Degradation effects on dichlorvos by a biocontrol strain,Trichoderma atroviride T23
2023-09-16SUNJiananSlGaoyueLlUHongyiLlYaqianWANGXinhuaCHENJie
SUN Jia-nan ,Sl Gao-yue ,LlU Hong-yi ,Ll Ya-qian ,WANG Xin-hua ,CHEN Jie #
1 School of Agriculture and Biology,Shanghai Jiao Tong University,Shanghai 200240,P.R.China
2 State Key Laboratory of Microbial Metabolism,Shanghai Jiao Tong University,Shanghai 200240,P.R.China
Abstract
Excessive use of organophosphate pesticides (OP),such as dichlorvos,in farming system poses a threat to human health through potential contamination of environment.To date,biodegradation has been prospected most promising approach to eliminate environmental OP residues.Trichoderma species as a biological control microorganism is often exposed to the chemical pesticides applied in environments,so it is necessary to understand the mechanism of degradation of dichlorvos by Trichoderma.In this study,dichlorvos significantly inhibited the growth,sporulation and pigmentation of T.atroviride T23,and the dichlorvos degradation activity of T23 required the initial induction effect of dichlorvos and the culture conditions,including the nutrient and pH values of the medium.Various changed primary and secondary metabolites released from T23 in the presence of dichlorvos were speculated as the energy and antioxidants for the strain itself to tolerate dichlorvos stress.The results showed that T23 could produce a series of enzymes,especially the intracellular enzymes,to degrade dichlorvos.The activities of the intracellular enzyme generated by T23 were differentially changed along time course and especially relied on initial dichlorvos concentration,ammonium sulfate and phosphate added in the medium.In conclusion,some dichlorvos-induced chemical degradation related enzymes of T23 were proved to be involved in the degradation of dichlorvos.
Keywords: Trichoderma atroviride T23,dichlorvos,intracellular enzyme,induced enzyme activity
1.lntroduction
Dichlorvos (DDVP) is an artificial synthetic 2,2-dichlorovinyldimethyl phosphate,which is commonly viewed as a kind of broad-spectrum insecticide and ubiquitously applied in various agricultural industries (Kazemietal.2012; Renetal.2015; Heidaretal.2017; Asemoloyeetal.2019).However,persistent residues of dichlorvos would potentially threat the ecological safety of food,water and soil owing to its highly water solubility and presence in aquatic products.Moreover,its degradationreleased metabolites transfer along the food chain through bioaccumulation process,thereby adversely impacting human health and beneficial insects,which have been reported to inhibit acetylcholinesterase of organisms (Larietal.2014; Gaonkaretal.2019; Mengetal.2019; Fuetal.2022).Due to the urgency of detoxification of the toxic compounds in environments by using ecological safety approaches,several bioremediation strategies have been preferred to be applied to the degradation of organic pollutants compared to the environmentally unfriendly physicochemical methods,and the elimination of OP residues can be completely achieved by bioremediation (Bhandarietal.2022; Bhattetal.2022; Fuetal.2022).Many microbes,includingFusariumsolani,Trichodermaspp.,Talaromycesatroroseus,Aspergillusoryzae,andPseudomonasaeruginosaenable to mineralize the organic pollutants dichlorvos as potential carbon source (Spinaetal.2018; Fengetal.2020; Huangetal.2020; Pangetal.2020; Zhangetal.2021; Priyankaetal.2023).Meanwhile,Trichodermahas been confirmed available in the environmental pollutant removal by biosorption (Luetal.2016) in the way of co-metabolism (Prenafeta-Bolduetal.2006) and enzymatic degradation (Lietal.2010).Trichodermahas been demonstrated to be either tolerant to an array of chemical pesticides or effective in the degradation of intractable chemical pesticides and other pollutants (Tripathietal.2013) containing polycyclic aromatic hydrocarbons and heavy metals.The maximum tolerance concentration ofTrichodermaatrovirideT23 was up to 2 000 μg mL–1(Tangetal.2010) and the resistance of T23 showed great potential in degrading carbendazim.Previously,someTrichodermaspecies are reported with the activities in the degradation of chlorpyrifos,chlordane,lindane and DDT residues in soil (Tabet and Lichtenstein 1976; Ezzi and Lynch 2005; Zhouetal.2007; Bosso and Cristinzio 2014),which then leadTrichodermato possible application for the remediation of pesticides-contaminated soil.
The tolerance and degradation mechanism of the chemical pesticides byTrichodermahas been only studied at physiological and biochemical level so far,for instance,it has been demonstrated that β-tubulin is closely involved in the tolerance ofTrichodermato fungicide benzimidazole (Goldmanetal.1993).Relevant study has also shown that the oxidation system ofTrichodermais responsible for the degradation of exogenous substances including organochlorine pesticides (Katayama and Matsumura 1993).Evidences on dichlorvos degradation byT.atrovirideT23 have suggested a positive link to paraoxonase 1 (PON1),ABC transporter and cytochrome P450 system (Sunetal.2019b,2022),whereas there has been nowadays no publication on the physiological basis for the multiple enzymes production related to the dichlorvos zymolytic degradation andTrichodermametabolic changes in the presence of dichlorvos.The object of this study is to reveal the induced production of dichlorvos degradation enzyme and action features byT.atrovirideT23.Our work illustrated that degradation related enzyme production was induced by dichlorvos which depended on nutrients in medium,and significant changes ofTrichdodermametabolism occurred in the induction process.
