Physicochemical properties and antibacterial mechanism of theabrownins prepared from different catechins catalyzed by polyphenol oxidase and peroxidase
2023-09-16CHENXiaoqiangLIUJiayanHUANGXuejunWEIYananSHAORuixiangCHENTingtingXIEJianchun
CHEN Xiao-qiang,LIU Jia-yan,HUANG Xue-jun,WEI Yan-an,SHAO Rui-xiang,CHEN Ting-ting,XIE Jian-chun
1 Cooperative Innovation Center of Industrial Fermentation (Ministry of Education & Hubei Province)/Key Laboratory of Fermentation Engineering (Ministry of Education)/School of Bioengineering and Food,Hubei University of Technology,Wuhan 430068,P.R.China
2 School of Light Industry,Beijing Technology & Business University (BTBU),Beijing 100048,P.R.China
Abstract
Theabrownins (TBs) are the characteristic functional and quality components of dark teas such as Pu’er tea and Chinbrick tea.TBs are a class of water-soluble brown polymers with multi-molecular weight distribution produced by the oxidative polymerisation of tea polyphenols during the fermentation process of dark tea,both enzymatically and non-enzymatically.TBs have been extracted and purified from dark tea all the time,but the obtained TBs contain heterogeneous components such as polysaccharides and caffeine in the bound state,which are difficult to remove.The isolation and purification process was tedious and required the use of organic solvents,which made it difficult to industrialise TBs.In this study,epigallocatechin (EGC),epigallocatechin gallate (EGCG),epigallocatechin gallate (ECG),EGC/EGCG (mass ratio 1:1),EGCG/ECG (mass ratio 1:1),EGC/ECG (mass ratio 1:1) and EGC/EGCG/ECG (mass ratio 1:1:1) as substrates and catalyzed by polyphenol oxidase (PPO) and peroxidase (POD) in turn to produce TBs,named TBs-dE-1,TBs-dE-2,TBs-dE-3,TBs-dE-4,TBs-dE-5,TBs-dE-6 and TBs-dE-7.The physicochemical properties and the antibacterial activity and mechanism of TBs-dE-1–7 were investigated.Sensory and colour difference measurements showed that all seven tea browning samples showed varying degrees of brownish hue.Zeta potential in aqueous solutions at pH 3.0–9.0 indicated that TBs-dE-1–7 was negatively charged and the potential increased with increasing pH.The characteristic absorption peaks of TBs-dE-1–7 were observed at 208 and 274 nm by UVvisible (UV-vis) scanning spectroscopy.Fourier transform infrared (FT-IR) spectra indicated that they were phenolic compounds.TBs-dE-1–7 showed significant inhibition of Escherichia coli DH5α (E.coli DH5α).TBs-dE-3 showed the strongest inhibitory effect with minimum inhibitory concentration (MIC) of 1.25 mg mL–1 and MBC of 10 mg mL–1,followed by TBs-dE-5 and TBs-dE-6.These three TBs-dEs were selected to further investigate their inhibition mechanism.The TBs-dE was found to damage the extracellular membrane of E.coli DH5α,causing leakage of contents,and increase intracellular reactive oxygen content,resulting in abnormal cell metabolism due to oxidative stress.The results of the study provide a theoretical basis for the industrial preparation and product development of TBs.
Keywords: theabrownins,catechins,enzymatic oxidation,physicochemical properties,antibacterial mechanism
1.Introduction
Theabrownins (TBs) are a class of water-soluble brown polymers with multi-molecular weight distribution produced by the oxidative polymerisation of tea polyphenols during the “pile-fermentation” process of dark tea such as Pu’er tea,Fu-brick tea,Chin-brick tea,Liupao tea,both enzymatically and non-enzymatically.It is the characteristic quality and function component of dark tea (Pengetal.2013a; Chenetal.2022b; Huetal.2022).Many studies have shown that theabrownins have anticancer,hypolipidemic,and bacteriostatic effects (Pengetal.2013b; Durazzoetal.2019; Wuetal.2020; Fuetal.2021; Xuetal.2022).While theabrownins are typically extracted from dark tea,they will often be combined with polysaccharides,protein,caffeine and other substances,and the compositions will be complex (Wangetal.2011; Gongetal.2012; Pengetal.2013a; Xiaoetal.2020; Wang Yetal.2021).Therefore,the mechanism and structure of oxidative polymerization remain unclear.At the same time,the separation and purification of the extracted theabrownins are difficult and require the use of organic solvents and other reagents,which creates additional pollution and can be hazardous.Therefore,it is of great significance to develop a simple and low-cost extraction method that yields high-purity theabrownins.
EscherichiacoliDH5α (E.coliDH5α) is a Gramnegative,faculty-anaerobic,rod-shaped bacterium commonly found in the lower digestive tract of endothermic organisms (Ebenezer and Pallen 2022).MostE.coliDH5α strains naturally found in the gut are not pathogenic,but virulent strains can cause gastroenteritis,hemorrhagic colitis,and Crohn’s disease (Khanetal.2017; Zhugeetal.2019).Nibiretal.(2017) found that the minimum antibacterial concentration of green tea againstE.coliDH5α was 575 μg mL–1.Duhetal.(2004) found that polyphenols found within Pu’er tea interact with microbial proteins to inhibit microbial growth.
In this study,(–)-epigallocatechin gallate (EGCG),(–)-epigallocatechin (EGC),and (–)-epicatechin gallate (ECG) underwent oxidative polymerization by polyphenol oxidase (PPO) and peroxidase (POD) to obtain a series of theabrownins (TBs catalyzed using double enzymes,TBs-dE).Then,the physicochemical properties of these TBs-dE were analyzed,and their antibacterial mechanisms againstE.coliDH5α were explored.We anticipate that these results will provide a practical basis for the further study of theabrownins and further promote the industrialization of theabrownins.
2.Materials and methods
2.1.Materials
EGC,ECG,and EGCG were purchased from Hangzhou Luyuan Technology Co.,Ltd.(Hangzhou,China).PPO (activity: 2 000 U g–1) was purchased from Hefei Bomei Biotechnology Co.,Ltd.(Hefei,China).POD (activity>150 U mg–1) was purchased from Suzhou Tianke Trading Co.,Ltd.(Suzhou,China).Yeast extract,peptone,and NaCl were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).2-Nitrophenyl-β-Dgalactopyranoside (ONPG) was purchased from Shanghai Aladdin Biochemical Technology Co.,Ltd.(Shanghai,China).The reactive oxygen species (ROS) assay kit was purchased from Beijing Solarbio Science and Technology Co.,Ltd.(Beijing,China).
