB.hyodysenteriae) was separated by SDS-PAGE and the protein bands were transferred onto PVDF membranes.After blocking with 5% nonfat dry milk in TBST (20 mmol L–1Tris-HCl,150 mmol L–1NaCl,0.1% Tween-20,pH 7.4) for 2 h at 37°C,the membranes were incubated with mAbs and pAb at 4°C overnight,followed by HRP-conjugated antimouse IgG and visualized using enhanced ECL chemiluminescence reagents (ZOMANBIO,Beijing,China).The second method for identifying the specificity of the mAbs was iELISA,the equal amount of bacterial proteins (L.intracellularis,E.coli,S.Choleraesuis,S.Typhimurium andB.hyodysenteriae) as coating antigen was diluted by carbonate buffer and added into the micro-titer plates,and the following steps are consistent with the protocol of ELISA.Meanwhile,normal ascites (generated by SP2/0 cells) were used as a negative control.
2.6.lmmunofluorescence assay to monitor L.intracellularis infection in vitro
An immunofluorescence (IF) assay using mAbs against Hsp60 was performed to evaluate the monolayer of McCoy cells infected withL.intracellularis.Briefly,McCoy cells were seeded on confocal laser dishes and cultured overnight to 30% confluence.The cells were infected withL.intracellularisat a multiplicity of infection (MOI) of 100 for 7 days in a tri-gas incubator with 83.2% N2,8.8% CO2and 8% O2at 37°C (Gene Science AG300 plus,USA) according to methods described by Vannuccietal.(2012).Uninfected monolayers were negative controls.The samples were fixed with 4% (v/v) paraformaldehyde in PBS at room temperature for 15 min then the dishes were rinsed and the cells were permeabilized with 0.3% TritonX-100 for 10 min.The fixed cells were incubated with mAbs and pAb at 4°C overnight and then rinsed in PBS buffer (5×5 min).The dishes were incubated with goat anti-mouse IgG AF594 second antibody (same as Alexa Fluor 594,1:500,Abmart,China) for 1 h at 37°C and then washed in PBS buffer (5×5 min).Finally,the nuclei were dyed with DAPI (4,6-diamidino-2-phenylindole dihydrochloride; Solarbio,China) and the dishes were immediately examined by Confocal Laser Scanning Microscope (Nikon ECLIPSE Ti,Japan).
2.7.lmmunofluorescence and immunohisto- chemistry to evaluate L.intracellularis infection in vivo
The ileum used in this study,collected from commercial pigs in a slaughterhouse in Jiangsu Province,China,was suspected of PPE according to the results of PCR.To identifyL.intracellularisantigen in paraffin-embedded intestinal tissues,IF and IHC assay were performed by using mAbs (3E5,4E2,9G6,1:100 diluted with PBS) and pAb (1:200 diluted with PBS) againstL.intracellularis-Hsp60 as primary antibodies according to a previous study (Szczotkaetal.2011; Huanetal.2017; Zhangetal.2018),and uninfected ileum was as a negative control.
2.8.Statistical analysis
The statistical analysis was performed using GraphPad Prism 8.0.1.Unpaired Student’st-test was used for the statistical analysis of the data (such as the reactivity of mAbs).AP-value of less than 0.05 was considered to indicate statistical significance.*,P<0.05;**,P<0.01;***,P<0.001.All experiments were performed at least three independent experiments.
3.Results
3.1.Expression and purification of the recombinant fusion protein Hsp60
SDS-PAGE results revealed that the recombinant Hsp60 protein was successfully expressed in BL21 (DE3) cells after being induced by IPTG (Fig.1-A,lane 3),and the size of the Hsp60 following the expected size,60.3 kDa.Further research found the target protein in the supernatant of bacterial lysates (Fig.1-B,lane 2),and the purity of the purified Hsp60 protein was approximately 90% after purification by a GSTRAP HP column (Fig.1-B,lane 4).Western blotting demonstrated that the anti-L.intracellularispositive serum at a 1:200 dilutions specifically recognized the Hsp60 protein (Fig.1-C).