2.Materials and methods
2.1.Strain,culture and reagents
TrichodermaatrovirideT23 strain was isolated from soil and stored at Agricultural Culture Collection of China (No.ACCC 32730).Firstly,T23 strain was maintained on potato dextrose agar medium at 28°C until sporulation occurred.To observe the tolerance ofTrichodermato DDVP,conidia of T23 strain were washed with 0.01% Tween 80 and the conidia suspension (105spores in 5 μL) was inoculated on PD medium containing Potato (200 g L–1) and glucose (20 g L–1) or bulk medium containing 0.2 g L–1K2HPO4,0.8 g L–1KH2PO4,0.2 g L–1MgSO4·7H2O,0.1 g L–1CaSO4·2H2O,0.0033 g L–1Na2MoO4·2H2O,0.005 g L–1FeSO4·7H2O,1.0 g L–1(NH4)2SO4and 1.0 g L–1glucose with pH 6.0 and different concentrations of DDVP.DDVP technical materials (98%) were provided by Jilin Academy of Agricultural Sciences,China and prepared into 100 mg mL–1stock solution with methanol and kept at 4°C.The DDVP methanol standard sample (1 000 μg mL–1)was purchased from Aladdin Holdings Group Co.,Ltd.(Beijing,China).
2.2.Extraction of intracellular enzyme and extracellular enzyme,activities analysis and hydrogen peroxide concentration determination
The enzymatic crude extract (ECE) was obtained as follows.A volume of 10 mL of fungal cultures were centrifuged at 8 000 r min–1for 20 min at 4°C,then the fungi mycelium and supernatant were collected respectively.The supernatant was precipitated in the solution from the saturation of 80% (NH4)2SO4.The dialysis process was carried out at 4°C.Then the dialysis tube was placed for 24 h and the precipitate was dissolved in 15 mL of phosphate buffer (pH 8.0,0.05 mol L–1),and the supernatant was discarded to obtain the extracellular enzyme crude extract and was stored at –80°C after the centrifugation.
The 1 g of wet mycelium was weighed and washed three times with sterilized water,and 5.0 mL 1.0 mol L–1NaCl extract solution (pH 7.4,20 mmol L–1Tris–HCl buffer solution) were added into the sample after ice bath-grounding and were transferred into 10 mL sterile centrifuge tubes for centrifuging at 10 000 r min–1for 15 min at 4°C,then the intracellular enzyme crude extract were collected and stored at –80°C.
The intracellular and extracellular degrading enzymes were induced by dichlorvos for 72 h and the protein concentration were determined using the Bradford method.The degrading enzymatic activity was measured at 12 h based on degrading DDVP amount with initial concentration of 100 μg mL–1by intracellular enzyme unit concentration.Activities of peroxidase (POD),superoxide diamutase (SOD),thioredoxin reductase (TrxR) and catalase (CAT) in intracellular enzymes were determined following the Solarbio Brand BC0090,BC0350,BC1155 and BC0200 Procedure.The PON1 concentration was detected using the double-antibody sandwich enzymelinked immunosorbent assay (ELISA) method,and the intracellular enzyme activity has been calculated according to the PON1-ELISA Kit manual of Wuhan Elabscience Biotechnology Co.,Ltd.(Wuhan,China).The concentration of hydrogen peroxide was examined through the approach of Solarbio Brand Kit BC3595.Enzyme activity was detected using the kit manual method as the standard.
2.3.Determination of dichlorvos residues
The 0.04 g of NaCl and equal volume ethyl were added into 2 mL medium and the oscillation extraction was conducted at 120 r min–1for 30 min.The ethyl acetate extracts from top layer were transferred to new centrifuge tubes after the medium static stratification,and this process was repeated twice.The ethyl acetate from extracted multiple times were mixed with 1 g of anhydrous magnesium sulfate to remove the dehydration,and 1 mL of pesticide residue grade n-hexane was also performed after the blowing dry of nitrogen at 30°C and then shaked for 30 min at 150 r min–1.After that,the dissolved products were filtered by 0.22-μm polytetrafluoroethylene milli-pore filters for detection.
A gas chromatography instrument with an FPD (GC 2010,USA),and a high polarity capillary column (HP-5,30 m×0.32 mm,0.25 μm film,USA) was used for degradation products analysis.Nitrogen was used as the carrier gas (nitrogen at 1 mL min–1with 99.9999% purity).Other conditions: split ratio was 1/10 and the injector temperate was set at 200°C and the injection volume was 1 μL.The oven temperature was initially set at 100°C for 0.5 min and then raised to 250°C at 15°C min–1and then kept 20 min.The FPD detector was used to increase the reliability of the analysis and the detector temperature was set at 250°C.
2.4.The identification and analysis of intracellular metabolites
The LC-MS technique was applied for the analysis of intracellular metabolic products.In brief,a portion of 60 mg of the mycelium were placed in 2 mL of sterile plastic centrifugal tubes,and 500 μL of precooling methanol (–20°C) and ultrapure water (4°C) were also added to these tubes.After 30 s vortex oscillation,100 mg of glass bead was added to the tubes and fixed on the instrument adaptor.The freeze–thawing tubes were prepared at room temperate after a 5-min freeze in liquid nitrogen.Next,the tubes were again placed in the instrument adaptor and a 2-min ground at 70 Hz was conducted twice.The tubes were removed and centrifuged at 13 000 r min–1for 10 min at 4°C,and the centrifugation,condensation and drying process were conducted for the sample supernatant.The dissolving reaction was started by adding 300 μL of DL-2-chlorophenylalanie (4 mg L–1) methanol aqueous solution (1:1,4°C) to the sample,and the mixed sample were filtered through 0.22-μm filter membrane for the purpose of the determination.