2.2.Preparation of TBs-dE using polyphenol oxidase and peroxidase
EGC,EGCG,ECG,EGC/EGCG (mass ratio 1:1),EGCG/ECG (mass ratio 1:1),EGC/ECG (mass ratio 1:1),and EGC/EGCG/ECG (mass ratio 1:1:1) were used as substrates for oxidation catalyzed by PPO and POD under certain conditions,respectively.Theabrownins were prepared by enzymatic oxidation according to a previously published protocol (Chen 2013).The obtained theabrownin samples were named TBs-dE-1,TBs-dE-2,TBs-dE-3,TBs-dE-4,TBs-dE-5,TBs-dE-6,and TBs-dE-7,respectively.
2.3.Detection of possible residues of catechins and PPO and POD in TBs-dE
A high performance liquid chromatography (HPLC) system equipped with an Agela-C18 column (4.6 mm×250 mm,5 μm; Agela Technologies,Tianjing,China) was used for separation and analysis of the purities of the enzymatically produced TBs-dE.The mobile phase consisted of 2% acetic acid in water (solvent A) and acetonitrile (solvent B).An elution gradient profile was utilized for separation,which consisted of 0–16 min,6.5–15% B; 16–25 min,15–25% B; and 25–30 min,25–6.5% B.The mobile phase flow rate was 1.00 mL min–1,the sample injection volume was 10 μL,the detection wavelength was 280 nm,and the column temperature was 40°C.The samples were centrifuged at 8 500 r min–1for 10 min at a concentration of 0.10 mg mL–1and then filtered through a 0.22-μm filter membrane.
Because the theabrownins were synthesized by aninvitroenzymatic oxidation method,it was necessary to determine whether there were PPO and POD residues in the samples.Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (Duhetal.2004; Chenetal.2022a) was used for the determination.With marker as the positive control,the additive amount was 3 μL.The concentration of the theabrownins was 10.00 mg mL–1.
2.4.Chrominance of the TBs-dE solid powder
L*,a*,and b* chromaticity system was used to analyze the chromaticity differences of EGC,EGCG,ECG,and TBs-dE-1–7 (Chenetal.2022a).A colorimeter (CHROMA METER CR-400,Konica Minolta,Shanghai,China) was used to measure the chromaticity of solid samples.
To quantitatively analyze the color differences between the TBs-dE samples 1–7; the color difference value of the lightest sample TBs-dE-1 was taken as the benchmark,and the color differences were calculated as follows:
ΔE*=[(ΔL*)2+(Δa*)2+(Δb*)2]1/2,
where ΔL*=L*(TBs-dE-X)–L*(TBs-dE-1),Δa*=a*(TBsdE-X)–a*(TBs-dE-1),Δb*=b*(TBs-dE-X)–b*(TBs-dE-1);Xrepresents 2,3,4,5,6,and 7.
2.5.Zeta potential of the TBs-dE aqueous solution
First,1.00 mg mL–1TBs-dE-1–7 aqueous solutions were prepared,and 0.10 mol L–1HCl and NaOH were used to adjust the pH of the solutions from 3 to 9 at intervals of 1.0 pH units.The Zeta potentials of the TBs-dE solutions at each pH were measured using a Zetasizer Nano-ZS particle diameter and potentiometric analyzer (Malvern,UK) to determine their acid-base stability (Hanetal.2020; Chenetal.2022b,c).
2.6.Spectral analysis of TBs-dE
Ultraviolet–visible (UV–vis) absorption spectra were acquired on a TU-1900 spectrophotometer (Persee,Beijing,China).First,solutions (10.00 μg mL–1) of the TBs-dE-1–7 in ultrapure water were prepared,and then spectra were acquired over the window between 200 and 800 nm; water was used as a blank (Chenetal.2019).
Fourier transform infrared (FT-IR) spectra of the TBsdE derivatives were acquired on a Nicolet Nexus 670 FT-IR spectrometer (Brook Instruments Inc.,Dresden,Germany) over the frequency range of 4 000–400 cm–1at a resolution of 4 cm–1and an average of 32 scans per sample (Xiaoetal.2020).
2.7.Micromorphological features of TBs-dE
Scanning electron microscopy (SEM,Japan NTC JSM-6390LV) was used to observe the microscopic morphologies of the TBs-dE derivatives.The solid powder of TBs-dE-1–7 were fixed on the sample table with conductive adhesive and sprayed with gold.SEM images were then taken at 1 500× magnification at a voltage of 10 kV (Wang L Betal.2021).
In addition to the powder,aqueous solutions (0.01 mg mL–1) of the TBs-dE-1–7 were prepared,of which 10 μL was dried on a mica sheet and observed by atomic force microscopy (AFM).The images were captured by a Bruker MultiMode 8 atomic force microscopy system (Karlsruhe,Germany) (Zhangetal.2019).
2.8.Inhibitory activity of TBs-dE against E.coli DH5α
Configuration of the culture mediumLB liquid medium was prepared with 1% sodium chloride,0.5% yeast extract,and 1% peptone.Following,the LB liquid medium was supplemented with 1.5% agar and then sterilized at 115°C for 20 min.
Determina t ion of t he minimum inhibi t o r yconcentration (MIC) and the minimum bactericidal concentration (MBC) of TBs-dE against E.coli DH5αSolutions (80.00 mg mL–1) of the TBs-dE-1–7 were prepared and then serial diluted to 40,20,10,5,2.5,1.25,0.625,and 0.3125 mg mL–1.Then,96-well plates were seeded with 100 μL of theE.coliDH5α bacterial suspension (bacterial density: 106CFU mL–1),which were supplemented with the sample solutions at different concentrations.The solutions were mixed and incubated in a constant temperature incubator at 37°C for 24 h,and the absorbances of each solution were measured at 600 nm.Sample solutions with the same concentration without bacterial suspension were used as blank.The MIC of the sample represented the lowest concentration whose absorption was not higher than the blank.Take 100 μL culture medium above the MIC coated on LB agar medium and continued to incubate in a constant temperature incubator at 37°C for 24 h to observe the generation of colonies.The MBC represented the minimum sample concentration corresponding to the sterile growing plate (Yazgan 2022).
Determination of growth curvesA certain amount of theabrownin powder was weighed and dissolved in LB liquid medium,so that the final concentrations of samples were 0MIC (control),0.1MIC,0.5MIC and 1MIC,respectively.The activated bacterial suspension was inoculated into the medium containing the sample at a ratio of 1:500.The medium without bacterial suspension but containing the TBs-dE samples was used as blank.The absorbance at 600 nm was measured every 1 h after incubation for 10 h to draw the growth curves (Zhouetal.2020).
Atomic force microscopy (AFM) of bacterial morphologyA certain amount of TB in LB medium was weighed so that the concentration of each sample was 1MIC.Then,20% activated bacterial solution was added to the control group and the experimental group media,which were cultured for 4 h (180 r min–1,37°C).The bacteria were collected by centrifugation (3 500 r min–1,5 min) and fixed in 2.5% (v/v)glutaraldehyde at 4°C for 4 h,after which the solutions were centrifuged,and the glutaraldehyde (supernatant) was discarded.The pellet was resuspended in sterile water,and an aliquot (10 μL) of the bacterial suspension was adsorbed onto mica sheet and allowed to dried naturally,after which the changes in cell morphology ofE.coliDH5α were observed by AFM (Alzahranietal.2020).