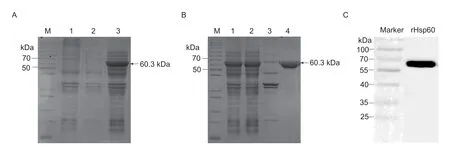
Fig.1 Expression and purification of recombinant heat shock protein 60 (Hsp60) of Lawsonia intracellularis.A,SDS-PAGE analysis of pET28a-Hsp60 transfected BL21 (DE3) cells.M,protein molecular weight marker; lane 1,Escherichia coli BL21 with empty vector pET-28a(+); lane 2,uninduced E.coli BL21 with pET-28a-Hsp60; lane 3,pET28a-Hsp60 induced by IPTG.B,purification of Hsp60 protein.M,protein molecular weight marker; lane 1,IPTG-induced pET28a-Hsp60 transfected cells prior to sonication; lane 2,supernatant of pET28a-Hsp60 transfected cells post sonication; lane 3,the precipitates of bacterium solution; lane 4,purified rHsp60 protein.C,Western blotting of E.coli expressed pET28a-Hsp60,probed with pig anti-L.intracellularis serum.
3.2.Production and screening of mAbs against Hsp60 of L.intracellularis
Three cell lines of hybridoma that secreted anti-Hsp60 mAbs termed 3E5,4E2,and 9G6 were established by the hybridoma technique.Western blotting results revealed that the mAbs were successfully purified from mouse ascites (Fig.2-A).The heavy and light chain of the mAbs (3E5,4E2 and 9G6) were IgG1 and k,respectively (55 and 25 kDa) (Fig.2-B).All hybridomas could stably secrete anti-Hsp60 antibodies at high titer for more than 10 passages.The titers (3E5,4E2,and 9G6) of ascites fluid were 1:1 024 000,1:2 048 000 and 1:2 048 000,respectively (Fig.2-C).
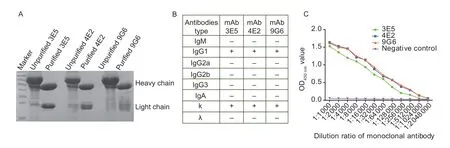
Fig.2 Preparation and identification of the monoclonal antibodies against Hsp60 of Lawsonia intracellularis.A,purification of the mAbs were determined by SDS-PAGE.B,the subtype of the mAbs (3E5,4E2 and 9G6) were IgG1 and k,respectively.C,titers of ascites fluid were evaluated using indirect enzyme linked immune sorbent assay.Data were presented as mean±SE (n=3).***,P<0.001.
3.3.Analysis of mAbs by Western blotting
As shown in Fig.3-A–D,Western blotting results demonstrated that all mAbs (3E5,4E2 and 9G6) and pAb reacted well with Hsp60,whereas Hsp60 protein could not recognize with normal ascites (Fig.3-E).Those results indicated that purified Hsp60 could be probed with mAbs,but not with control normal ascites,confirming that Hsp60 contains specific epitopes recognized by mAbs.
