右美托咪定滴鼻在小儿肺功能检查中的镇静效果
2023-08-31刘娜朴美英孟繁峥刘万超
刘娜,朴美英,孟繁峥,刘万超
·药物研究·
右美托咪定滴鼻在小儿肺功能检查中的镇静效果
刘娜1,朴美英1,孟繁峥1,刘万超2
1.吉林大学第一医院小儿呼吸科,吉林长春 130021;2.中国人民武装警察部队吉林省总队医院麻醉科,吉林长春 130052
评价右美托咪定滴鼻在小儿肺功能检查中的临床效果。选取2021年6月至2021年12月于吉林大学第一医院小儿呼吸科行肺功能检查的患儿120例,采用随机数字表法将患儿分为A组(=40)、B组(=40)和C组(=40)。A组患儿采用10%的水合氯醛按50~80mg/kg保留灌肠,B组和C组患儿分别于检查前30min将右美托咪定原液按照2.0μg/kg和3.0μg/kg进行滴鼻。待患儿睫毛反射消失后开始肺功能检查。记录3组患儿镇静前(T0)、镇静后10min(T1)、20min(T2)和30min(T3)时的平均动脉压(mean arterial pressure,MAP)、心率(heart rate,HR)、呼吸频率(respiratory rate, RR)及脉搏血氧饱和度(pulse oxygen saturation,SpO2)。记录3组患儿镇静前、镇静后10min、20min和30min时的警觉/镇静观察评分(observer’s assessment of alertness/sedation scale,OAA/S)和镇静成功率,以及3组患儿镇静期间不良反应发生情况。T0时,3组患儿的MAP、HR比较,差异均无统计学意义(>0.05)。T1时,3组患儿MAP、HR均显著低于T0(<0.05),A组和B组患儿在T1时的MAP、HR显著高于C组(<0.05)。T2、T3时,3组患儿的MAP和HR比较,差异均无统计学意义(>0.05)。在不同时间点3组患儿的RR及SpO2比较,差异均无统计学意义(>0.05)。与A组和B组比较,C组患儿镇静成功率显著升高(<0.05)。与B组比较,A组和C组镇静起效时间显著缩短(<0.05);3组患儿的苏醒时间比较,差异无统计学意义(>0.05)。镇静期间B组和C组的不良反应总发生率显著低于A组(<0.05)。右美托咪定2μg/kg滴鼻用于小儿肺功能检查可有效提高患儿的检查依从性,镇静成功率明显高于水合氯醛,且镇静期间生命体征平稳,不良反应发生率低,镇静失败补救追加右美托咪定至3μg/kg,可安全应用于临床。
右美托咪定;水合氯醛;滴鼻;肺功能检查;小儿
小儿肺功能检查是评估患儿肺部疾病进展和治疗效果的有效手段,可儿童依赖性强、合作性差,不能很好的配合完成检查,往往需要镇静辅助。水合氯醛、咪达唑仑、戊巴比妥等药物常被应用于小儿无痛性检查的镇静。水合氯醛具有催眠作用,被广泛应用于儿童无痛性检查的辅助,其机制可能是通过激活γ-氨基丁酸(γ-aminobutyric acid,GABA)受体而实现镇静[1],但其对儿童胃肠道具有刺激且影响上气道力学,近年来在临床的使用率明显下降。右美托咪定是一种高选择性α2肾上腺素能受体激动剂,能拮抗交感神经兴奋性,具有明显的镇静作用和一定的镇痛作用,且无呼吸抑制,已经逐渐应用于小儿麻醉[2]。因此,本研究旨在观察右美托咪定滴鼻和水合氯醛保留灌肠在小儿肺功能检查中的镇静效果。
