真武汤对阿霉素诱导的慢性心力衰竭大鼠肠黏膜细菌多样性的影响
2023-09-07张曦宁,董振华,赵震宇,彭娜姿,胡志希,李琳
张曦宁,董振华,赵震宇,彭娜姿,胡志希,李琳
〔摘要〕 目的 检测真武汤对慢性心力衰竭大鼠肠道菌群的影响,探讨真武汤治疗慢性心力衰竭的作用机制。方法 24只大鼠分为空白組、模型组、真武汤组、美托洛尔组,每组6只,模型组、真武汤组及美托洛尔组采用腹腔注射阿霉素(3 mg/kg,每周1次,连续7周)的方法制备心力衰竭模型,造模成功后,真武汤组进行真武汤18 g/(kg·d)灌胃治疗,美托洛尔组用美托洛尔溶液10 mg/(kg·d)灌胃治疗,空白组和模型组进行蒸馏水10 mL/(kg·d)灌胃,每天1次,连续21 d。干预结束后采集黏膜刮片,进行Miseq高通量测序。结果 治疗后,模型组左室射血分数(left ventricular ejection fraction, LVEF)和左心室短轴缩短率(left ventricular ejection fractional shortening, LVFS)均明显低于空白组(P<0.01),真武汤组LVEF、LVFS均明显高于模型组(P<0.01)。与空白组相比,模型组大鼠肠道菌群Chao、Shannon、ACE指数显著升高(P<0.05,P<0.01),Simpson指数显著下降(P<0.05);与模型组相比,真武汤组各项指标出现回调。与空白组相比,模型组变形菌显著减少(P<0.01),脱硫杆菌显著增加(P<0.01),拟杆菌门、疣微菌门、酸杆菌门、螺旋菌门增加(P<0.05);与模型组相比,真武汤组变形菌、脱硫杆菌、疣微菌、酸杆菌及蛭弧菌门出现回调(P<0.05)。与空白组比较,模型组大肠志贺菌属、瘤胃球菌科显著下降(P<0.05,P<0.01),未分类的绒毛杆菌属、罗姆布茨菌属、杜氏杆菌、狭义梭菌属、颤螺旋菌科、双歧杆菌属、普雷沃菌属显著增加(P<0.05,P<0.01);与模型组比较,真武汤组大肠志贺菌属、乳酸杆菌属、杜氏杆菌呈显著回调趋势(P<0.05,P<0.01)。结论 真武汤可有效改善慢性心力衰竭大鼠肠道菌群多样性及丰富度,调节肠黏膜细菌组成与结构,这或为真武汤治疗慢性心力衰竭的潜在作用机制。
〔关键词〕 心力衰竭;真武汤;肠黏膜细菌;中医药;16S rRNA;高通量测序;生物信息学
〔中图分类号〕R285.5 〔文献标志码〕A 〔文章编号〕doi:10.3969/j.issn.1674-070X.2023.08.005
Effects of Zhenwu Decoction on bacterial diversity of intestinal mucosa in rats with doxorubicin-induced chronic heart failure
ZHANG Xining1, DONG Zhenhua1, ZHAO Zhenyu1, PENG Nazi1, HU Zhixi1,2*, LI Lin1,2*
1. Hunan University of Chinese Medicine, Changsha, Hunan 410208, China; 2. Institute of TCM Diagnostics,
Hunan University of Chinese Medicine, Changsha, Hunan 410208, China
〔Abstract〕 Objective To investigate the effects of Zhenwu Decoction on the intestinal flora of rats with chronic heart failure (HF), and to explore the mechanism of action of Zhenwu Decoction in treating this disease. Methods Twenty-four rats were divided into blank group, model group, Zhenwu Decoction group, and metoprolol group, with six rats in each group, and HF models in model group, Zhenwu Decoction group, and metoprolol group were established by intraperitoneal injection of doxorubicin (3 mg/kg, once a week, for 7 consecutive weeks). After successful modeling, Zhenwu Decoction group was given Zhenwu Decoction 18 g/(kg·d) by gavage, metoprolol group metoprolol solution 10 mg/(kg·d), and blank group and model group distilled water 10 mL/(kg·d), once a day, for 21 consecutive days. After the intervention, the intestinal mucosal scrapings of the rats were collected for high-throughput sequencing of Miseq. Results After treatment, the left ventricular ejection fraction (LVEF) and left ventricular fractional shortening (LVFS) of rats in model group were significantly lower than those in blank group (P<0.01); compared with model group, LVEF and LVFS of rats in Zhenwu Decoction group were significantly higher (P<0.01). Compared with blank group, the Chao, Shannon and ACE indexes of rat intestinal flora in model group were significantly higher (P<0.05, P<0.01), while Simpson index was significantly lower (P<0.05); compared with model group, those indexes in Zhenwu Decoction group showed a callback. Compared with blank group, the intestinal flora of rats in model group showed a significant decrease in the abundance of Proteobacteria (P<0.01), a significant increase in the abundance of Desulfobacterota (P<0.01), and an increase in the abundance of Bacteroidetes, Verrucomicr?鄄obia, Acidobacteria, and Spirochaetes (P<0.05); compared with model group, the abundance of Proteobacteria, Desulfobacterota, Verrucomicrobia, Acidobacteria, and Bdellovibrionota in Zhenwu Decoction group showed a callback (P<0.05). Compared with blank group, the intestinal flora of rats in model group showed a significant decrease in the abundance of Escherichia-Shigella and norank_f__Ruminococcaceae (P<0.05, P<0.01), and a significant increase in the abundance of norank_f__Muribaculaceae, Romboutsia, Dubosiella, Clostridium_sensu_stricto_1, norank_f__Oscillospiraceae, Bifidobacterium, and Prevotella (P<0.05, P<0.01); compared with model group, the abundance of Escherichia-Shigella, Lactobacillus, and Dubosiella showed a significant callback in Zhenwu Decoction group (P<0.05, P<0.01). Conclusion Zhenwu Decoction can effectively improve the diversity and abundance of intestinal flora in rats with chronic HF, and regulate the composition and structure of intestinal mucosal bacteria, which may be the potential mechanism of Zhenwu Decoction in treating chronic HF.