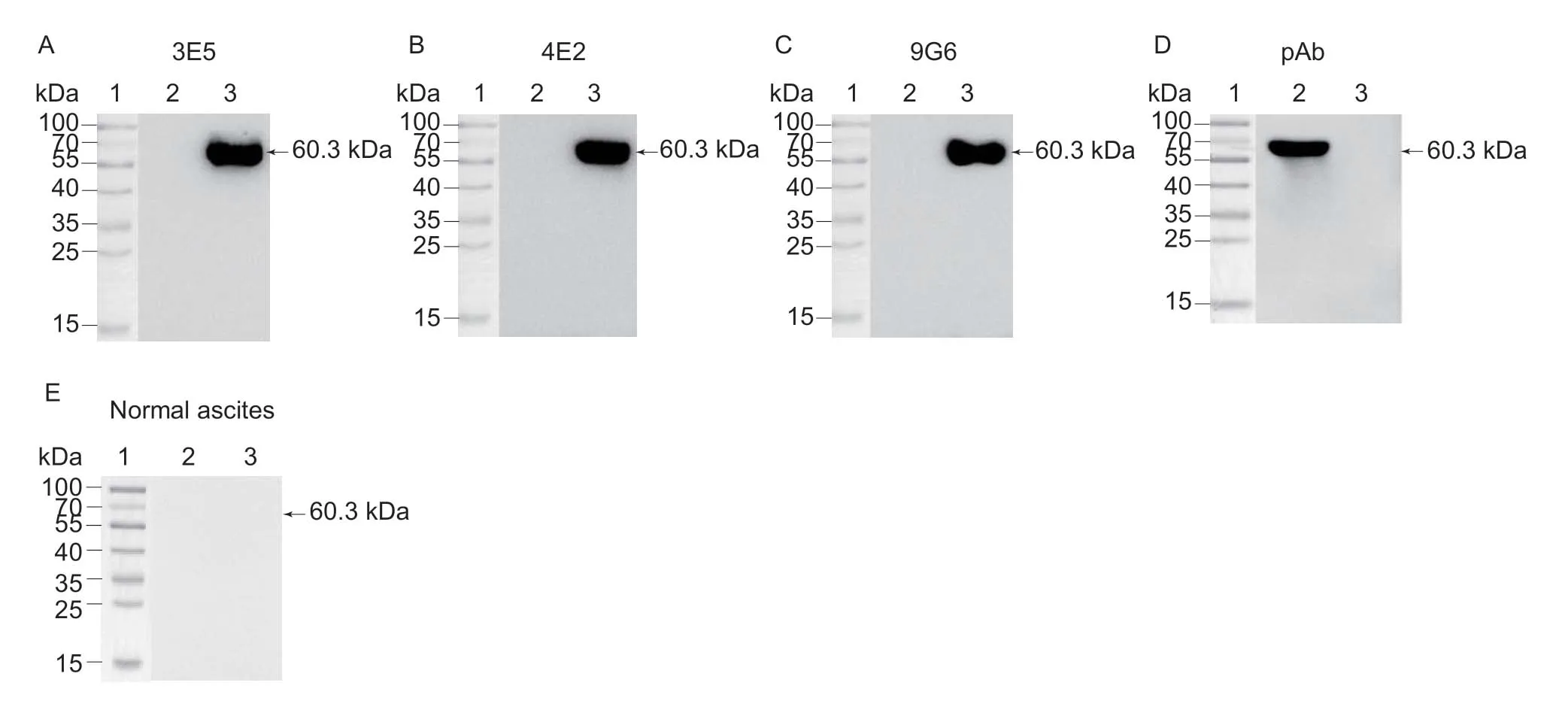
Fig.3 Monoclonal antibodies against Lawsonia intracellularis Hsp60 were identified by Western blotting analysis.A–E,3E5,4E2,9G6,pAb and normal ascites served as primary antibodies,respectively.1,marker; 2,His-tag; 3,His-Hsp60.
3.4.Assessment of the specificity of mAbs
The specificity of mAbs forL.intracellularisHsp60 was evaluated by Western blotting.3E5,4E2 and 9G6 specifically recognized the target protein ofL.intracellularisrather than other enteric bacterial proteins (Fig.4-A).However,pAb could bind with many proteins fromE.coli,S.Choleraesuis,S.Typhimurium andB.hyodysenteriae.The results demonstrated that the specificity of mAbs was better than pAb.Furthermore,the specificity of mAbs was also tested by iELISA,and the results showed that all three mAbs were sensitive in response toL.intracellularisprotein,and not cross-react to other enteric bacterial protein commonly found in the ileum of pigs,such asE.coli,S.Choleraesuis,S.Typhimurium andB.hyodysenteriae(Fig.4-B),indicating the mAbs had high specificity forL.intracellularis.These data indicated that the 3E5,4E2 and 9G6 were more specific toL.intracellularis.