1 资料与方法
1.1 一般资料
选取2021年6月至2021年12月于吉林大学第一医院小儿呼吸科行肺功能检查的患儿120例,采用随机数字表法将患儿分为A组(=40)、B组(=40)和C组(=40)。纳入标准:①患儿存在反复咳嗽、上呼吸道感染、支气管炎或喘息,经临床评估需要进行肺功能检查;②年龄1~3岁;③体质量指数(body mass index,BMI)18.0~24.0kg/m2;④美国麻醉医师协会(American Society of Anesthesiologists,ASA)分级为Ⅰ~Ⅱ级。排除标准:①有严重心动过缓或房室传导阻滞;②近1周内有严重腹泻;③有精神系统疾病。3组患儿年龄、性别、ASA分级、BMI及肺功能检查时间比较,差异均无统计学意义(>0.05),具有可比性,见表1。本研究经吉林大学第一医院医学伦理委员会审批通过(伦理审批号:2021-746),患儿家属签署知情同意书。
1.2 方法
患儿检查前禁食6h、禁饮2h。肺功能检查当天由家长陪同进入麻醉准备室,由1名不知情的麻醉护士实施给药。A组患儿采用10%的水合氯醛(生产单位:特丰制药有限公司,批准文号:H20193425,规格:1.34g:0.5g)按照50~80mg/kg的剂量给药。灌肠时患儿取侧卧位,充分暴露肛门后,使用一次性无菌注射器抽取药液后,连接导尿管,并将导尿管缓慢插入肛门7~12cm,推入药液后,用止血钳夹紧尿管尾部,然后分离注射器,并抽吸空气再次进入肛门,将药物全部推入直肠,夹紧患儿肛门并抬高其臀部,药物保留10~15min;B组和C组患儿分别于检查前30min将未稀释的右美托咪定(生产单位:江苏恒瑞医药股份有限公司,批准文号:H20090248,规格:2ml:200μg)按照2.0μg/kg和3.0μg/kg剂量,通过1ml注射器分次滴入患儿两侧鼻孔,所有患儿滴鼻后保持仰卧平躺1~2min,并轻轻按摩患儿两侧鼻翼,以确保药物吸收良好。
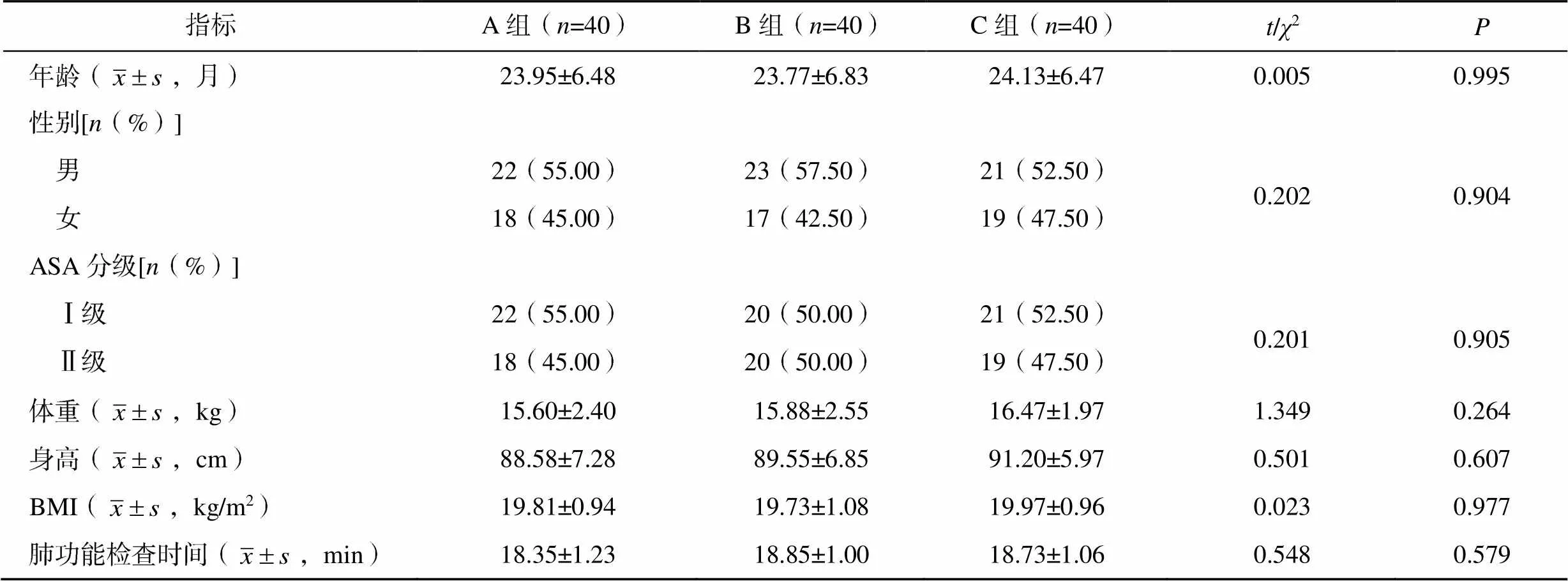
表1 三组患儿一般资料比较
1.3 观察指标
记录3组患儿镇静前(T0)、镇静后10min(T1)、20min(T2)和30min(T3)时的平均动脉压(mean arterial pressure,MAP)、心率(heart rate,HR)、呼吸频率(respiratory rate,RR)、脉搏血氧饱和度(pulse oxygen saturation,SpO2)。
记录3组患儿镇静起效时间(从患儿实施镇静开始到睫毛反射消失的时间)和苏醒时间(从患儿睫毛反射消失到患儿出现体动,呼之睁眼)。记录3组患儿镇静期间低血压、心动过缓、恶心呕吐、嗜睡、呼吸抑制(RR<10次/分或SpO2<90%持续1min)等不良反应发生情况。