The quality control (QC) sample consisting of 20 μL aliquots of each testing sample were used to correct and analyze the deviation of the outcome and the error caused by experiment alone,respectively.The sample loading was conducted in the order of blank solvents,QC sample and the testing sample.Experiments were performed on an Ultimate 3000 with QExactiveFocus liquid chromatography-mass spectrometry coupled with detector,using an ACQUITYUPLC®HSST chromatography column (2.1 μm×150 mm,31.8 μm; Shimadzu,Kyoto,Japan),and the auto-sampler temperate was at 8°C and the flow rate was set to 0.25 mL min–1and the column temperate was at 40°C and the injection volume was 2 μL and gradient elution was carried out.Positive ion mode 0.1% formic water (C)–0.1% formic acetonitrile (D) and negative ion 5 mmol L–1ammonium formate water (A)–acetonitrile (B) buffer solutions were used as mobile phases.The gradient solution program was determined as follows: 0–1 min,2% B/D: 1–9 min,2–50% B/D; 9–12 min,50–98% B/D;12–13.5 min,98% B/D; 13.5–14 min,98–2% B/D; 14–20 min,2% D-positive ion mode (14–17 min,2% B-negative ion mode).
The spectrometry conditions were equipped with electrospray ion source (ESI),following positive and negative ions mode,3.80 kV positive ionization spray voltage and 2.50 kV negative ionization spray voltage and the sheath gas were set at 45 unit,and the auxiliary gas was set at 15 unit.The capillary column temperate was at 325°C and the scanning resolution was 7 000,the scanning coverage were from 81 to 1 000,the HCD was used in secondary fragmentation under the collision energy (30 eV),and unnecessary MS/MS information were removed by dynamic exclusion.
2.5.Data analysis
The DDVP residues,DDVP degradation rate and fungal biomass data from three independent experiments were processed by means of ANOVA and SPSS 23.0 Software (IBM Predictive Software,USA).Statistical differences of the treatments were determined by the Duncan’s test (P<0.05) and plots were created in GraphPad Prism 7.0 (GraphPad Software).In addition,the possible correlations among DDVP degradation content and intracellular antioxidant enzymes activity results were calculated based on Pearson correlation analyses.
3.Results
3.1.The tolerance analysis of T23 under the DDVP stress
The growth rate and sporulation of T23 were significantly affected by DDVP at different concentrations.The different growth and sporulation of T23 were revealed on PDA and Burk medium containing DDVP at 84 h (Fig.1).It was suggested that the growth of T23,conidial production and pigment synthesis were evidently depressed along the elevated concentrations of DDVP (Fig.1-A).It was clearly seen that T23 was unable to grow on Burk medium when the concentration of dichlorvos reached 1 000 μg mL–1,but the growth of T23 was slowed on the PDA medium containing the same DDVP concentration,indicating that the tolerance of T23 to dichlorvos substantially depended on the kinds of medium or nutrients in whichTrichodermawas grown.
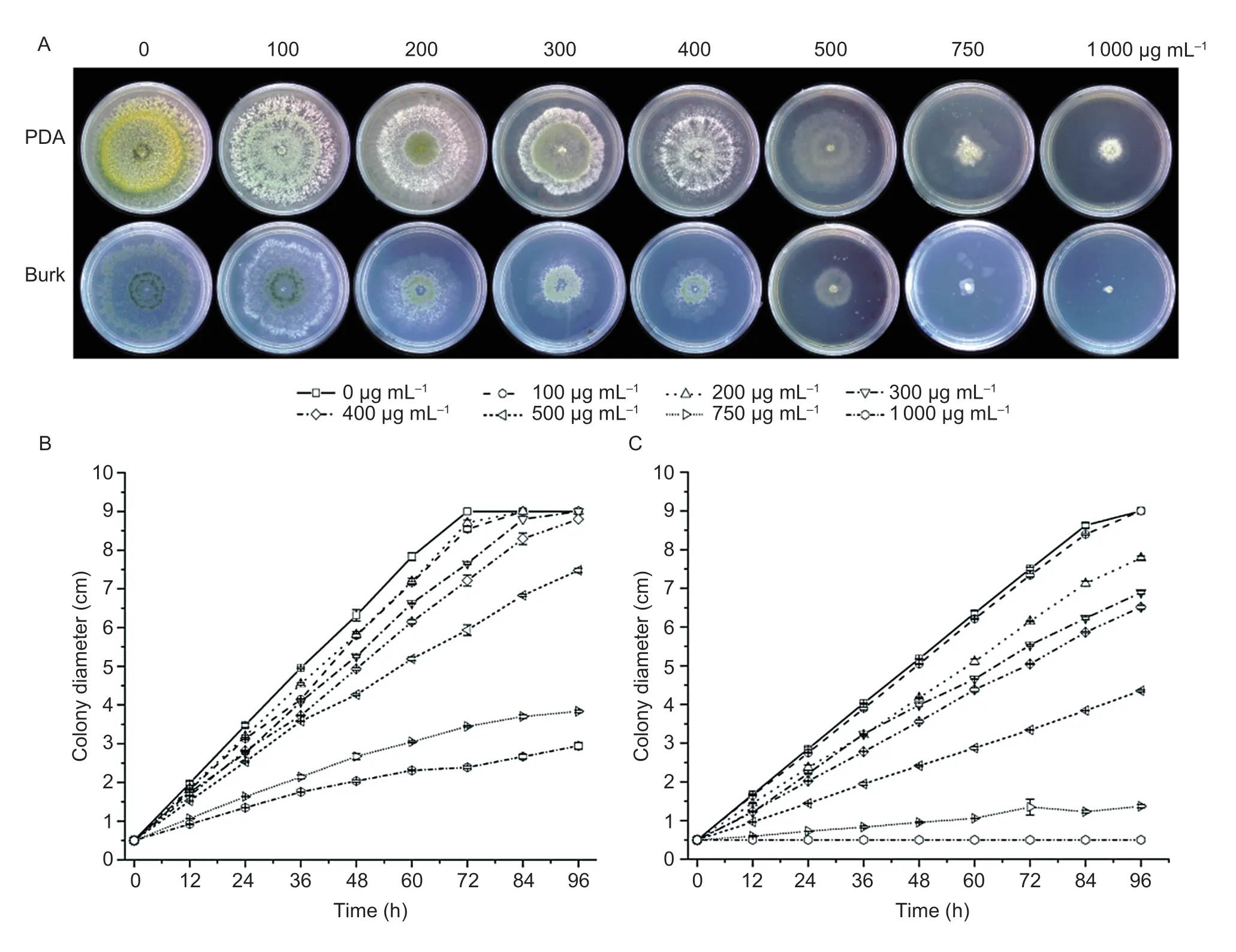
Fig.1 Effect of different concentrations of dichlorvos (DDVP) on Trichoderma atroviride T23.A,the colony morphology of T23 at 84 h on PDA and Burk media containing different concentrations of DDVP.B and C,colony diameter changes of T23 after exposure to different concentrations of DDVP every 12 h interval from 0–96 h on PDA (B) and Burk medium (C).Each value is expressed as the mean±SE of three replicates.