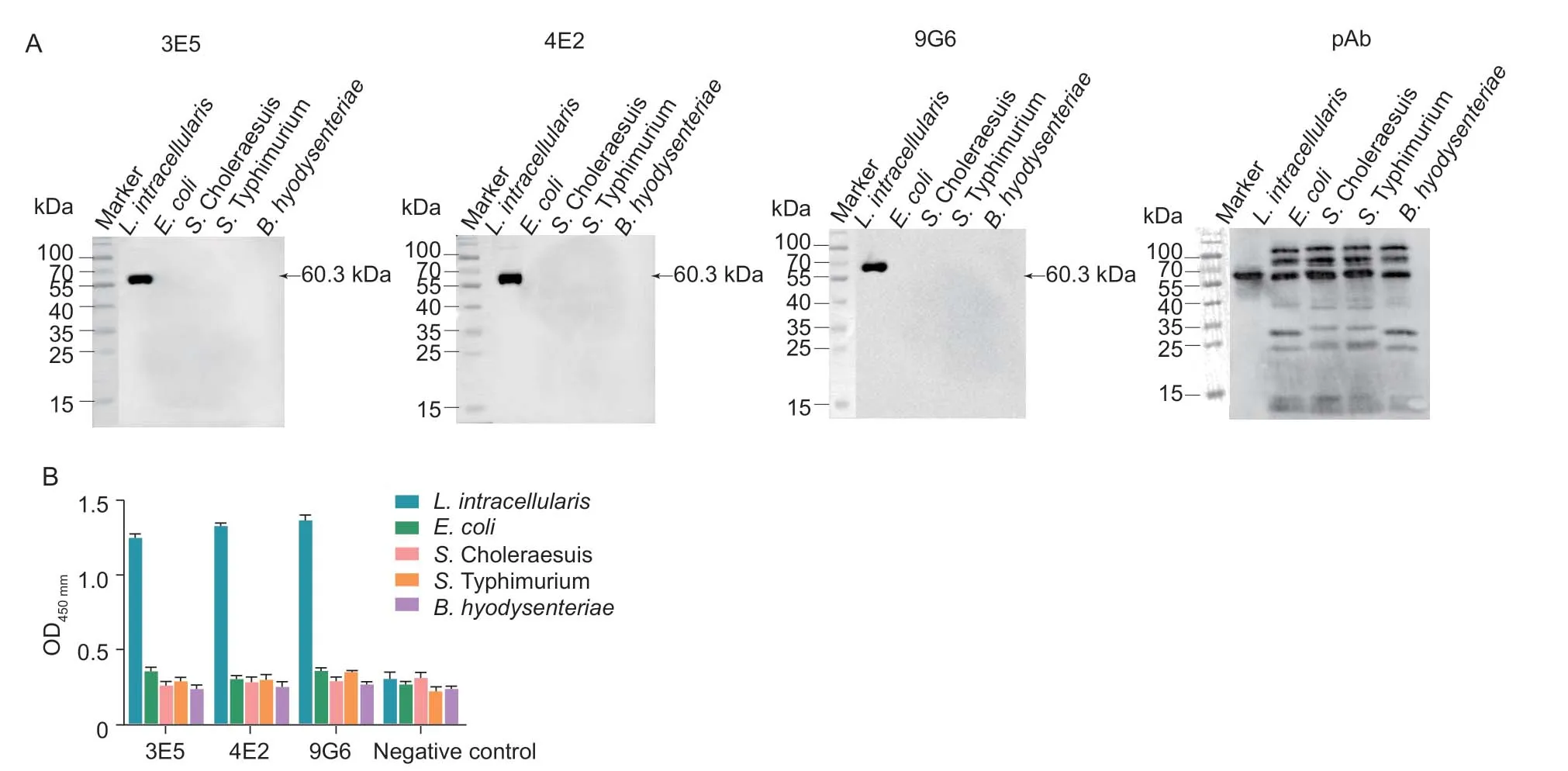
Fig.4 Cross-reaction of anti-Hsp60 monoclonal antibody (mAb).A,Western blot results using 3E5,4E2,and 9G6 to evaluate the cross-reactivity with the whole bacterial protein of Escherichia coli,Salmonella Choleraesuis,Salmonella Typhimurium and Brachyspira hyodysenteriae.B,the reactivity of mAbs with the whole bacteria of E.coli,S. Choleraesuis,S.Typhimurium and B.hyodysenteriae confirmed by indirect ELISA,respectively.Data were presented as mean±SE (n=3).