记录3组患儿镇静前(T0)、镇静后10min(T1)、20min(T2)和30min(T3)时警觉/镇静评分(observer’s assessment of alertness/sedation scale,OAA/S)和镇静成功率。0分为对伤害性刺激无反应;1分为对伤害性刺激有应答,对拍身体无应答;2分为对轻拍身体有应答,对反复大声呼喊无应答;3分为对反复大声呼喊有应答,对正常呼喊无应答;4分为对正常呼喊反应迟钝;5分为对正常呼喊反应正常,处于完全清醒状态。给药后30分钟内OAA/S>3分,肺功能检查无法顺利完成,为镇静失败;OAA/S≤3分肺功能检查可顺利完成,为镇静成功。在数据处理上将0~3分归为1组,将4分和5分归为1组。
1.4 统计学方法
2 结果
2.1 不同时间点MAP、HR、RR及SpO2的比较
T0时,3组患儿的MAP、HR比较,差异均无统计学意义(>0.05)。T1时,3组患儿MAP、HR均显著低于T0(<0.05),A、B组患儿在T1时的HR显著高于C组(<0.05)。T2、T3时,3组MAP和HR比较,差异均无统计学意义(>0.05)。不同时间点3组患儿的RR及SpO2比较,差异均无统计学意义(>0.05),见表2。
2.2 不同时间点OAA/S情况及镇静成功率比较
与A组和B组比较,C组患儿镇静成功率显著升高(<0.05),见表3。
2.3 镇静起效时间和苏醒时间比较
与B组比较,A组和C组镇静起效时间显著缩短(<0.05);3组患儿的苏醒时间比较,差异无统计学意义(>0.05),见表4。
表2 三组患儿不同时间点MAP、HR、RR及SpO2的比较()
注:1mmHg=0.133kPa;与同组T0时间点比较,*<0.05;与C组比较,#<0.05
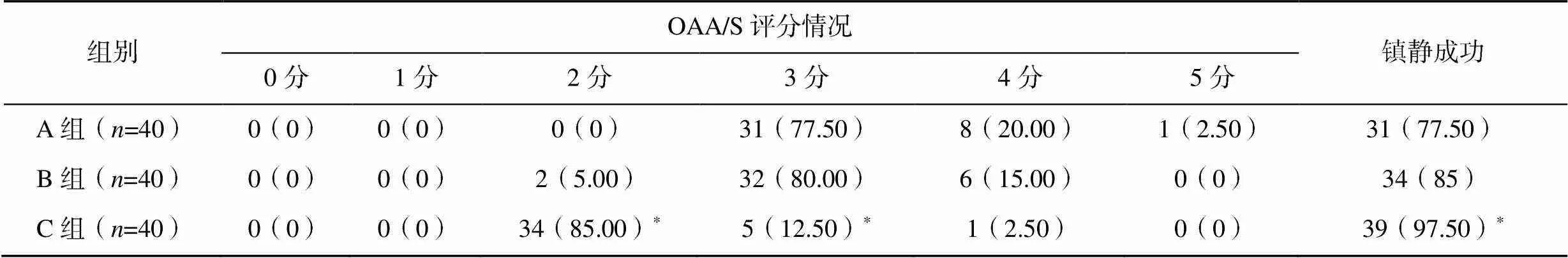
表3 三组患儿不同时间点OAA/S情况及镇静成功率比较[n(%)]
注:与A组比较,*<0.05
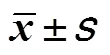
表4 三组患儿镇静起效时间和苏醒时间比较(,min)
注:与B组比较,*<0.05
2.4 三组患儿镇静期间不良反应发生率比较
镇静期间B组和C组的不良反应总发生率显著低于A组(2=6.384,=0.041),见表5。
3 讨论
儿童肺功能检查可用于评定患有哮喘、囊性纤维化和慢性肺病的儿童对治疗的反应[3]。但低龄幼儿心智尚不成熟,不能自主配合检查,依从性较差,为肺功能检查带来了一定的难度,因此临床通常需要应用镇静药物使患儿入睡后再进行检查。为确保肺功能检查顺利完成,需患儿达到中、深度镇静,即入睡状态,对轻度刺激无反应,对疼痛刺激有反应,呼吸循环无需特殊处理可以维持稳定,所以由麻醉医师根据患儿特点进行规范化镇静是小儿镇静的必然趋势[4]。