Permeability of the outer membraneA certain amount of TB in LB medium was weighed so that the final concentration of the samples was 0MIC (control),0.1MIC,0.5MIC,and 1MIC.Then,20% activated bacterial solution was added,and the mixtures were cultured for 5 h (180 r min–1,37°C).A total of 1 mL centrifuged supernatant was sampled at 1 h intervals.After a 100-fold dilution,the absorbance was measured at 260 nm to determine the leakage of nucleic acids inE.coliDH5α (Zengetal.2021).
Permeability of the inner membraneA certain amount of TB in LB medium was weighed so that the final concentration of the samples was 0MIC (control),0.1MIC,0.5MIC,and 1MIC.Then,20% activated bacterial solution was added,and the mixtures were cultured for 4 h (180 r min–1,37°C).The bacteria were washed with PBS (0.1 mol L–1,pH 7.4) and collected by centrifugation.The OD420of the bacterial suspension was adjusted to 1.0 by enzyme labeling instrument,then 190 μL of the bacterial suspension was taken and mixed with 10 μL of ONPG (30 mmol L–1).The mixture was placed at room temperature for 20 min and centrifuged (3 500 r min–1,5 min),and the absorbance of the supernatant was measured at 420 nm (Je and Kim 2006; Zhouetal.2020).
Intracellular reactive oxygen species (ROS)The procedure for handling bacterium were the same as above.The OD600of the bacteria suspension was adjusted to 1.0 with 0.85% saline solution,and 1.00 mL of the bacterial solution was removed and centrifuged (3 500 r min–1,2 min).The pelleted bacteria were resuspended in a 10.00 μmol L–1solution of the DCFHDA probe and cultured for 1 h (180 r min–1,37°C).A 200-μL aliquot of each sample was pipetted onto a dark microporous fluorescent plate,and the fluorescence intensity was measured with a Fluorescent Enzyme Labeler.The excitation wavelength was 488 nm,the emission wavelength was 525 nm,the slit width was 10 nm,and the voltage was 300 V (Gomesetal.2005).
2.9.Statistical analysis
Data were analyzed by Origin 2019 and SPSS Statistics 21.To determine the statistical significance between individual groups of data obtained from three independent assays performed in duplicate for each sample,non repetitive one-way ANOVA was used.Statistical significance was set atP<0.05.
3.Results
3.1.Determination of possible residues of catechins and PPO and POD in TBs-dE
HPLC chromatograms of the three catechin substrates are shown in Fig.1-A–C.The mass percentage of EGC,EGCG,and ECG were 97.95,85.01,and 83.51%,respectively.HPLC chromatograms of the TBs-dE-1–7 are shown in Fig.1-D–J.Neither TBs-dE-1 nor TBs-dE-6 had substrate residues.However,there was 5.85% GA residue in TBs-dE-2,while TBs-dE-3 contained 1.74% GA,11.51% ECG,and 2.36% EGCG.Furthermore,there was 1.71% GA residue in TBs-dE-4,and TBs-dE-5 contained 3.17% GA and 4.83% ECG.Lastly,TBs-dE-7 contained 2.23% GA and 1.06% ECG residue.
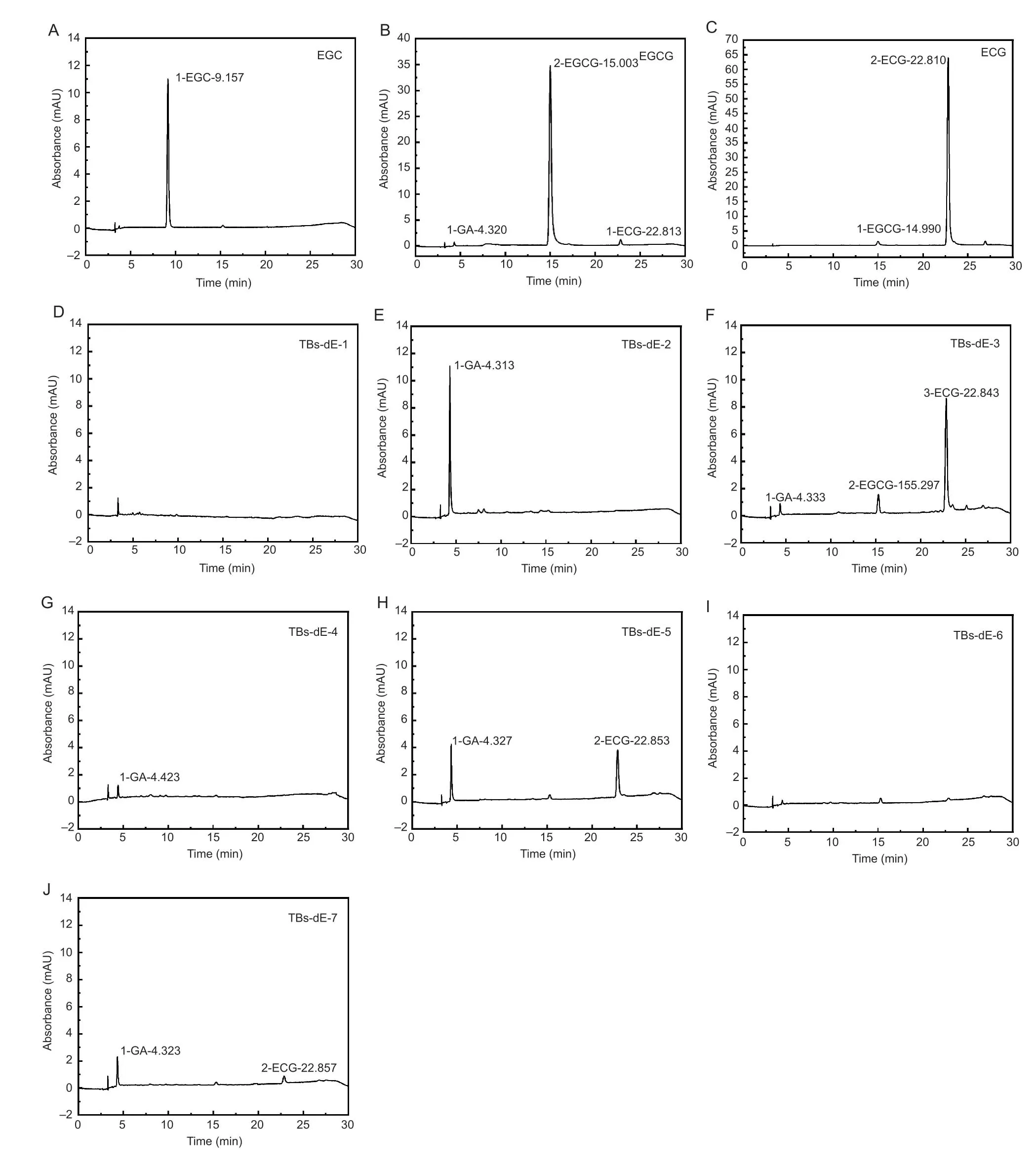
Fig.1 High performance liquid chromatography (HPLC) chromatograms of (–)-epigallocatechin (EGC),(–)-epigallocatechin gallate (EGCG),(–)-epicatechin gallate (ECG),and theabrownins (TBs) catalyzed using double enzymes (TBs-dE).