〔Keywords〕 heart failure; Zhenwu Decoction; intestinal mucosal bacteria; Chinese medicine; 16S rRNA; high-throughput sequencing; bioinformatics
心力衰竭(heart failure, HF)是一种复杂的临床综合征,常继发于心脏结构或功能异常,其特征主要为心室功能受损和外周血供不足[1-2],临床主要表现为呼吸困难、液体潴留等,严重威胁患者生命安全[3]。目前,HF患者的治疗用药多低于有效剂量,并且大部分患者未接受可改善HF预后的药物治疗,这或许是出于对不良反应的担忧[4],HF的临床治疗仍具有局限性。中国传统医药运用天然药物治疗疾病,其多种成分对多靶点发挥同时和/或协同治疗作用,具有广泛的有益作用及毒副作用小等特点[5]。有研究表明,中医药可以在HF患者中发挥对血管的保护作用及血管生成作用[6]。真武汤作为中医药经方之一,被证实可通过激活Nrf2信号减轻心脏内皮损伤[7],通过AGE-RAGE信号通路影响HF的进展[8]。
近年来,肠黏膜细菌及其代谢产物被证实在心血管疾病中起到重要作用[9]。同时,中医药通过胃肠达到对肠黏膜细菌的作用被发现[10],中药复方对肠道菌群具有调节作用,改善人体疾病状态。利用细菌基因测序、生物信息学分析等方法,研究疾病与治疗中肠黏膜细菌变化,中医药对疾病治疗作用将更加清楚地观测。因此,本实验基于16S rRNA高通量测序的方法,探讨阿霉素诱导HF大鼠肠黏膜细菌变化及真武汤治疗HF的作用机制。
1 实验材料
1.1 实验动物
SPF级SD雄性大鼠24只,体质量(200±20) g,购自湖南斯莱克景达实验动物有限公司(动物合格证号:ZS-202101160013)。本实验经湖南中医药大学动物实验中心动物实验伦理委员会审批(审批号:LLBH-201912160001),并按照实验动物伦理学标准执行。
1.2 实验材料
真武汤遵循原方由茯苓、白芍、生姜、白术、附子组成,比例为3∶3∶3∶2∶1。茯苓(批号:CX20091402)、白芍(批号:TH20080602)、白术(批号:NG20090401)均购自湖南中医药大学第一附属医院;附子(批号:DX200201)购自安徽亳药千草中药饮片有限公司。将药材用纱布袋包好置于不锈钢桶中,注水至淹没药材2~3 cm,大火烧开,小火慢煮,煎煮2次,合并药液继续煎煮至生药含量1 g/mL。注射用盐酸阿霉素(APExBIO公司,批号:A39662337769),以0.9%氯化钠溶液配制为2 mg/mL浓度备用。美托洛尔(AstraZeneca公司,批号:0902066),以0.9%氯化钠溶液配制为2 mg/mL浓度备用。戊巴比妥钠(美国SIGMA公司,批号:P3761);4%多聚甲醛(长沙威舍生物科技有限公司,批号:WB03004A)。SonoScape-S2N超声系统(深圳市开立科技有限公司);PCR仪(美国ABI公司,型号:ABI GeneAmp■ 9700 型);电泳仪(北京六一仪器厂,型号:DYY-6C)。
2 实验方法
2.1 动物分组及模型制备
24只大鼠分为8笼,每笼3只,饲养于湖南中医药大学动物实验中心,室温23~25 ℃,相对湿度40%~70%,适量给食,自由饮水。适应性培养1周,随机分为空白组(6只)、造模组(18只)。根据前期研究复制慢性HF大鼠模型[11],进行腹腔注射阿霉素(2 mg/L,1.5 mL/kg),每周1次,连续7周。将造模成功后的大鼠随机分为美托洛尔组、真武汤组、模型组,每组6只。
2.2 給药方法
模型制备后次日,给与实验组药物治疗。真武汤组进行真武汤18 g/(kg·d)治疗[12],美托洛尔组进行美托洛尔10 mg/(kg·d)治疗,模型组和空白组均给予同体积蒸馏水10 mg/(kg·d),灌胃操作每天1次,连续3周。