水合氯醛保留灌肠是临床上常用的儿童镇静方法,但其半衰期较长、镇静治疗窗较短,且对呼吸系统和上气道力学存在损伤可导致相关的低氧血症,因此限制了其在临床的广泛应用。右美托咪定作为高选择性α2受体激动剂,其与α2受体的亲和力约为可乐定的8倍,具有镇静、镇痛、抗焦虑等临床效应,且效应呈剂量相关性[5-6]。镇静剂量的右美托咪定可达到自然睡眠的状态,表明镇静剂量的右美托咪定对呼吸抑制轻微[7-8];此外还可降低麻醉患者的气道分泌物,减少对黏膜的刺激[9]。研究显示,患儿采用不同剂量右美托咪定镇静,对上呼吸道的各解剖部位的轴径影响差异不大,发生呼吸道梗阻的风险均较小[10]。
本研究结果显示,右美托咪定2μg/kg组镇静成功率明显优于水合氯醛灌肠组,且右美托咪定2μg/kg组的不良反应发生率明显降低。究其原因,这与水合氯醛本身的药理作用有关,水合氯醛会导致儿童呼吸抑制伴延迟镇静、恶心呕吐和腹泻[11-13],虽然右美托咪定3μg/kg组镇静成功率更高,但右美托咪定剂量的增加导致心动过缓等不良反应随之增加,从安全性角度来说,右美托咪定2μg/kg组不良反应总发生率仅为2.5%,明显低于其他两组,而且作为镇静失败补救措施追加右美托咪定至3μg/kg,此种给药方式更为合理。右美托咪定2μg/kg组有效的降低了OAA/S评分,说明右美托咪定2μg/kg滴鼻可使患儿获得较佳的镇静水平及深度,能够较好地耐受肺功能检查时面罩对脸部的刺激,进而镇静效果优于水合氯醛组。究其原因,右美托咪定对呼吸系统干扰较小,且无臭、无味、无刺激性,滴鼻给药患儿容易接受,同时鼻腔内黏膜毛细血管较为丰富,经鼻药物可较快地吸收进入血液,因而镇静成功率也相应升高。有研究表明采用2.0μg/kg以上剂量的右美托咪定滴鼻,其镇静成功率显著高于水合氯醛镇静[14],本研究结果与其相符。OAA/S评分≤3分即可完成肺功能检查,虽然右美托咪定3μg/kg能降低OAA/S评分至2分,但随之风险也会增加,因此本研究推荐应用右美托咪定2μg/kg。
综上所述,右美托咪定2μg/kg滴鼻用于小儿肺功能检查可有效地提高患儿的检查依从性,镇静成功率明显高于水合氯醛,且镇静期间生命体征平稳,不良反应发生率低,镇静失败补救追加右美托咪定至3μg/kg,可安全应用于临床。
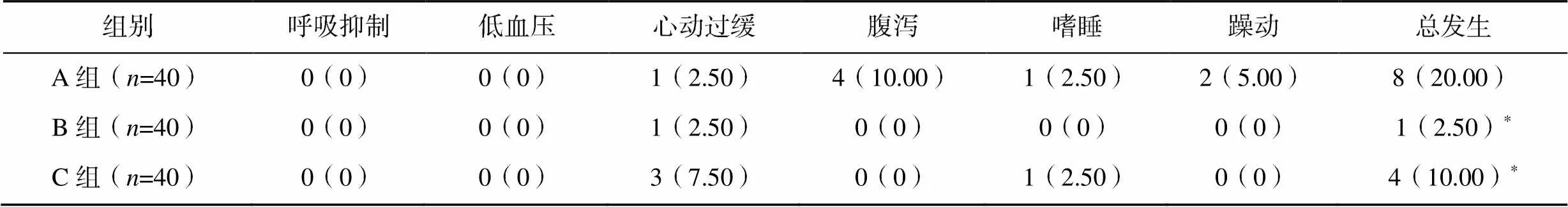
表5 三组患儿镇静期间不良反应发生率比较[n(%)]
注:与A组比较,*<0.05
[1] CAO Q Z, LIN Y Q, XIE Z B, et al. Comparison of sedation by intranasal dexmedetomidine and oral chloral hydrate for pediatric ophthalmic examination[J]. Paediatr Anaesth, 2017, 27(6): 629–636.