Fig.2-A showed the SDS-PAGE results.Bands 1 and 10 showed the electrophoretic phenomenon of protein marker.Band 9 was the blank control.Bands 2–8 showed the electrophoretic phenomenon of TBs-dE-1–7,and no protein showed up,indicating that there was no residual PPO and POD in TBs-dE.
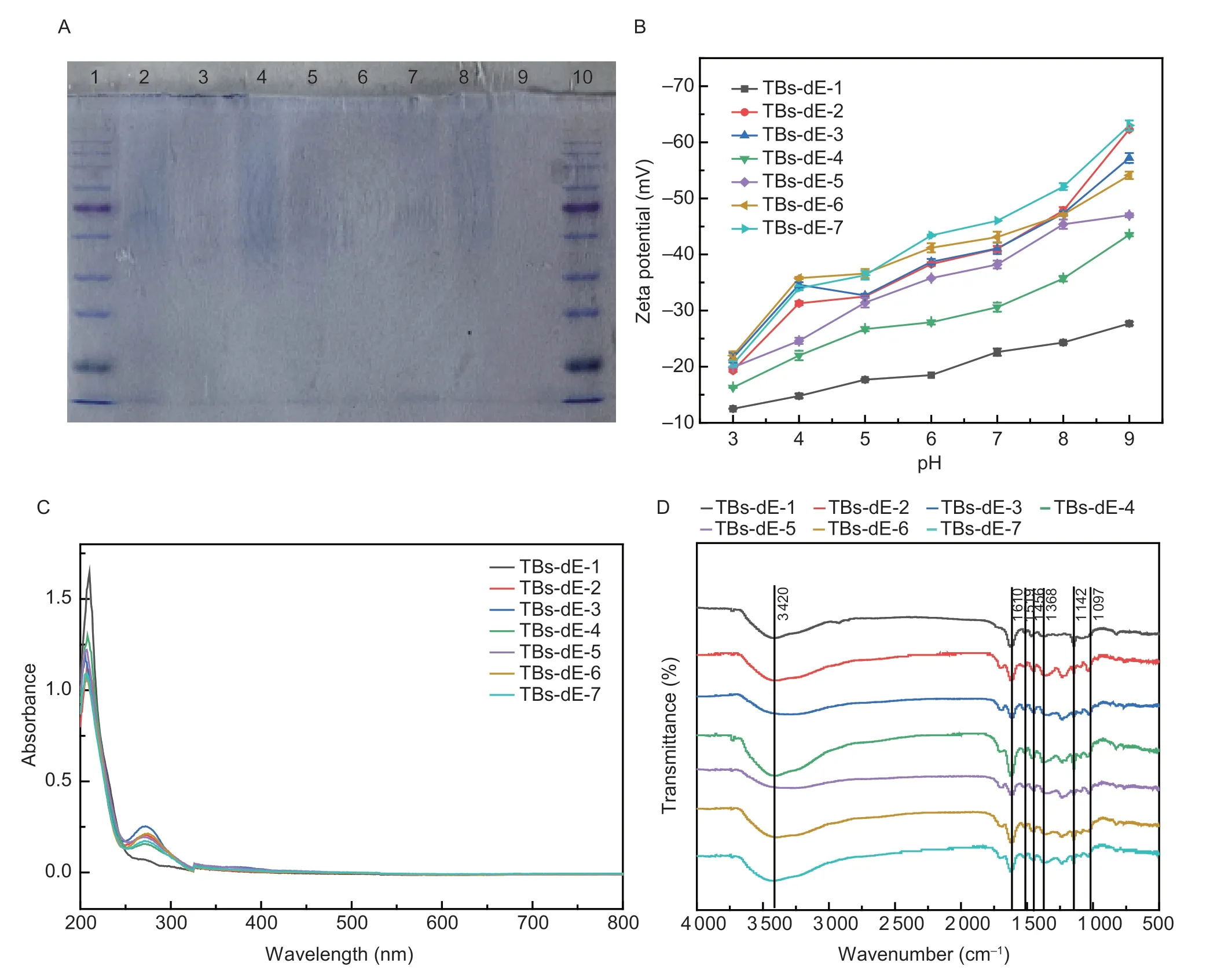
Fig.2 Physicochemical properties of theabrownins.A,polyphenol oxidase (PPO) and peroxidase (POD) residues in theabrownins (TBs) catalyzed using double enzymes (TBs-dE) detected by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDSPAGE).Lanes 1 and 10 were markers,while lanes 2–8 were TBs-dE-1,TBs-dE-2,TBS-dE-3,TBs-dE-4,TBs-dE-5,TBs-dE-6,and TBs-dE-7,in order.B,Zeta potential values of aqueous solutions of TBs-dE-1–7 from pH 3.0–9.0.C,UV-visible (UV-vis) spectra of TBs-dE-1–7.D,Fourier transform infrared (FT-IR) spectra of TBs-dE-1–7.
3.2.Chrominance analysis
Except for TBs-dE-1,the other six TBs-dE-2–7 showed a distinct brownish hue that differed significantly from the white,yellowish-white colour of the raw material catechin (Fig.3).The LAB chromatic difference system quantitatively characterized the chrominance of these TBs-dE and the raw material catechin,as shown in Table 1.Since TBs-dE-1 was the lightest in colour,using it as a benchmark,the other TBs-dE-2–7 had a chromatic difference ΔE* of 28.94,21.81,10.84,24.25,20.16,and 26.20 in that order.