2.3 超声心动图检测
使用戊巴比妥钠致大鼠完全麻醉,并将其仰卧位置固定于鼠板上,予以胸部脱毛处理并均匀涂抹超声耦合剂,使用SonoScape-S2N超声系统进行超声心动图检查,得到各组大鼠左室射血分数(left ventricular ejection fraction, LVEF)及左心室短轴缩短率(left ventricular ejection fractional shortening, LVFS)。
2.4 样本收集
各组大鼠禁食12 h,使用戊巴比妥钠致大鼠完全麻醉后,用手术镊剥离部分回肠部黏膜,放至冻存管,于-80 ℃冰箱中低温保存备用。取部分心肌组织,于4%多聚甲醛溶液中固定保存,经乙醇脱水、二甲苯透明后,将其浸蜡、包埋、切片、烘干、染色,并置于光镜下观察心肌病理学改变。
2.5 PCR扩增和16s rRNA测序
扩增程序:(1)预变性95 ℃ 3 min;(2)27个循环(95 ℃变性 30 s,55 ℃退火 30 s,72 ℃延伸30 s);(3)稳定延伸72 ℃ 10 min;(4)在4 ℃进行保存。PCR产物经2%琼脂糖凝胶电泳检测,纯化后的产品利用上海美吉生物医药科技有限公司Miseq PE300/NovaSeq PE250平台进行测序。
2.6 数据处理
使用fastp[13]软件对原始测序序列进行质控,使用FLASH[14]软件进行操作,过程包括过滤、拼接、拼接筛选、修正、分析。
2.7 统计学分析
实验数据均采用SPSS 25.0统计软件进行处理,采用“x±s”表示:若数据满足正态性和方差齐性,则两组间比较采用t检验,多组间比较采用单因素方差分析;若不满足,则采用秩和检验。均以P<0.05为差异有统计学意义。
3 结果
3.1 真武汤对慢性HF大鼠心功能影响
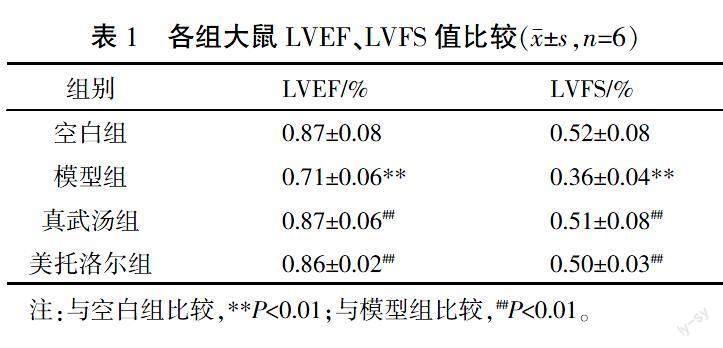
治疗后,与空白组相比,模型组大鼠LVEF、LVFS值下降,差异有统计学意义(P<0.05),提示HF造模成功;与模型组比较,真武汤组、美托洛尔组大鼠LVEF、LVFS值均上升(P<0.05)。详见表1、图1。
空白组大鼠心肌纤维排列整齐,结构完整,横纹清晰,细胞形态大小正常,细胞质染色均匀清晰;模型组大鼠心肌纤维排列紊乱,横纹不清晰,出现溶解、断裂,心肌细胞形态不规整,走形紊乱,出现水肿、肥大,细胞质染色不均匀,炎性细胞浸润明显;与模型组大鼠相比,经过真武汤和美托洛尔治疗后的大鼠心肌纤维排列、心肌细胞形态及炎性浸润均出现改善。详见图2。
3.2 真武汤对慢性HF大鼠肠道菌群多样性的影响
每组选择6只小鼠,共24个大鼠肠黏膜样本,通过16S rRNA高通量测序分析,共获得1 108 375条有效检测序列。稀释曲线可用来说明样本的测序数据量是否合理,图3曲线趋向平坦,说明测序数据量合理。
Alpha多样性反映不同样本中物种的丰富度和均匀度。与空白组相比,模型组Chao、Shannon指数升高(P<0.05),ACE指数显著升高(P<0.01),Simpson指数下降(P<0.05);与模型组相比,美托洛尔组及真武汤组各值虽有改变,但变化差异无统计学意义(P>0.05)。与美托洛尔组相比,真武汤组各数值呈回调趋势,与空白组水平接近。详见表2。
3.3 真武汤对慢性HF大鼠肠道菌群结构的影响
主坐标分析(principal co-ordinates analysis, PCoA)可用来研究样本的相似性或差异性,样品的群落组成越相似,在图中距离越接近。基于bray_curtis距离算法,ANOSIM组间差异检验绘制图4。横坐标PC1对总体菌群代表性为24.11%,纵坐标PC2为10.81%。基于PC1轴可见,模型组与空白组点位差距较大。与模型组相比,美托洛尔组点位与模型组相近,而真武汤组点位与空白组相近,说明真武汤组与空白组大鼠肠道菌群结构更为相近,这提示HF可一定程度上影响大鼠肠道菌群结构,而真武汤可调整其结构接近正常。