[2] AFONSO J, REIS F. Dexmedetomidine: current role in anesthesia and intensive care[J]. Rev Bras Anestesiol, 2012, 62(1): 118–133.
[3] BEYDON N, DAVIS S D, LOMBARDI E, et al. An official American Thoracic Society/European Respiratory Society statement: pulmonary function testing in preschool children[J]. Am J Respir Crit Care Med, 2007, 175(12): 1304–1345.
[4] SURY M, BULLOCK I, RABAR S, et al. Sedation for diagnostic and therapeutic procedures in children and young people: summary of NICE guidance[J]. BMJ, 2010(341): c6819.
[5] 屈双权, 肖婷. 小儿门诊镇静药物选择的研究进展[J]. 医学临床研究, 2014, 31(12): 2448–2451.
[6] LIU X T, LI Y Q, KANG L, et al. Recent advances in the clinical value and potential of dexmedetomidine[J]. J Inflamm Res, 2021, 30(14): 7507–7527.
[7] PANSINI V, CURATOLA A, GATTO A, et al. Intranasal drugs for analgesia and sedation in children admitted to pediatric emergency department: a narrative review[J]. Ann Transl Med, 2021, 9(2): 189.
[8] CHIMA A M, MAHMOUD M A, NARAYANASAMY S. What is the role of dexmedetomidine in modern anesthesia and critical care? [J]. Adv Anesth, 2022, 40(1): 111–130.
[9] LIAN X H, LIN Y Z, LUO T, et al. Comparison of dexmedetomidine with chloral hydrate as sedatives for pediatric patients: a systematic review and meta- analysis[J]. Medicine, 2020, 99(31): e21008.