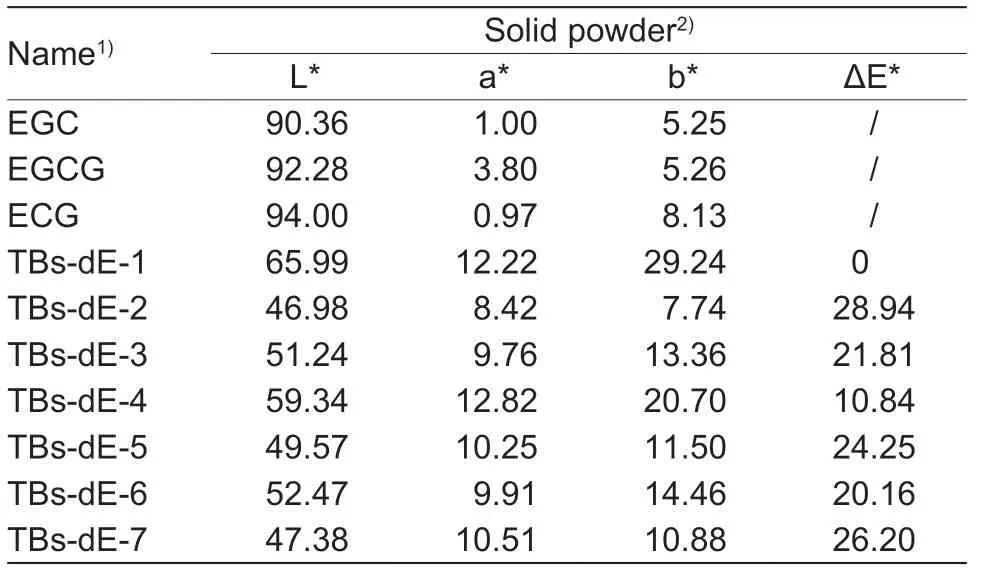
Table 1 Differences in color among TBs-dE-1–7 solid powders

Fig.3 Colors of TBs-dE solid powders.EGC,(–)-epigallocatechin; EGCG,(–)-epigallocatechin gallate; ECG,(–)-epicatechin gallate; TBs-dE,theabrownins catalyzed using double enzymes.
3.3.Zeta potential of TBs-dE
Theabrownins are polymers with surface charges when prepared in aqueous solutions.As shown in Fig.2-B,the zeta potentials of TBs-dE-1–7 in both acidic and alkaline solution were negative,indicating that they are both acidic oxidation products.The absolute value of TBs-dE-1–7 increased with increasing pH in the range of 3.0 to 9.0,which indicated that the theabrownin was more stable and less prone to precipitation in alkaline solution.The biggest change was TBs-dE-2,which increased from 19.3 to 62.3 mV.
3.4.Spectral characteristics of TBs-dE
To understand the structural properties of the TBs-dE,ultraviolet and infrared spectra were analyzed.The UV spectra are shown in Fig.2-C.The UV absorption properties of all seven TBs-dE derivatives were similar,demonstrating strong characteristic absorption peaks at 208 and 274 nm,while no obvious absorption was observed in the visible region.This indicated that the aromatic C=C content in TBs-dE was high (Xiaoetal.2023).
As shown in Fig.2-D,the FT-IR spectra of TBs-dE-1–7 featured absorption bands at 3 420,1 610,1 519,1 456,1 368,1 142,and 1 097 cm–1.The strong and broad peaks near 3 400 cm–1were characteristic absorption peaks of O–H tensile vibrations.The absorption peak at 1 368 cm–1indicated the presence of carboxyl and carbonyl groups,while the absorption bands at 1 142 and 1 097 cm–1were attributed to C–O stretching vibrations.Except for TBsdE-1,the FT-IR spectra of TBs-dE-2–7 featured absorption peaks at 1 697 and 1 038 cm–1,which were the result of C=O stretching vibrations and C–O stretching vibrations,respectively.Additionally,the FT-IR spectra of TBs-dE-1,TBs-dE-3,TBs-dE-4,TBs-dE-6,and TBs-dE-7 featured absorption bands at 820 cm–1,while only the spectra of TBs-dE-2,TBs-dE-3,TBs-dE-4,and TBs-dE-5 featured absorption bands at 767 cm–1.These bands were attributed to out-of-plane C–H bending vibrations,represented by the difference in the number and position of substituents on the benzene ring (Xiaoetal.2020).These results show that,despite the difference in raw materials,the TBs-dE were endowed with similar spectral properties.Furthermore,these results indicated that the TBs-dE derivatives were a polyhydroxy-carboxyl phenolic polymers containing benzene rings,which is consistent with the results reported by previous researchers (Pengetal.2013a; Xiaoetal.2020).
3.5.Morphological characteristics of TBs-dE
As shown in the SEM images of the TBs-dE derivatives in Fig.4-A,all seven TBs-dE samples were aggregated amorphous polymers with an irregular flakey structure and relatively smooth surface.Among them,TBs-dE-4 and TBs-dE-6 were flocculent and velvety,which might have been a result of the loss of water during the lyophilization process.
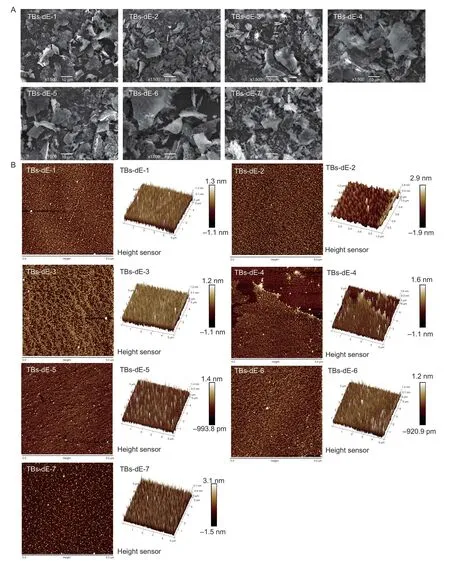
Fig.4 Scanning electron microscopy (SEM) (A) and atomic force microscopy (AFM) (B) images of theabrownins (TBs-dE-1–7).
AFM converts the height of the surface of an object by the change of the force between the microprobe and the atoms on the surface of the sample,and thus obtains the surface topography of the sample.The planar and three-dimensional structures of the TBs-dE derivatives observed by AFM are shown in Fig.4-B.All theabrownin samples had a large number of spherical clumps,indicating that the molecules were in an aggregated state.The aggregation might have been caused by the intermolecular and intramolecular hydrogen bonds between the hydroxyl groups in the TBs-dE molecules and the water molecules as well as intermolecular hydrogen bonding interactions between TBs-dE molecules (Jietal.2018).This result is consistent with the observation of significant O–H tensile vibrations observed in the FTIR spectra of the theabrownins,which has important effects on the conformation,molecular weight,and even biological activity of TBs-dE.
3.6.lnhibitory effects of TBs-dE on E.coli DH5α
The MIC and MBC of TBs-dE against E.coli DH5αTBsdE-1–7 all had inhibitory effects on the growth ofE.coliDH5α.The MICs of TBs-dE-1–7 were 20.00,20.00,1.25,20.00,1.25,2.50,and 20.00 mg mL–1,respectively,and the MBCs were 20.00,20.00,10.00,20.00,20.00,40.00,and 40.00 mg mL–1,respectively.Based on the consideration of MICs and MBCs,the antibacterial effect of TBs-dE-3 was the best,followed by TBs-dE-5 and TBs-dE-6.Therefore,these three theabrownins (TBsdE-3,TBs-dE-5,TBs-dE-6) were selected to explore their antibacterial mechanisms in follow-up experiments.
Effect of TBs-dE on the growth of E.coli DH5αAs depicted in Fig.5,compared to the control group (0MIC),the growth trend ofE.coliDH5α in the 0.1MIC group and 0.5MIC group were similar to that of the control group,and there were no obvious antibacterial effects,while in the 1MIC group,the growth rate decreased significantly,and the growth log period lag phenomenon appeared.