3.4 真武汤对慢性HF大鼠肠道菌群组成的影响
4组大鼠肠道微生物群落构成及其相对丰度的门水平分析中,所有样本均包含10种菌门:厚壁菌门(Firmicutes)、变形菌门(Proteobacteria)、unclassified_k_norank_d_Bacteria、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)、脱硫杆菌门(Desulfob?鄄acterota)、疣微菌门(Verrucomicrobia)、弯曲杆菌门(Campilobacterota)、酸杆菌门(Acidobacteria)和其他。4组中占比最高的为厚壁菌门,与空白组相比,模型组中厚壁菌占比升高;与模型组相比,美托洛尔组厚壁菌占比进一步升高,而经真武汤治疗后其占比下降。详见图5。
与模型组相比,厚壁菌/拟杆菌比值在经真武汤治疗后出现回调(P<0.05),且回调趋势较美托洛尔组更为显著。详见图6。
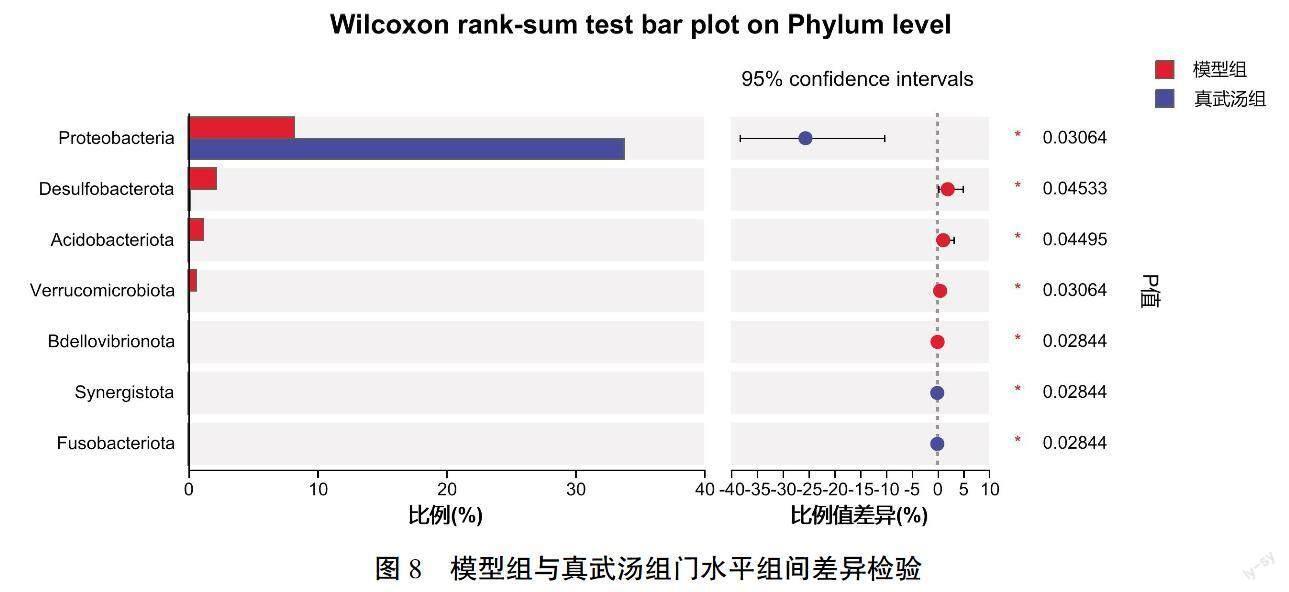
与空白组比较,模型组变形菌显著减少(P<0.01),脱硫杆菌显著增加(P<0.01),拟杆菌门、疣微菌门、酸杆菌门、螺旋菌门(Spirochaetes)增加(P<0.05);与模型组比较,真武汤组大鼠肠道中变形菌、脱硫杆菌、疣微菌、酸杆菌及蛭弧菌门(Bdellovibrionota)数量出现回调(P<0.05);而美托洛尔治疗后,未出现各菌群回调。详见图7—8。
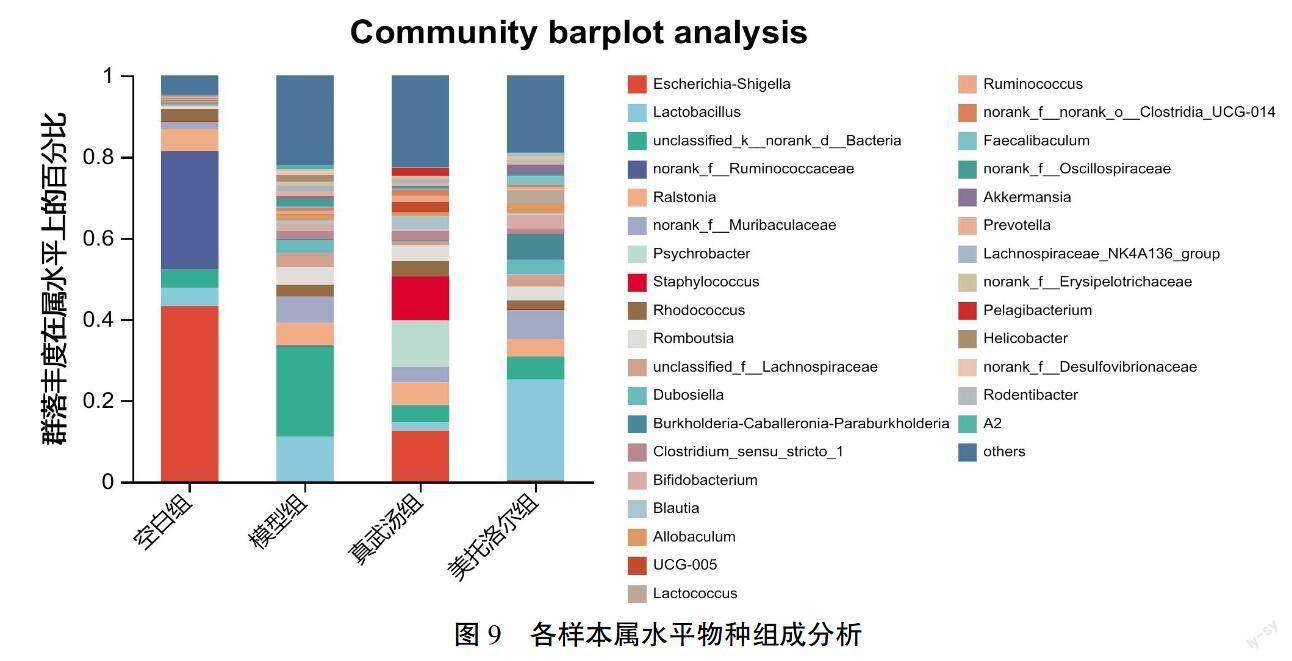
属水平上,空白组优势菌群为大肠志贺菌属(Escherichia-Shigella)40.27%、未分类的瘤胃球菌(norank_f__Ruminococcaceae)29.02%、罗尔斯通菌属(Ralstonia)6.28%;模型组的优势菌群为unclassified_
k__norank_d__Bacteria 21.77%、乳酸杆菌属(Lacto?鄄bacillus)11.24%、未分类的绒毛杆菌属(norank_f__
Muribaculaceae)6.66%;真武汤组优势菌群为大肠志贺菌属(Escherichia-Shigella)12.81%、嗜冷杆菌属(Psychrobacter)8.04%、葡萄球菌属(Staphylococcus)8.24%;美托洛尔组优势菌群为乳酸杆菌属(Lactobacillus)26.03%、未分类的绒毛杆菌属(norank_f__
Muribaculaceae)7.55%、urkholderia-Caballeronia-Para?鄄
burkholderia 4.89%。與空白组比较,模型组大肠志贺氏杆菌属(Escherichia-Shigella)、未分类的瘤胃球菌(norank_f__Ruminococcaceae)含量明显降低(P<0.01,P<0.05),norank_f__Muribaculaceae、罗姆布茨菌属(Romboutsia)、杜氏杆菌(Dubosiella)、狭义梭菌属(Clostridium_sensu_stricto_1)、未分类的颤螺旋菌科(norank_f__Oscillospiraceae)、双歧杆菌属(Bifidobacterium)、普雷沃菌属(Prevotella)含量增加显著(P<0.01,P<0.05),同时乳酸杆菌属(Lactobacillus)也出现了增长。与模型组比较,真武汤组大肠志贺菌属(Escherichia-Shigella)、乳酸杆菌属(Lactobacillus)、杜氏杆菌(Dubosiella)呈显著回调趋势(P<0.01,P<0.05),提示真武汤可调节HF大鼠肠道菌群趋近正常大鼠。详见图9—11。