3.5.Applications of the mAbs in immunological tests
The reactivity of mAbs toL.intracellularisin a monolayer of McCoy cells was observed by IF analyses.As shown in Fig.5,three mAbs showed great reactivity with the Hsp60 in infected McCoy cells.The results revealed that theL.intracellularisin a monolayer of eukaryotic cells could be monitored using 3E5,4E2,9G6 and pAb as primary antibodies,and HIC may be observed when the bacteria cultivated up to 7 days post-incubation.
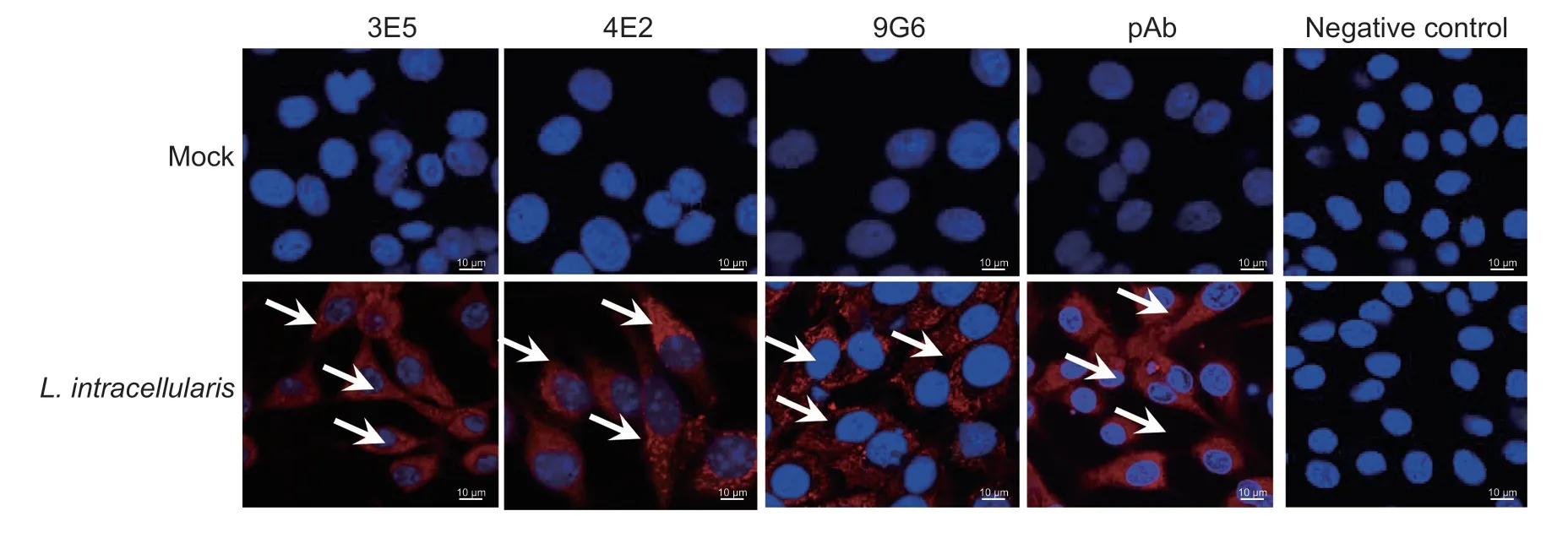
Fig.5 Immunofluorescence assay of the three mAbs in Lawsonia intracellularis-infected McCoy cells.The 3E5,4E2,9G6,and pAb using as primary antibodies to evaluate the reactivity with L.intracellularis after incubating with McCoy cells for 7 days,and the presence of L.intracellularis was stained red (arrow).Nuclei were counterstained with DAPI (blue).Uninfected McCoy cells were as negative control.Scale bar: 10 μm.
Moreover,L.intracellularisinfection was evaluated in infected pig ileum samples using IF.Lawsoniaintracellularisbacteria were primarily observed in the apical cytosol of epithelial cells of crypts (Fig.6-A,white arrows).IHC results as shown in Fig.6-B,L.intracellulariswas only found in the crypt of the ileum with pathological changes typical of PPE after reacting withL.intracellularis-specific three mAbs and pAb,and no signal was detected in the uninfected ileum.Our results demonstrated that mAbs could also be applied in the immunofluorescent and immunohistochemical diagnosing ofL.intracellularisin infected tissues of PPE.Altogether,these results revealed that the mAbs were useful for diagnosis purposes by IF and IHC.