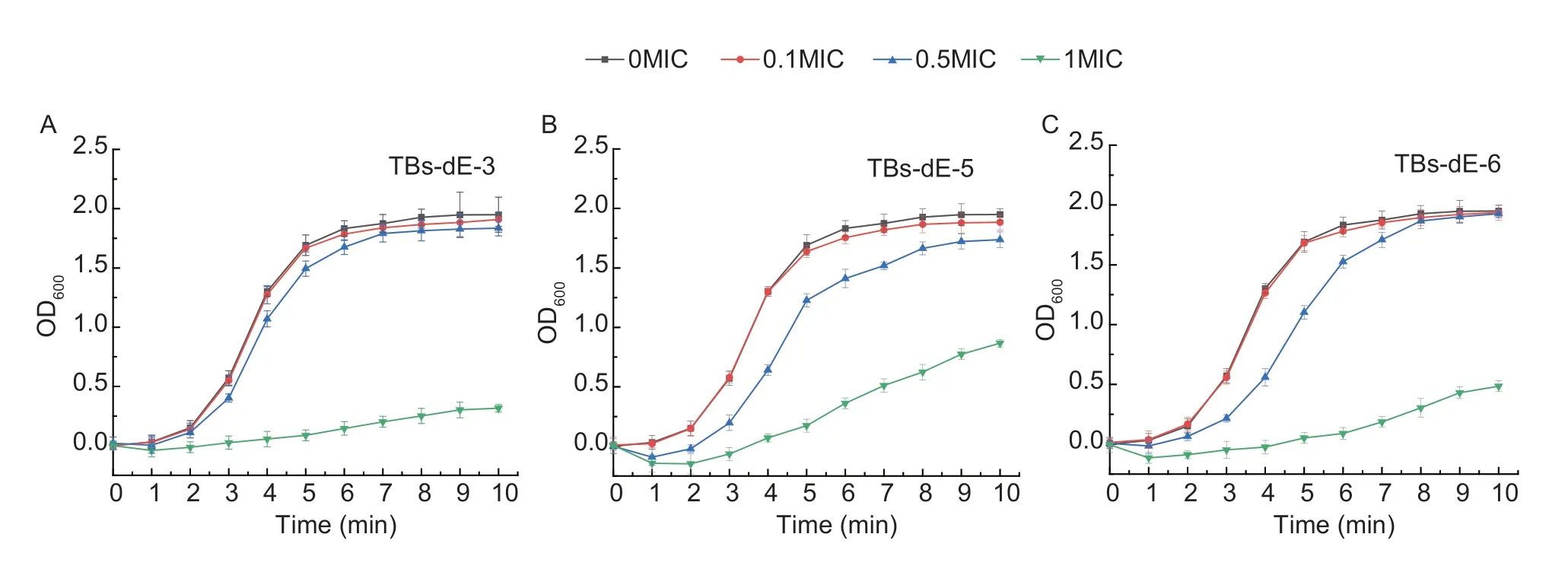
Fig.5 Growth curves of Escherichia coli DH5α.Inhibitory effects of TBs-dE-3 (A),TBs-dE-5 (B),TBs-dE-6 (C) on the growth of E.coli DH5α.TBs-dE,theabrownins catalyzed using double enzymes.MIC,minimum inhibitory concentration.
Effect of TBs-dE on the outer wall structureThe planar and three-dimensional structures ofE.coliDH5α are shown in Fig.6.In the control group,theE.coliDH5α were rod-shaped with a complete morphology and smooth surface.However,after exposure to TBsdE,most of theE.coliDH5α showed obvious shrinkage,intracellular material leakage,and cell adhesion.Among them,after treatment with TBs-dE-6,some of the bacterial cells were morphologically intact,but they also showed shrinkage.This indicated that TBs-dE interfered with the normal metabolic activities ofE.coliDH5α,making the cells shrink and eventually leading to abnormal cracking or death of the bacteria.
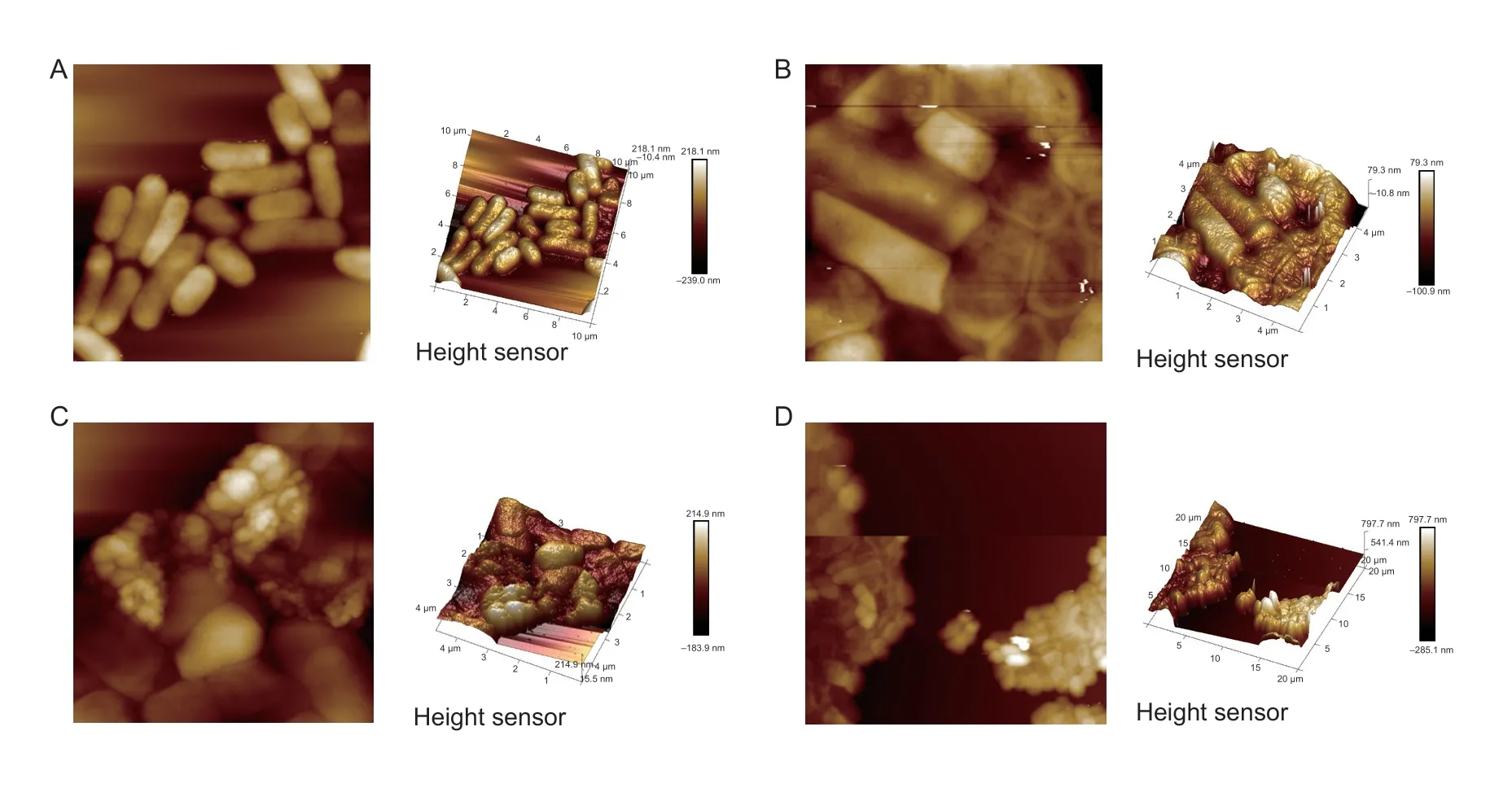
Fig.6 Atomic force microscopy (AFM) analysis.A,2D (left) and 3D (right) images of untreated Escherichia coli DH5α.B,2D (left) and 3D (right) images of TBs-dE-3-treated E.coli DH5α.C,2D (left) and 3D (right) images of TBs-dE-5-treated E.coli DH5α.D,2D (left) and 3D (right) images of TBs-dE-6-treated E.coli DH5.TBs-dE,theabrownins catalyzed using double enzymes.