The DDVP residues in medium inoculated with T23 were detected relative to the uninoculated control at 72 h.It was demonstrated that the DDVP residues in T23 inoculated on PDA or Burk medium were impressively lower than the control (Fig.2),and the DDVP degradation on PDA medium were notably higher than that on the Burk medium,suggesting that more vigorous growth of T23 on PDA medium might result in more active degradation of DDVP than that on the Burk medium.Thus,the degradation of DDVP was affected by theTrichodermagrowth biomass level.In addition,a more DDVP degradation could be achieved byTrichodermawhen grown on the media containing more nutrients.
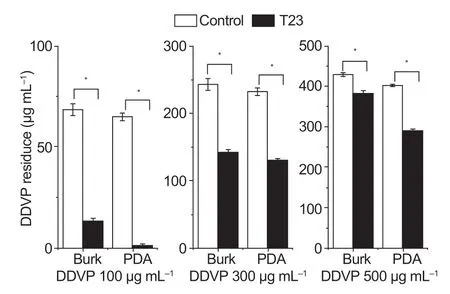
Fig.2 Changes of dichlorvos (DDVP) degradation by T23 in media containing different concentrations of DDVP.The medium were Burk and PDA,and the initial concentrations of dichlorvos were 100,300 and 500 μg mL–1.Data are mean±SD of three replicates.*,P<0.05.
3.2.The effect of DDVP on T23 metabolism
To understand the effect of DDVP on the T23 metabolism,the changes of intracellular metabolites induced by different times of DDVP stress onT.atrovirideT23 were investigated.A total of 248 differential metabolites were identified after the DDVP treatment from 24 to 72 h,among which the primary metabolic pathway mainly included amino acid metabolism,glycosaminoglycan and nucleotide sugar metabolism,glycolic acid and dicarboxylic acid metabolism,and the tricarboxylic acid cycle.Intracellular fatty acid compounds,such as 10-hydroxy-2E-decenoic acid,succinic acid andcis-Aconitic acid,12-hydroxydodecanoic acid and 10-hydroxydodecanoic acid significantly changed,implying that DDVP stress affected the ω-oxidation pathway ofT.atrovirideT23.Secondary metabolic pathway was mainly related to riboflavin metabolism,methane metabolism,pantothenic acid and CoA biosynthesis,from which the bio-oxidation-related riboflavin,and the resistance-associated flavonoids and alkaloids significantly increased,indicating thatT.atrovirideT23 had a high resistance to DDVP stress (Fig.3).