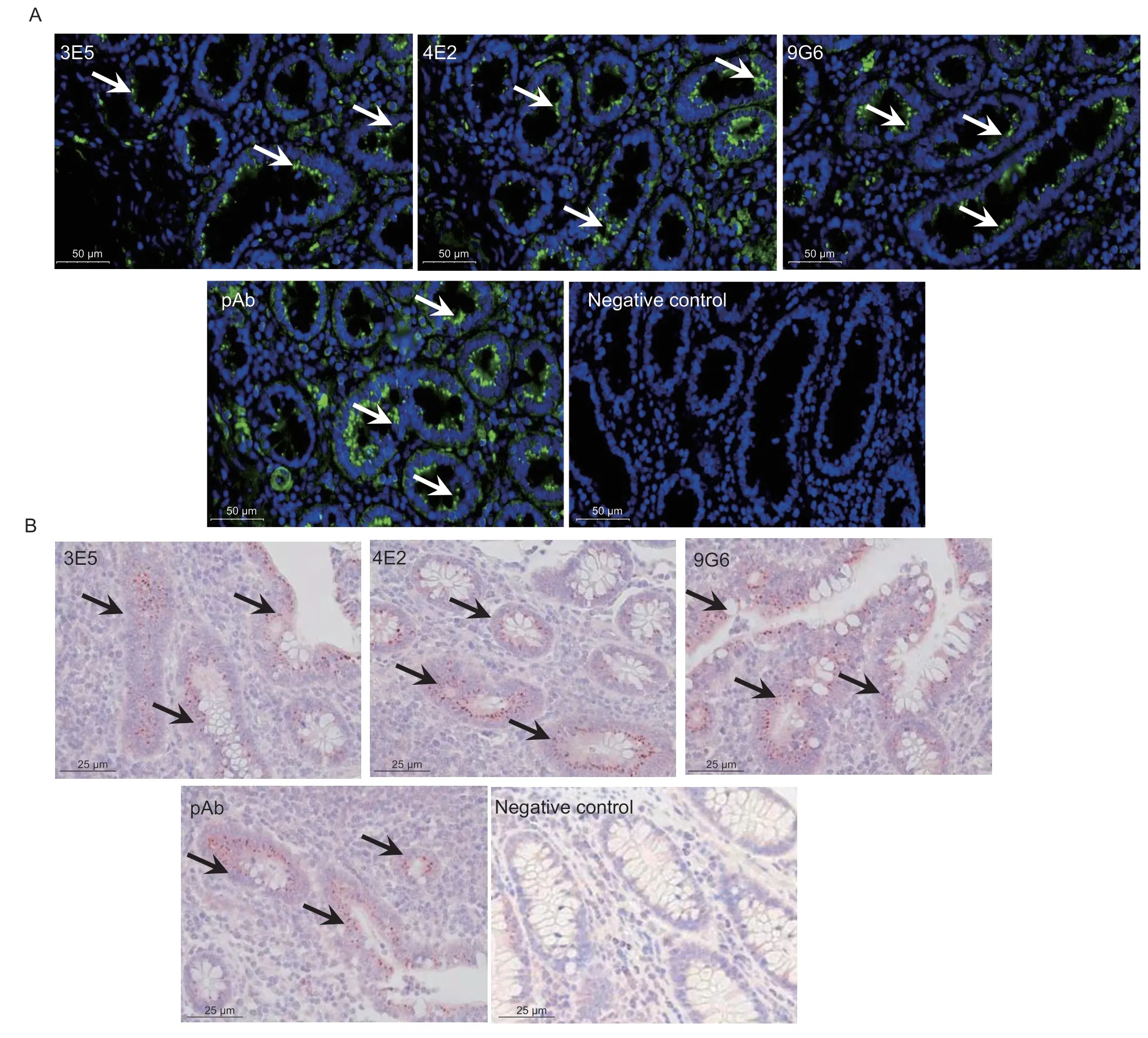
Fig.6 Immunofluorescence and immunohistochemistry assay to evaluate Lawsonia intracellularis in infected tissues of PPE.A,IF detection of L.intracellularis bacteria using mAbs (3E5,4E2,and 9G6) and pAb in infected ileum.Uninfected ileum was a negative control.Lawsonia intracellularis was detected using FITC-conjugated secondary antibodies (green).Nuclei counterstained with DAPI (blue).White arrows indicate L.intracellularis labeling along the apical cytosol of crypts cells.Scale bar: 50 μm.B,the presence of L.intracellularis by IHC in infected tissues was conducted using L.intracellularis Hsp60 mAbs (3E5,4E2,and 9G6) and pAb,respectively.Uninfected ileum was a negative control.Lawsonia intracellularis was stained red (arrow); nuclei were counterstained with hematoxylin (blue).Scale bar: 25 μm.