选定LDA>4为标准,进行线性判别分析(linear discriminant analysis effect size, LefSe),检验各组菌群属水平丰富度,可见各组显著差异菌群。空白组以γ-变形菌(Gammaproteobacteria)、肠杆菌(Enterobacterales)、大肠志贺菌属(Escherichia-Shigella)、变形菌(Proteobacteria)等为主;模型组以芽孢菌(Blastocatellaceae)、Peptostreptococcales-Tissierellales、普雷沃菌(Prevotellaceae)等有害菌或条件致病菌为主;真武汤组以莫拉氏菌(Moraxellaceae)、葡萄球菌、罗姆布茨菌(Romboutsia)、Blautia、UCG-005等为主;美托洛尔组以Bacilli、乳酸杆菌等为主。详见图12—13。
4 讨论
胃肠黏膜负责营养吸收与废物分泌[15],其作用是由定植在黏膜上的大量微生物实现的,这些微生物群被称为肠道微生物群(gut microbiota, GM),具有消化食物、合成必需维生素、调节免疫系统、支持肠道功能等功能,在维持宿主代谢稳态和健康方面有着重要作用[16-17]。其中,肠道细菌被称为肠道菌群,大部分被归类为厚壁菌门和拟杆菌门。研究认为,肠道菌群与HF有密切的关系。HF患者因静脉充血会出现肠壁水肿,导致肠道内细菌的过度生长[18]。
超声心动图结果表示,与空白组相比,模型组大鼠的LVEF、LVFS显著下降(P<0.01);通过心肌组织HE染色可见,与空白组大鼠心肌组织相比,模型组大鼠心肌组织发生病理学异常改变,提示模型组大鼠心功能可能出现了损伤。经真武汤、美托洛尔治疗后,大鼠LVEF、LVFS值均显著升高(P<0.01),心肌组织心肌细胞形态及炎性浸润均改善,提示真武汤、美托洛尔可恢复大鼠心肌组织及心脏功能,从而改善HF。本实验采用16S rRNA高通量测序技术探究真武汤对慢性HF大鼠肠黏膜细菌多样性的影响。Alpha多样性中,Chao、ACE指数代表丰富度,其值越高代表丰富度越高;Shannon、Simpson指数代表多样性,Shannon值越高、Simpson值越低代表多样性越低。与空白组相比,模型组的大鼠Chao、Shannon值升高(P<0.05),ACE值显著升高(P<0.01),Simpson值下降(P<0.05),提示与空白组相比较,模型组大鼠肠道菌群丰富度与多样性升高。有害菌的过度生长也可以引起多样性的升高,这与前人研究一致[19]。经真武汤治疗后,Chao、Shannon、ACE值下降,Simpson值升高,尽管其变化差异无统计学意义,但结合β多样性PCoA图可见,与模型组相比,真武汤组点位更接近于空白组,而美托洛尔组更接近于模型组,说明经治疗后的大鼠肠黏膜细菌丰富度及多样性出现回调,相较于美托洛尔组,真武汤引起的回调更接近于空白组。
进一步研究發现,各组HF大鼠肠黏膜细菌组成发生变化。在门水平上,厚壁菌门、拟杆菌门占比最大[20],其余优势菌门为变形菌门、放线菌门、梭杆菌门和疣状杆菌门。本研究结果显示,模型组大鼠肠黏膜细菌中出现了厚壁菌和拟杆菌数量的增加,但厚壁菌/拟杆菌比例出现了下降。厚壁菌/拟杆菌相对丰度的比例变化,成为决定健康与疾病的生物标记物[21]。其比例的下降可导致短链脂肪酸(short-chain fatty acid, SCFA)的减少,促进炎症发展[22]。SCFA是代谢产物之一,在免疫与体循环方面有积极作用,被应用于压力过载引起的心脏损伤,而HF患者中生产其能力降低[23-24]。厚壁菌的增加可见于酒精性脂肪肝大鼠体内[25],同时,拟杆菌中许多物种被认为是肠道外条件致病菌,其可激活激肽释放酶-激肽系统,增加缓激肽的生成,从而导致血管渗漏[26]。除此以外,模型组大鼠肠黏膜中出现脱硫菌、放线菌的增加,脱硫弧菌被证实可以促进肠道炎症[27],放线菌的增加可见于强直性脊柱炎,可能与体内炎症状态有关[28]。在属水平上,各组大鼠肠黏膜菌群比例发生变化。