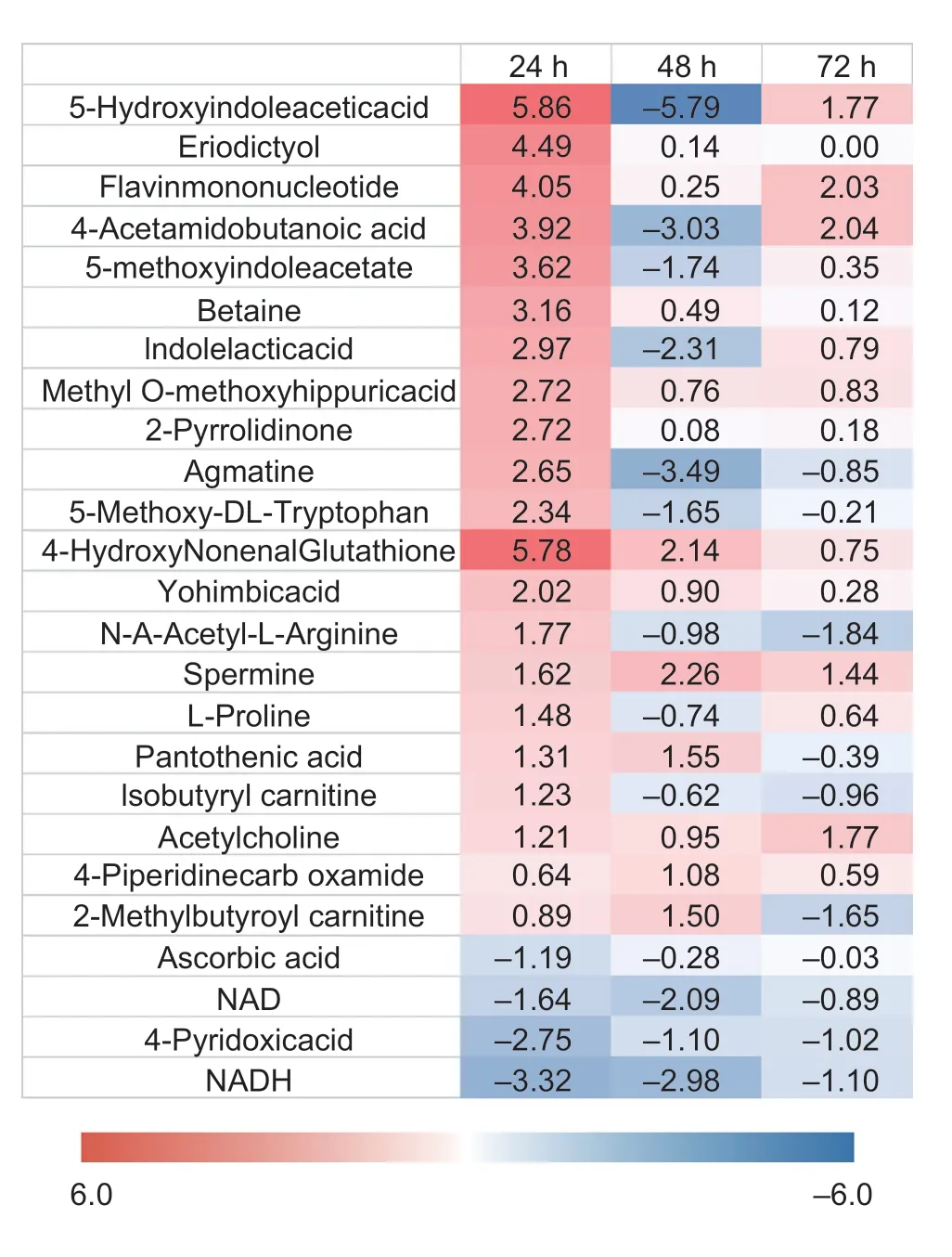
Fig.3 Changes of secondary metabolites of strain T23 in the process of the degradation of dichlorvos (DDVP).Fold change is the ratio of the mean value of mutant strain to the mean value of wild type,|log2(|Fold change)|>1 has significant difference.
The compounds associated with tolerance to DDVP was significantly changed along the secondary metabolism process (Fig.3).Most compounds were increased at 24 h in the existence of DDVP,and 5-methotyindole acetate,indole acetic acid and indolelactic acid compounds vastly increased at 24 h and decreased at 48 h.2-Pyrrolidone and betaine distinctly increased at 24 h,yet the effect of DDVP on the two secondary metabolites disappeared later on.The two compounds belonged to secondary metabolites of alkaloids and the study declared that microbial can improve the alkaloid compounds production on the tolerance to extreme environments,such as drought and heavy metal stress (Huetal.2019; Jochumetal.2019),and there were similar features among the degradation process of DDVP by T23.
4-Pyridoxic acid and pantothenic acid were usually known as the degradation metabolites from vitamins B6,and 4-pyruvate was the degradation products of vitamins B6 and the metabolites production required the combined action of oxidase,dehydrogenase and NAD-dependent aldehyde dehydrogenase (Geetal.2008).The change trends of metabolites concentration were consistent with those of NAD and NADH presenting with the trends in long duration decline.In addition,the pantothenic acid was an antioxidant aqueous solubility vitamin,and the concentration sustained increasing during the degradation process of DDVP,and the pantothenic acid participated in the fatty acid metabolism annotated as precursors for CoA (Tahiliani and Beinlich 1991).The results showed that the degradation of DDVP by T23 was affected by the type of T23 medium.Fig.2 showed that the degradation level of DDVP in T23-PDA was higher than that in T23-Burk at 72 h.
3.3.Factors affecting the degradation of DDVP by T23
The induction assay of different initial DDVP concentration on T23 was conducted by measuring the degradation activities of extracted intracellular and extracellular enzymes from T23 on DDVP at 220 μg mL–1(1 μmol mL–1) to define the contribution level of intracellular and extracellular degradation enzyme on DDVP degradation.The degradation activity of intracellular and extracellular enzymes was found to increase as the induction of DDVP and the degradation enzyme activity was increased with the elevated initial DDVP induction concentration.The intracellular enzyme activity in the presence of DDVP were much higher than that in the absence of the chemicals (Fig.4).

Fig.4 Comparison of T23 extracellular and intracellular enzymes activity in the degradation of dichlorvos (DDVP).
The T23 was induced by DDVP to produce degradation enzyme at the concentration of 100 μg mL–1,and almost 18.79 and 67.17 μg mL–1of DDVP were degraded by intracellular and extracellular enzyme within 12 h,respectively and 3.67 μg mL–1of DDVP were degradedviaenzyme hydrolysis.The DDVP at the concentration of 500 μg mL–1induced the T23 to generate degradation enzyme and intracellular and extracellular enzyme degraded 120.75 and 31.86 μg mL–1of DDVP,respectively (Fig.4).The result illustrated that the DDVP affects T23 characteristic to produce degradation enzyme,and the degradation process was dominated by intracellular enzymes,whereas the extracellular enzyme was adjuvant.In terms of the intracellular enzyme being predominant in T23,the enzyme degradation activity was mainly contributed to DDVP degradation.However,the optimal induction condition would determine degradation rate of DDVP by T23,which involved DDVP concentrations and induction time.