Effect of TBs-dE on the permeability of the outer membraneThe nucleic acid material inE.coliDH5α is fixed in the nucleus.This material has an absorbance maximum at 260 nm,so quantifying the changes in the amount of nucleic acids in the cells before and after exposure to the theabrownins could be determined by measuring the changes in the absorbance at this wavelength (Zengetal.2021).As depicted in Fig.7,the absorbance of the control group (0MIC) did not change significantly within 5 h,indicating that the membrane permeability of untreatedE.coliDH5α did not change much.After treatment,the absorbance of the three samples was significantly higher than that of the control group (0MIC),and the longer the culture time,the greater the absorbance value.The overall absorbance of TBsdE-3,TBs-dE-5 and TBs-dE-6 in the 1MIC group was higher than that in the 0.1MIC and 0.5MIC groups,indicating that indicating that the membrane permeability ofE.coliDH5α was the strongest after the sample concentration was 1MIC.These results showed that theabrownins destroyed the bacterial structure and made the outer membrane of theE.coliDH5α more permeable,promoting the leakage of nucleic acids from the cells.
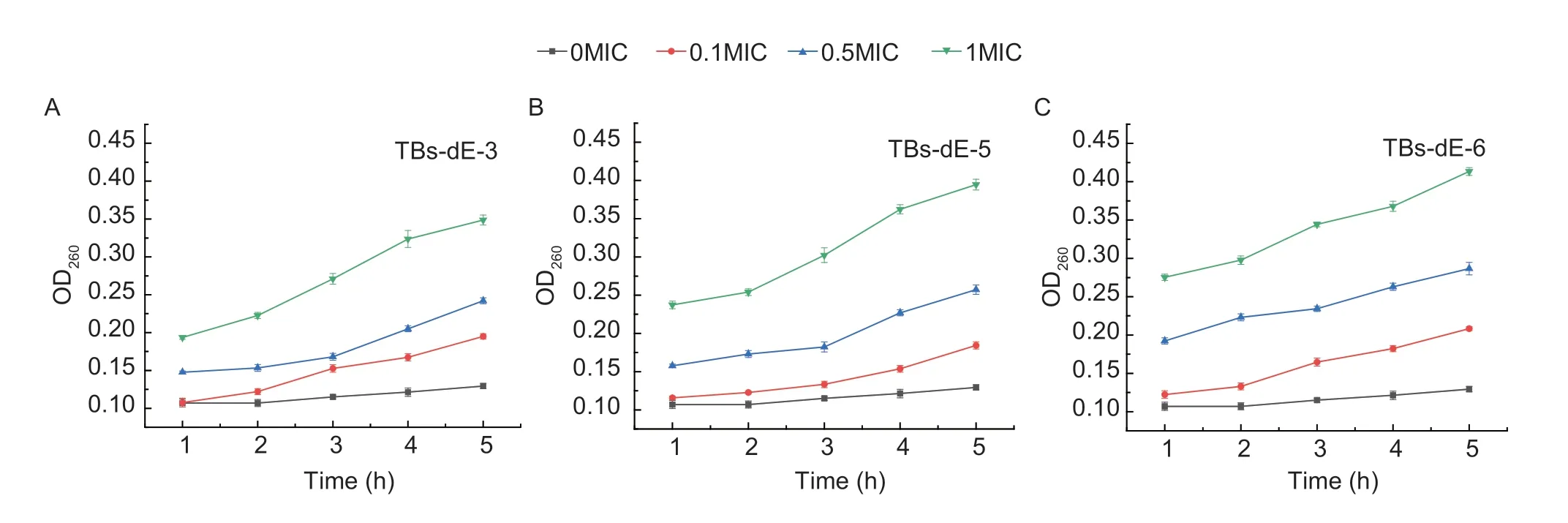
Fig.7 Effects of TBs-dE-3 (A),TBs-dE-5 (B),TBs-dE-6 (C) on the cell membrane permeability of Escherichia coli DH5α.TBs-dE,theabrownins catalyzed using double enzymes.MIC,minimum inhibitory concentration.
Effect of TBs-dE on the permeability of the inner membraneEscherichiacoliDH5α produces β-Galactosidase,which breaks down o-Nitrophenyl β-Dgalactopyranoside (ONPG) to generate o-Nitrophenol (ONP),a yellow compound that has a strong absorption at 420 nm (Je and Kim 2006; Zhouetal.2020).Therefore,the leakage of β-galactosidase in bacterial fluid is measured to judge whether the cell membrane is damaged.As depicted in Fig.8-A,compared to the control group (0MIC),after exposure ofE.coliDH5α to TBs-dE-3,TBs-dE-5,and TBs-dE-6 at each concentration,the OD420did not increase significantly,nor was it similar to the control group.These results indicated that the theabrownin had no significant effect on the inner membrane permeability ofE.coliDH5α.