与空白组相比,模型组中大肠志贺菌、瘤胃球菌减少,Muribaculaceae、罗姆布茨菌、杜氏杆菌、普雷沃菌及双歧杆菌增加。大肠志贺菌属丰度与血脂水平呈正相关[29],管腔内的长链脂肪酸(long-chain fatty acid, LCFA)或许与其有关。LCFA包括饱和脂肪酸与不饱和脂肪酸,其丰度与宿主饮食、微生物代谢、胆汁酸分泌等有关,其氧化过程是大多数心脏的能量来源[24, 30]。同时,瘤胃球菌是SCFA产生菌,在模型组大鼠中减少,这与前人研究结果相同[31],这种现象也与肠易激综合征的发生发展相关联[32]。双歧杆菌可以抑制脂多糖(lipopolysaccharides, LPS)[33],LPS可以通过上调IL-1α、HIF-α等炎症因子诱导炎症发生[34]。乳酸杆菌可以产生乳酸改善肠道环境,阻止有害细菌的黏附[35],罗姆布茨菌可能通过调节脂肪酸合成、纠正脂质代谢紊乱和减少炎症发生来发挥作用[36]。这些益生菌的升高或许是机体对炎症反应的代偿性反应,尤其是双歧杆菌,其升高被认为与抗吲哚酚酸盐相关[37]。Muribaculaceae的丰度与炎症细胞因子水平呈负相关[38];杜氏杆菌被认为与饮食中高糖基化终产物有关,并最终导致炎症[39];普雷沃菌被发现在黏膜部位的增加与疾病之间存在相关性,如代谢紊乱及全身低度炎症等[40]。由LDA判别表可见,与空白组相比,模型组大鼠肠黏膜细菌仍主要以有害菌或条件致病菌为主,除普雷沃菌外,Peptostreptococcales-Tissierellales在牙周炎患者中数量增加[41]。经过真武汤治疗后,HF大鼠肠黏膜优势菌群发生显著改变。有研究表明,莫拉氏菌在川崎病患者体内减少[42]。Blautia与体内IL-10水平呈正相关[43],而IL-10是公认的炎症抑制因子。UCG-005是属于瘤胃球菌的有益菌,是预防糖尿病的关键细菌[44]。由此推测,差异菌群可以通过诱导炎症发生、促进氧化损伤、降低脂质代谢等促进HF的发生发展,而这些过程或许与肠道菌群对免疫细胞组成、迁移和功能的调控作用及肠道菌群的易位有关[45];在HF发生发展过程中,TNF-α、IL-6、血管紧张素Ⅱ等炎症因子及TLR4/NF-κB等信号通路起到了重要作用[46],真武汤能够恢复肠道菌群,被恢复的肠道菌群或许对以上炎症因子及信号通路具有正向调节作用,从而实现真武汤对HF的治疗作用。
综上所述,真武汤对阿霉素诱导的HF大鼠肠黏膜细菌具有调节作用,使其组成与结构更接近于正常大鼠。同时,真武汤可以促使HF大鼠肠黏膜优势菌发生改变,促进有益菌增长,使菌群重新恢复稳定,从而达到治疗作用。通过16S rRNA测序技术,肠黏膜细菌与HF之间的相互作用被认识,同时也逐渐了解中医药对调节肠黏膜细菌、治疗HF的作用原理,这对疾病的预防、诊断与治疗以及中医的进步发展都有着深远的意义。随着基因测序技术的完善与发展,越来越多的肠道微生物被发现与分类,但它们的作用尚待讨论。同时,中医药对独立菌群的影响被不断证实,但仍然缺乏对肠黏膜整体作用的阐述。在未来,应进行更多验证性实验,以确定真武汤通过恢复肠黏膜菌群治疗HF的具体作用机制。
参考文献
[1] NHFA CSANZ HEART FAILURE GUIDELINES WORKING GROUP, ATHERTON J J, SINDONE A, et al. National heart foundation of Australia and cardiac society of Australia and New Zealand: Guidelines for the prevention, detection, and management of heart failure in Australia 2018[J]. Heart, Lung & Circulation, 2018, 27(10): 1123-1208.