As observed in Fig.5,the intracellular enzyme activity was substantially affected by incubation time and initial DDVP concentration.The degradation activity of intracellular enzyme increased with the elevated initial DDVP concentration at the same controlling time,except for the 2 h treatment conditions; the degradation enzyme activity increased firstly and then decreased over time under the same initial DDVP treatment concentration condition.There was increasing degradation activity of intracellular enzyme on DDVP under the treatment condition with initial DDVP concentration (300 μg mL–1) at 24 h.
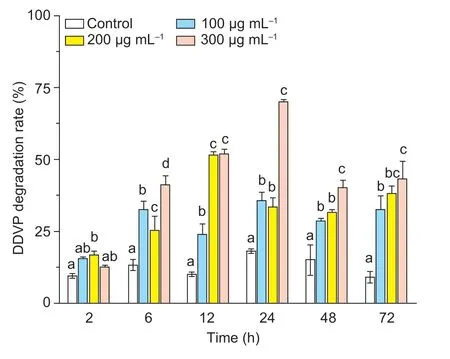
Fig.5 Degradation of dichlorvos (DDVP) by T23 intracellular enzymes induced by different concentration of DDVP.The induction time of intracellular enzyme was 2,6,12,24,48,and 72 h,and the induced concentrations of DDVP were 100,200,and 300 μg mL–1.Each value is expressed as the mean±SD of three replicates.
Furthermore,the effect of different carbon,nitrogen and phosphate sources on fungal biomass and degrading enzyme activity were determined after the induction of DDVP at 300 μg mL–1at 72 h to clarify the response of nutrition elements on DDVP degradation induced by T23.As shown in Fig.6-A,the mycelium biomass increased and the degrading enzyme activity increased firstly and then declined regardless of enhanced glucose concentration.The T23 biomass reached the maximum value (8.78 mg mL–1) at the glucose concentration (20 g L–1) in medium; the DDVP degrading activity by T23 was the highest at the glucose concentration of 10 g L–1in medium.The plausible reasons may be that the elevated glucose promoted the increase on the T23 biomass,but the degrading ability of T23 on DDVP was not altered along the elevating the carbon concentration,the 10 g L–1glucose was defined as optimal carbon source condition (Fig.6).
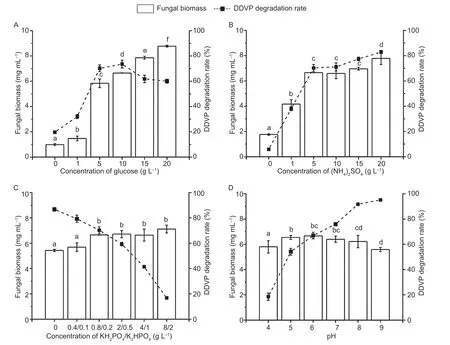
Fig.6 Effects of different concentrations of medium components and pH on T23 growth and dichlorvos (DDVP) degradation.Each value is expressed as the mean±SD of three replicates.Different letters mean significant difference at P<0.05.
The Fig.6-B exhibited that there were salient variations among biomass and degrading enzyme activity with the changes of ammonium sulfate concentration.The biomass constantly increased with elevated ammonium sulfate concentration and there were insignificant differences among biomass (P>0.05) at the ammonium sulfate concentration of 1–5 g L–1.The degradation activity of T23 on DDVP kept rising states and what can be explained that the nitrogen sources were the key element that affect protein synthesis,and DDVP degradation enzyme were substantially synthesized by living organisms with adequate nitrogen sources.Therefore,the 1 g L–1of ammonium sulfate concentration was selected for the following study considering of the changes among DDVP biomass and degradation situation (Fig.6-B).This treatment supposed that the DDVP degradation rates became slower with elevated phosphate concentration,suggesting that excessive exogenous phosphate element highly inhibited the degrading enzyme activities (Fig.6-C).Collectively,0.8/0.2 of appropriate phosphate proportion in medium was applied for subsequent trials.
The pH had distinct effects on enzyme-producing and enzyme activity of microbes.The results in Fig.6-D described that the growth of T23 is basically not affected when the pH ranged from 5 to 8,but the DDVP degrading level was increased with the elevated pH in the range 5–8 and the DDVP degrading enzyme produced by T23 preferred alkaline environment (Fig.6-D).Hence,the pH 6.0 was used in medium for the following experiment to suppress the interference from alkaline environment on biodegradation.In brief,it was examined that the liquid medium formulation consisted of glucose at 10.0 g L–1,ammonium sulfate at 1.0 g L–1,KH2PO4at 0.8 g L–1,K2HPO4at 0.2 g L–1and pH at 6.0,with the requirement on the optimal DDVP degrading efficiency.
3.4.The correlation analysis between intracellular antioxidant enzymes activity with the degradation effect of T23
The oxidoreductases,lactonase and arylesterase activity have been reported correlation with the phosphate degradation in previous study (Sunetal.2019a),as a result,T23 intracellular enzyme system may contain some related to the tolerance or antioxidation helpful to DDVP degradation.Fig.7 revealed the activities of the four related intracellular enzymes was changed at different DDVP concentrations for induction.The POD activity was activated in the presence of DDVP,showing the first increasing and then decreasing trends and the enzyme activity grew to the peak value at 24 h,as depicted in Fig.7.The POD activities increased by 1.37,2.02 and 3.05 times compared with control sample under the addition amount of DDVP at 100,200 and 300 μg mL–1,respectively.POD enzyme has been verified with function in catalyzing the hydrogen peroxide to oxidate diverse substrates (H2O2+substrates–H2→substrates+2H2O) (Gagnéetal.2014).