4.Discussion
Lawsoniaintracellularisis an obligate intracellular microorganism.However,it is challenging to isolate and grow the organismsinvitro(Krolletal.2005).Therefore,detecting,tracking,and quantifying the obligate intracellular bacteria remains challenging if antibodies are unavailable (Obradovicetal.2016).Despite the commercial availability of a monoclonal antibody againstL.intracellularis(SVANOVIR,Germany),the commercial monoclonal antibody is extremely expensive.Therefore,studies ofL.intracellularisare limited in some veterinary laboratories due to the lack of a specific antibody againstL.intracellularis.A previous study used live avirulentL.intracellularisstrain to generate rabbit hyperimmune serum,Western blotting results indicated that the antibodies in the hyperimmune serum had specificity forL.intracellularis,but the rabbit antibodies could bind with many proteins from total protein isolation ofL.intracellularis(Vannuccietal.2012; Obradovicetal.2016).Another study prepared mAb against whole cellL.intracellularis(VPB4 strain,isolated from the USA) and found the 2001 MAb could specifically react withL.intracellularisfrom pure culture (Guedes and Gebhart 2003c).Boesenetal.(2005) developed a mAb (Law1-DK) by immunization with mucosa scrapings of the intestinal mucosa from anL.intracellularis-infected pig (Boesenetal.2005).Furthermore,Hwangetal.(2012) used the whole bacterial protein ofL.intracellularis(isolated from Korea) to generate a monoclonal antibody.However,a monoclonal antibody againstMycoplasmahyorhiniswas also identified,indicating that theL.intracellularisstrain isolated from Korea was contaminated byMycoplasmahyorhinis(Hwangetal.2012).However,in many reports of mAbs against clinical isolates ofL.intracellularis,information on monoclonal antibodies against eukaryotic proteins is limited in peer-reviewed publications.
Hsp60 is one of the Hsps family members,which are widely spread in nature and highly conserved proteins found in all eukaryotic and prokaryotic organisms (Bajzert and Stefaniak 2015).This protein plays a critical role in bacterial pathogenesis and has extensive applications in diagnosis (Equilsetal.2006; Okadaetal.2007; Yuetal.2019).Although Hsp60 (also known as Cpn60,GroEL) is a class of proteins with roles in the post-translational assembly of oligomeric protein structures (Hemmingsenetal.1988),it is also a stress response protein that has undergone changes in the localization from the cytosol to the cell surface,making it a potential immunogen (Fourie and Wilson 2020; Fourieetal.2021).