[2] ABASSI Z, KHOURY E E, KARRAM T, et al. Edema formation in congestive heart failure and the underlying mechanisms[J]. Frontiers in Cardiovascular Medicine, 2022, 9: 933215.
[3] GEDELA M, KHAN M, JONSSON O. Heart failure[J]. South Dakota Medicine, 2015, 68(9): 403-405, 407-409.
[4] ABDIN A, BAUERSACHS J, SOLTANI S, et al. A practical approach to the guideline-directed pharmacological treatment of heart failure with reduced ejection fraction[J]. ESC Heart Failure, 2023, 10(1): 24-31.
[5] MENG T W, LI X H, LI C J, et al. Natural products of traditional Chinese medicine treat atherosclerosis by regulating inflammatory and oxidative stress pathways[J]. Frontiers in Pharmacology, 2022, 13: 997598.
[6] WU C N, CHEN F, HUANG S, et al. Progress on the role of traditional Chinese medicine in therapeutic angiogenesis of heart failure[J]. Journal of Ethnopharmacology, 2023, 301: 115770.
[7] LIU X, LI Y, NI S H, et al. Zhen-Wu Decoction and lactiflorin, an ingredient predicted by in silico modelling, alleviate uremia induced cardiac endothelial injury via Nrf2 activation[J]. Journal of Ethnopharmacology, 2022, 298: 115579.
[8] MA C Y, MA Y Q, DENG M. Mechanism of Zhen Wu decoction in the treatment of heart failure based on network pharmacology and molecular docking[J]. Evidence-Based Complementary and Alternative Medicine, 2022, 2022: 4877920.
[9] HUANG Y, ZHANG H, FAN X, et al. The role of gut microbiota and trimethylamine N-oxide in cardiovascular diseases[J]. Journal of Cardiovascular Translational Research, 2023, 16(3): 581-589.
[10] LIN T L, LU C C, LAI W F, et al. Role of gut microbiota in identification of novel TCM-derived active metabolites[J]. Protein & Cell, 2021, 12(5): 394-410.
[11] 趙震宇, 胡炎芝, 董振华, 等. 参附注射液对阿霉素诱导的心力衰竭大鼠肠道细菌多样性的影响[J]. 中成药, 2022, 44(7): 2334-2340.
[12] 劉中勇, 李 林, 方 家. 真武汤对心力衰竭模型大鼠心室重构及心肌细胞凋亡、纤维化的影响[J]. 中医杂志, 2017, 58(14): 1218-1223.
[13] CHEN S F, ZHOU Y Q, CHEN Y R, et al. Fastp: An ultra-fast all-in-one FASTQ preprocessor[J]. Bioinformatics, 2018, 34(17): i884-i890.
[14] MAGO■ T, SALZBERG S L. FLASH: Fast length adjustment of short reads to improve genome assemblies[J]. Bioinformatics, 2011, 27(21): 2957-2963.
[15] SODERHOLM A T, PEDICORD V A. Intestinal epithelial cells: At the interface of the microbiota and mucosal immunity[J]. Immunology, 2019, 158(4): 267-280.
[16] FAN Y, PEDERSEN O. Gut microbiota in human metabolic health and disease[J]. Nature Reviews Microbiology, 2021, 19(1): 55-71.
[17] LONG D, MAO C H, ZHANG X Y, et al. Coronary heart disease and gut microbiota: A bibliometric and visual analysis from 2002 to 2022[J]. Frontiers in Cardiovascular Medicine, 2022, 9: 949859.
[18] FP P, DVOROKOV M, VACHALCOV?譧 M, et al. Gut microbiome in heart failure and aortic stenosis[J]. Vnitrni Lekarstvi, 2022, 68(E-2): 4-10.
[19] ZHAO Z Y, LIU J H, HU Y Z, et al. Bacterial diversity in the intestinal mucosa of heart failure rats treated with Sini Decoction[J]. BMC Complementary Medicine and Therapies, 2022, 22(1): 93.
[20] HUTTENHOWER C, GEVERS D, KNIGHT R, et al. Structure, function and diversity of the healthy human microbiome[J]. Nature, 2012, 486(7402): 207-214.
[21] VAISERMAN A, ROMANENKO M, PIVEN L, et al. Differences in the gut Firmicutes to Bacteroidetes ratio across age groups in healthy Ukrainian population[J]. BMC Microbiology, 2020, 20(1): 221.
[22] CAI X S, DENG L, MA X G, et al. Altered diversity and composition of gut microbiota in Wilson's disease[J]. Scientific Reports, 2020, 10: 21825.
[23] PAKHOMOV N, BAUGH J A. The role of diet-derived short-chain fatty acids in regulating cardiac pressure overload[J]. American Journal of Physiology Heart and Circulatory Physiology, 2021, 320(2): H475-H486.
[24] PALM C L, NIJHOLT K T, BAKKER B M, et al. Short-chain fatty acids in the metabolism of heart failure-rethinking the fat stigma[J]. Frontiers in Cardiovascular Medicine, 2022, 9: 915102.