Fig.7 The changes of T23 intracellular enzymes activities induced by dichlorvos (DDVP) along time course.A,peroxidase (POD).B,superoxide diamutase (SOD).C,catalase (CAT).D,thioredoxin reductase (TrxR).E,arylesterase.F,lactonase.Each value is expressed as the mean±SD of three replicates.
As shown in Fig.8,the intracellular H2O2concentration reached its peak (35.21 μmol mL–1) at 6 h,then declined gradually following time course,while at the time point,antioxidative enzymes (POD,CAT,SOD,and TrxR) just start to increase and still allow more H2O2production to promote oxidative degradation of DDVP.Totally majority of antioxidative enzymes showed higher activities at the DDVP concentration of 300 μg mL–1than other DDVP concentrations (Fig.7).The SOD activity was increased at DDVP induction concentration of 300 μg mL–1,0.66 times over the control.The CAT activity was usually related to the removal of intracellular H2O2,however,in present study the CAT activity was the lower in the treated samples than that in the control sample at all tested concentration of DDVP,indicating DDVP might significantly restrain the CAT activity (Fig.7-C).The thioredoxin TrxR played a critical role on regulating the intracellular redox balance (Saccocciaetal.2014).According to Fig.7-D,the TrxR activity increased firstly and then decreased as the increase of DDVP concentration,and the thioredoxin activity grew to peak with 15.608 and 9.227 U at the DDVP concentration of 100 and 200 μg mL–1at 12 h,respectively,and the thioredoxin activity reached the peak with 13.510 U at DDVP concentration of 300 μg mL–1at 24 h.Similarly,activities of arylesterase and lactonase reached a peak at 24 h when the DDVP concentration was 300 μg mL–1(Fig.7-E and F).
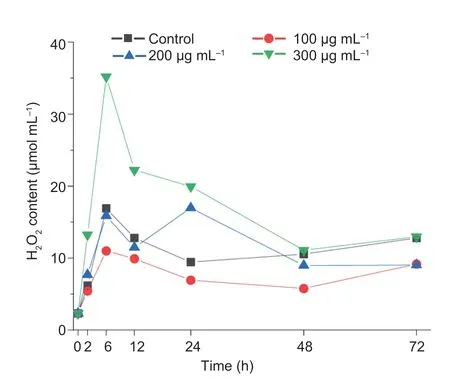
Fig.8 Time-course of intracellular H2O2 accumulation of T23 exposed to 0,100,200 and 300 μg mL–1 dichlorvos (DDVP),respectively.
Previous study demonstrated that the DDVP degradation by aquatic mammals were closely associated with paraoxonase1 activity (Sunetal.2019a),and the paraoxonase1 content in intracellular enzyme of T23 mycelium was 0.215 ng mL–1,remarkably higher than the 0.087 ng mL–1in the uninduced treatment under DDVP stress through the double-antibody sandwich ELISA detection method.The paraoxonase1 enzyme had shown activities of both arylesterase and lactonase,interestingly in the study the two intracellular enzyme activities was simultaneously increased to the highest value at 24 h in the presence of DDVP (Fig.7-E and F).
The results in Fig.9 showed that the correlation coefficients between arylesterase,lactonase,POD and TrxR activity of T23 with DDVP degradation level were 0.6915,0.7085,0.5268 and 0.1492,respectively,in which lactonase and arylesterase activity were closely relevant to DDVP degradation in the presence of the DDVP.Nevertheless,the SOD and CAT enzyme activity were not linked with DDVP degradation (P>0.5) (Fig.9-C and D).Thus,there were the closest connections between lactonase,arylestrase activity and DDVP degradation; yet the paraoxonase1 enzyme which hydrolyzed the P–O bonds also impacted the aryletrase and lactonase activity.
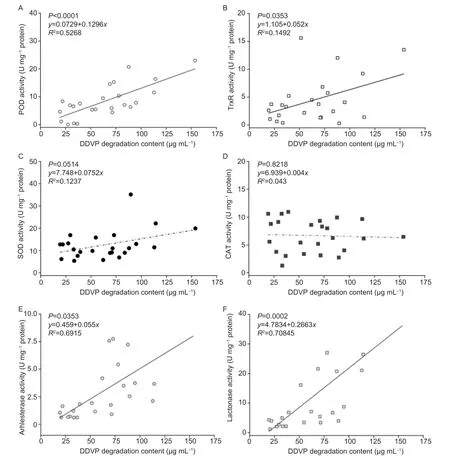
Fig.9 Correlations between the intracellular enzyme activity and the dichlorvos (DDVP) degradation content in T23.A,peroxidase (POD).B,thioredoxin reductase (TrxR).C,superoxide diamutase (SOD).D,catalase (CAT).E,arylesterase.F,lactonase.
4.Discussion
Microorganisms are recognized as a key approach for low-cost and efficient OPs bioremediation.In this study,the T23 strain was identified to be effective in the degradation of DDVP,while the degradation level depended on appropriate initial DDVP induction concentration,temperature and pH.Intracellular enzymes including the antioxidative enzymes from T23 were crucial in the dichlorvos degradation.In the early induction period of DDVP to T23,majority of amino acid and lipid metabolites were up-regulated in T23,then downregulated in later period.The increased amino acids and lipid metabolites at the early period were probably the positive response to the DDVP stress allowing to balance or alleviate the damages by DDVP stress.Similarly,5-methoxyindole acetate,indole acetic acid and indole lactic acid were all up-regulated in the early stage of T23 interaction with DDVP,and down-regulated in later stage.As previously described,DDVP degradation enzymes production commonly need energy to support in the early stage of T23-DDVP interaction.