Moreover,Hsp60 has been previously reported as a potential activator of the immune system and is the immunodominant bacterial antigen (Bajzert and Stefaniak 2015; Vinod Kumaretal.2017; Rauchetal.2021).It has been shown that Hsp60 obtained from Gram-negative bacteria can stimulate the acquired and innate immune system cells.Therefore,bacterial Hsp60 is an interesting candidate for a subunit vaccine.Bajzertetal.(2018) demonstrated that the recombinant Hsp60 proteins obtained from four common pathogenic bacteria:Escherichiacoli,Histophilussomni,Pasteurellamultocida,andSalmonellaEnteritidis could induce a humoral immune response in DBA/2J mice.Subsequently,a new subunit vaccine against salmonellosis based on Hsp60 has been developed,suggesting that the subunit vaccine provides a good immune protection effect on DBA/2 J mice (Bajzertetal.2019).It shows that the Hsp60 protein is a good immune protective antigen.A previous study demonstrated that Hsp60 was identified duringL.intracellularisinfectioninvitroandinvivo(Koyamaetal.2006).ElevenL.intracellularisproteins interacting with IPEC-1 cell’s surface proteins were identified,including Hsp60 (Obradovicetal.2019).Another study has observed that Hsp60 was also previously identified from two isolates ofL.intracellularisusing a shot-gun proteomic approach (Watsonetal.2014).After the challenge,mice immunized with Hsp60 ofL.intracellularisreduced fecal shedding (Lietal.2021).Thus,it could be speculated that Hsp60 would be a promising candidate antigen for developing a universal vaccine againstL.intracellularis.
The current study used a prokaryotic system to express the Hsp60 antigen.Compared with the eukaryotc system,Hsp60 was easily and quickly acquired using anE.colihost cell.Therefore,an effective and reliable method has been established to prepare the anti-Hsp60 mAbs by expressing soluble recombinant fusion Hsp60 inE.coli,and then the highly specific mAbs named 3E5,4E2,and 9G6 were successfully used to detectL.intracellularisantigen in the infected monolayer cell and infected intestinal tissue.These results revealed thatL.intracellularisfrom the infected cells or tissue could be precisely detected after incubation with the specific mAbs 3E5,4E2,and 9G6.Our results are consistent with a previous study (Boesenetal.2005; Koyamaetal.2006; Szczotkaetal.2011).The results of the present study further confirm the usefulness of monoclonal antibodies in detectingL.intracellularisantigen in monolayer cells and intestinal tissues.
5.Conclusion
The recombinant Hsp60 ofL.intracellulariswas successfully expressed in this study through prokaryotic expression technology.Western blotting results showed that the protein could react with the positive serum ofL.intracellularis.Subsequently,the recombinant Hsp60 was used to immunize BALB/mice,and three monoclonal antibodies against Hsp60 were screened by indirect ELISA,the monoclonal antibody specific againstL.intracellulariswill be useful for the evaluation ofL.intracellularisinfectioninvitroandinvivo.
Acknowledgements
We thank Mrs.Zhou Hong (Nanjing Agricultural University,China) for her help in reagent ordering and Mr.Li Haolei (Nanjing Agricultural University) for helping with the animal experiments.This work was supported by the National Key Research and Development Program of China (2021YFD1800400),the National Natural Science Foundation of China (31872480),the Jiangsu Agriculture Science and Technology Innovation Fund,China (CX (19)2020),and the Priority Academic Program Development of Jiangsu Higher Education Institutions,China (PAPD).
Declaration of competing interest
The authors declare that they have no conflict of interest.
Ethical approval
The animal study was reviewed and approved by the Animal Care Committee at Nanjing Agricultural University,China.Experimental protocols for the preparation of monoclonal antibody and obtaining swine clinical samples used in this study were performed according to the Animal Ethics Committee of the responsible authority from the College of Veterinary Medicine,Nanjing Agricultural University,China.
杂志排行
Journal of Integrative Agriculture的其它文章
- Comparison of cell wall changes of two different types of apple cultivars during fruit development and ripening
- Colonization by Klebsiella variicola FH-1 stimulates soybean growth and alleviates the stress of Sclerotinia sclerotiorum
- Degradation effects on dichlorvos by a biocontrol strain,Trichoderma atroviride T23
- Novel 18β-glycyrrhetinic acid amide derivatives show dual-acting capabilities for controlling plant bacterial diseases through ROSmediated antibacterial efficiency and activating plant defense responses
- Effects of methionine treatment on storage quality and antioxidant activity of postharvest jujube fruit
- Physicochemical properties and antibacterial mechanism of theabrownins prepared from different catechins catalyzed by polyphenol oxidase and peroxidase