[25] LIU J Y, KONG L Z, SHAO M T, et al. Seabuckthorn polysaccharide combined with astragalus polysaccharide ameliorate alcoholic fatty liver by regulating intestinal flora[J]. Frontiers in Endocrinology, 2022, 13: 1018557.
[26] WANG X, CAO Y, ZHI Y X. Throat microbiota alterations in patients with hereditary angioedema[J]. The World Allergy Organization Journal, 2022, 15(10): 100694.
[27] ZHANG Y W, CHEN L Y, HU M J, et al. Dietary type 2 resistant starch improves systemic inflammation and intestinal permeability by modulating microbiota and metabolites in aged mice on high-fat diet[J]. Aging, 2020, 12(10): 9173-9187.
[28] LIU B, DING Z H, XIONG J H, et al. Gut microbiota and inflam?鄄
matory cytokine changes in patients with ankylosing spondylitis[J]. BioMed Research International, 2022, 2022: 1005111.
[29] VAVRECKOVA M, GALANOVA N, KOSTOVCIK M, et al. Specific gut bacterial and fungal microbiota pattern in the first half of pregnancy is linked to the development of gestational diabetes mellitus in the cohort including obese women[J]. Frontiers in Endocrinology, 2022, 13: 970825.
[30] MITCHELL M K, ELLERMANN M. Long chain fatty acids and virulence repression in intestinal bacterial pathogens[J]. Frontiers in Cellular and Infection Microbiology, 2022, 12: 928503.
[31] SUN W J, DU D B, FU T Z, et al. Alterations of the gut microbiota in patients with severe chronic heart failure[J]. Frontiers in Microbiology, 2021, 12: 813289.
[32] BLAD C C, TANG C, OFFERMANNS S. G protein-coupled receptors for energy metabolites as new therapeutic targets[J]. Nature Reviews Drug Discovery, 2012, 11(8): 603-619.
[33] HORWAT P, KOPE■ S, GARCZYK A, et al. Influence of enteral nutrition on gut microbiota composition in patients with Crohn's disease: A systematic review[J]. Nutrients, 2020, 12(9): 2551.
[34] WEI Y Z, WANG K, ZHANG Y F, et al. Potent anti-inflammatory responses: Role of hydrogen in IL-1α dominated early phase systemic inflammation[J]. Frontiers in Pharmacology, 2023, 14: 1138762.
[35] HUI H Y, WU Y, ZHENG T, et al. Bacterial characteristics in intestinal contents of antibiotic-associated diarrhea mice treated with qiweibaizhu powder[J]. Medical Science Monitor: International Medical Journal of Experimental and Clinical Research, 2020, 26: e921771.
[36] ZHANG F Q, WU J S, RUAN H N, et al. ZeXie Decoction alleviates non-alcoholic fatty liver disease in rats: The study of genes, lipids, and gut microbiotas[J]. Biochemical and Biophysical Research Communications, 2022, 632: 129-138.
[37] SHENG Y, ZHENG S J, MA T S, et al. Mulberry leaf alleviates streptozotocin-induced diabetic rats by attenuating NEFA signaling and modulating intestinal microflora[J]. Scientific Reports, 2017, 7: 12041.
[38] WANG K, LI W C, WANG K, et al. Litchi thaumatin-like protein induced the liver inflammation and altered the gut microbiota community structure in mice[J]. Food Research International, 2022, 161: 111868.
[39] VAN DONGEN K C W, LINKENS A M A, WETZELS S M W, et al. Dietary advanced glycation endproducts (AGEs) increase their concentration in plasma and tissues, result in inflammation and modulate gut microbial composition in mice; evidence for reversibility[J]. Food Research International, 2021, 147: 110547.
[40] LARSEN J M. The immune response to Prevotella bacteria in chronic inflammatory disease[J]. Immunology, 2017, 151(4): 363-374.
[41] KAWAMOTO D, BORGES R, RIBEIRO R A, et al. Oral dysbiosis in severe forms of periodontitis is associated with gut dysbiosis and correlated with salivary inflammatory mediators: A preliminary study[J]. Frontiers in Oral Health, 2021, 2: 722495.
[42] S?魣NCHEZ-MANUBENS J, HENARES D, MU?譙OZ-ALMAGRO C, et al. Characterization of the nasopharyngeal microbiome in patients with Kawasaki disease[J]. Anales De Pediatria, 2022, 97(5): 300-309.
[43] CHEN Q X, YIN Q L, XIE Q G, et al. 2'-fucosyllactose promotes the production of short-chain fatty acids and improves immune function in human-microbiota-associated mice by regulating gut microbiota[J]. Journal of Agricultural and Food Chemistry, 2022, 70(42): 13615-13625.
[44] LI Q Q, HU J L, NIE Q X, et al. Hypoglycemic mechanism of polysaccharide from Cyclocarya paliurus leaves in type 2 diabetic rats by gut microbiota and host metabolism alteration[J]. Science China Life Sciences, 2021, 64(1): 117-132.
[45] ZHAO J X, ZHANG Q, CHENG W, et al. Heart-gut microbiota communication determines the severity of cardiac injury after myocardial ischaemia/reperfusion[J]. Cardiovascular Research, 2023, 119(6): 1390-1402.
[46] ZHANG Z, CHEN F, WAN J J, et al. Potential traditional Chinese medicines with anti-inflammation in the prevention of heart failure following myocardial infarction[J]. Chinese Medicine, 2023, 18(1): 28.
(本文編辑 匡静之)