Several studies have reported that the optimum physiochemical parameters for OPs dichlorvos were obtained at pH 7.0 and incubation temperature was at 37°C (Jiangetal.2019; Parteetal.2020) and the degradation rates of dichlorvos were reduced along with increasing dichlorvos concentrations (Ningetal.2012).The growth of microbialAeromonasbivalviumandBacillusspp.can be improved or impressed when the DDVP concentration is below or above the 300 mg L–1(Zhangetal.2022).In particular,the OP biodegradation rate was closely connected to the microbial genetic composition and the metabolites variation were determined by microbial and physiological properties (Saurabhetal.2022).Therefore,these fungal strains possess organo-phosphorus hydrolase genesmpd,cbh,afk2andafk4,which could promote the production of degrading enzymes and enhance the mineralization of dichlorvos (Asemoloyeetal.2019).
T23 intracellular enzymes have been shown to be essential for DDVP degradation,and a group of reactive oxygen scavenging enzymes is closely involved in protectingTrichodermafrom DDVP oxidative stress damage during its degradation.For instance,the relatedredox intracellular enzyme POD,TrxR and SOD from T23 mycelium were correlated with DDVP degradation,implying that T23 applied oxidoreductase enzyme to scavenge the accumulated intracellular reactive oxygen species during the DDVP degradation process,in whichTrichodermaand the intracellular enzyme system were fully prevented from DDVP oxidation damages.Several enzymes can degrade OP and different ways are being conducted to improve their efficiency and stability (Candidaetal.2022).
Similar to our previous study,the mineralization effect was confirmed as the major way to degrade organophosphorus pesticides byT.atrovirideT23 (Sunetal.2019a),in which we speculated that glucose is not just conducive to T23 growth,but also beneficial for the synthesis of degradation enzyme by providing exogenous energy.Actually,the T23 produced massive cellulase in natural soil (Fanetal.2015) and decomposed the crop residues from soil to release the glucose,suggesting that the character of T23 satisfied the conditions of intensified degradation enzyme production to accomplish efficient DDVP degradation in the natural environment.Although DDVP were more prone to conduct self-degradation at the defined alkaline condition,it should be noted that DDVP degradation enzyme were highly active within the pH range from 5 to 7,which is also fitted the T23 growth.Based on our work,it was supposed that DDVP degradation plausibly depended on the synergistic action of multiple enzymes instead of single enzyme.The present study only reflects integrating degradation capability of degradation enzyme mixture from T23,therefore the different components in the multiple enzymes will be identified separately in the future.
5.Conclusion
TrichodermaatrovirideT23 has been confirmed to degrade organophosphate chemical pesticide DDVP,indicating T23 has multiple functions involved in biocontrol of plant diseases and soil remediation to remove chemicals pollution.This study firstly investigated that the DDVP degrading enzyme is a kind of induced enzymes,implying that DDVP specific induction of T23 was necessary for the activation of degrading enzymes,however,the induction effect was dependent on the concentration of DDVP.T23 can produce amount of degradation enzymes specifically induced by DDVP,in which the metabolism of T23 was also affected by DDVP stress which then leaded to high production of antioxidants beneficial to protect T23 in the DDVP stress.
The degradation enzyme system of T23 on DDVP was classified as inducible enzymes and the intracellular enzyme activity was persistently higher than extracellular enzyme activity.The peak values of intracellular lactonase,arylesterase and POD activity presented the escalation from 6 to 48 h and then gradual decline in the presence of DDVP.In addition,DDVP triggered the increase on the concentration of organophosphorus hydrolase PON1.The protease consisting of PON1,lactonase and arylesterase activity were intimately associated with the DDVP degradation.Overall,this study provides detailed insights into the physiological basis related to DDVP degradation byTrichoderma.The relevant literature on the development of DDVP biodegradable enzymes andTrichodermastrains keeps deficient.Potential genomic and proteomic technologies can be fully considered further in-depth study in the biodegradation mechanisms and metabolites of DDVP.
Acknowledgements
The authors are thankful to Dr.Xu Lurong,School of Agriculture and Biology,Shanghai Jiao Tong University,China,for providing guidance with the operation of the GC–MS instrument and the data analysis.This work was supported by the National Natural Science Foundation of China (31872015),the Shanghai Science and Technology Innovation Action Program of the Shanghai Science and Technology Commission,China (21N41900200),the Shanghai Agricultural Applied Technology Development Program (2022-02-08-00-12-F0-01143),the China Agriculture Research System of MOF and MARA (CARS-02),and the National Key R&D Program of China (2017YFD0200403).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Combining nitrogen effects and metabolomics to reveal the response mechanisms to nitrogen stress and the potential for nitrogen reduction in maize
- Natural variations and geographical distributions of seed carotenoids and chlorophylls in 1 167 Chinese soybean accessions
- Carbon sequestration rate,nitrogen use efficiency and rice yield responses to long-term substitution of chemical fertilizer by organic manure in a rice–rice cropping system
- ldentification and epitope mapping of anti-p72 single-chain antibody against African swine fever virus based on phage display antibody library
- Dissecting the genetic basis of grain color and pre-harvest sprouting resistance in common wheat by association analysis
- The PcERF5 promotes anthocyanin biosynthesis in red-fleshed pear (Pyrus communis) through both activating and interacting with PcMYB transcription factors