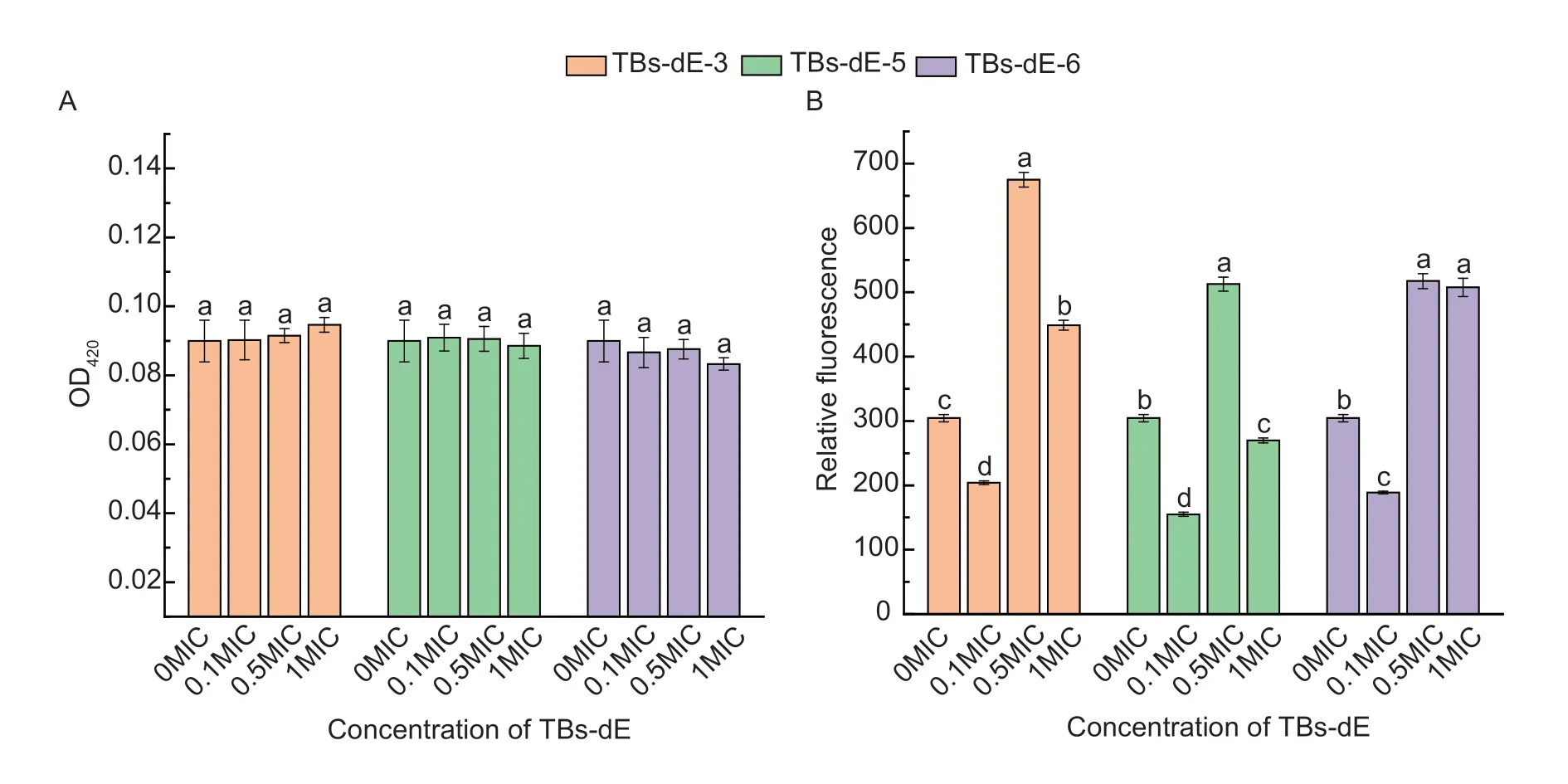
Fig.8 Effects of TBs-dE on the content of intracellular β-galactosidase leakage (A) and intracellular ROS levels (B) in Escherichia coli DH5α.TBs-dE,theabrownins catalyzed using double enzymes.MIC,minimum inhibitory concentration.All data are expressed as the mean±standard deviation (n=3).The lowercase letters indicate statistically significant differences (P<0.05).
Effect of TBs-dE on the intracellular ROSThe levels of intracellular ROS were determined using an ROS Assay Kit.The reagent that detects ROS is 2´,7´-dichlorodihydrofluorescein diacetate,which is not fluorescent.Upon entering the cell,it is hydrolyzed to DCFH by intracellular esterases.DCFH is not membrane-permeable,and intracellular ROS oxidize DCFH it to produce fluorescent DCF.Therefore,by measuring the changes in the fluorescence of DCF,it could be determined whether theabrownins caused changes in the level of ROS inE.coliDH5α (Gomesetal.2005).As depicted in Fig.8-B,at a concentration of 0.1MIC,the ROS levels in the cell were all lower than that of the control group (0MIC).When the concentration was increased to 0.5MIC,the ROS levels in the cells were significantly higher than that of the control group (P<0.05),reaching the highest at this concentration.These results indicated that the effect of TBs-dE on the active oxygen level ofE.coliDH5α was not concentration-dependent,and all three samples promoted oxidative stress inE.coliDH5α,with TBs-dE-3 having the strongest effect.
4.Discussion
Theabrownins are formed during the fermentation of dark tea.However,the presence of polysaccharides,proteins,and other macromolecular substances makes extracting the theabrownins through traditional methods very difficult and costly,so the development and application of theabrownins are limited as a result.In this study,we employed EGC,ECG,and EGCG as substrates and oxidized them by PPO and POD to prepare the theabrownins (TBs-dE).The preparation was trivial and did not involve the extraction of the theabrownins by organic reagents,making the process more environmentally friendly.This study highlighted that the theabrownins enzymatically synthesized could inhibit the growth ofE.coliDH5α,providing a practical basis for the development and application of theabrownins as natural antibacterial active substances.
We invented green technologies including dual enzyme method (polyphenol oxidase PPO,peroxidase POD) and single enzyme method (polyphenol oxidase,baking soda) to prepare TBs using tea polyphenols as raw materials,and conducted physicochemical characterization and activity studies (Chenetal.2022b; Wangetal.2022).These TBs showed significant inhibitory effects on lung cancer tumors (exvivoandinvivolevels),colon cancer cells,StaphylococcusaureusandE.coli.These series of studies will lead to the industrialization of TBs (Chenetal.2022b; Wangetal.2022).
5.Conclusion
The enzymatic preparation of theabrownins utilized in this study has several advantages over traditional theabrownin extraction methods,including lower costs and enabling higher purity,which is beneficial for product research and development.Studying the antibacterial activity and mechanism of TBs-dE is only one case to evaluate the function of enzymatic TBs production.Its activity evaluation can be carried out in more experimental models.
Acknowledgements
This research was financially supported by the National Natural Science Foundation of China (31871813).
Declaration of competing interest
The authors declare that they have no conflict of interest.
杂志排行
Journal of Integrative Agriculture的其它文章
- Comparison of cell wall changes of two different types of apple cultivars during fruit development and ripening
- Colonization by Klebsiella variicola FH-1 stimulates soybean growth and alleviates the stress of Sclerotinia sclerotiorum
- Degradation effects on dichlorvos by a biocontrol strain,Trichoderma atroviride T23
- Novel 18β-glycyrrhetinic acid amide derivatives show dual-acting capabilities for controlling plant bacterial diseases through ROSmediated antibacterial efficiency and activating plant defense responses
- Effects of methionine treatment on storage quality and antioxidant activity of postharvest jujube fruit
- A Gγ allele makes alkaline tolerance real