[10] MAHMOUD M, RADHAKRISHMAN R, GUNTER J, et al. Effect of increasing depth of dexmedetomidine anesthesia on upper airway morphology in children[J]. Paediatr Anaesth, 2010, 20(6): 506–515.
[11] GANIGARA M, SRIVASTAVA S, MALIK P, et al. Comparison of chloral hydrate and pentobarbital sedation for pediatric echocardiography[J]. Echocardiography, 2019, 36(4): 766–769.
[12] NECULA V, STAMATE M C, BLEBEA C, et al. Safety and effectiveness of chloral hydrate in outpatient paediatric sedation for objective hearing tests[J]. Int J Pediatr Otorhinolaryngol, 2019, 126: 109605.
[13] FONG C Y, LIM W K, LI L M, et al. Chloral hydrate as a sedating agent for neurodiagnostic procedures in children[J]. Cochrane Database Syst Rev, 2021, 8(8): CD011786.
[14] LEWIS J, BAILEY C R. Intranasal dexmedetomidine for sedation in children; a review[J]. J Perioper Pract, 2020, 30(6): 170–175.
Effect of dexmedetomidine nasal drops on pulmonary function tests in children
LIU Na, PIAO Meiying, MENG Fanzheng, LIU Wanchao
1.Department of Pediatric Respiratory, the First Hospital of Jilin University, Changchun 130021, Jilin, China; 2.Department of Anesthesiology, Jilin Provincial Corps Hospital of the Chinese People’s Armed Police Force, Changchun 130052, Jilin, China
To evaluate the clinical effect of dexmedetomidine nasal drops on pulmonary function tests in children.A total of 120 children who underwent pulmonary function tests in pediatric respiratory department of the First Hospital of Jilin University from June 2021 to December 2021 were selected and divided into group A (=40), group B (=40) and group C (=40) according to random number table method. group A was given 10% chloral hydrate 50-80mg/kg retention enema, and children in group B and group C received nasal drops of dexmedetomidine stock solution at 2.0μg/kg and 3.0μg/kg respectively 30 minutes before the examination. After eyelash reflex of children disappeared, the examination was performed. Mean arterial pressure (MAP), heart rate (HR), respiratory rate (RR) and pulse oxygen saturation (SpO2) of children were recorded before sedation (T0), 10min (T1), 20min (T2) and 30min (T3) after sedation in three groups. The observer’s assessment of alertness/sedation scale(OAA/S) and the success rate of sedation were recorded before sedation, at 10min, 20min and 30min after sedation. The adverse reactions during sedation was recorded in the three groups.At T0, the MAP and HR of the three groups of children were compared, and the differences were not statistically significant (>0.05). At T1, the MAP and HR of the three groups of children were significantly lower than those of T0 (<0.05), and the MAP and HR of the children of group A and group B were significantly higher than those of group C at T1 (<0.05). At T2 and T3, the MAP and HR of the three groups were compared, and the differences were not statistically significant (>0.05). Comparison of RR and SpO2of children in the three groups at different time points showed no statistically significant difference (>0.05). Compared with groups A and B, the sedation success rate of children in group C was significantly higher (<0.05). Compared with group B, the onset of sedation in group A and group C was significantly shorter (<0.05). The difference in the awakening time of the children in the three groups was not statistically significant (>0.05). The total incidence of adverse reactions during sedation in Groups B and C was significantly lower than that in group A (<0.05).Dextrmedetomidine 2μg/kg nasal drops can effectively improve the compliance of children's pulmonary function examination. The success rate of sedation is significantly higher than that of chloral hydrate. During sedation, the vital signs are stable, and the incidence of adverse reactions is low. Dextrmedetomidine is added to 3μg/kg to remedy sedation failure, which can be safely used in clinical practice.
Dexmedetomidine; Chloral hydrate; Pulmonary function test; Sedation; Children
R614
A
10.3969/j.issn.1673-9701.2023.23.019
刘万超,电子信箱:865755754@qq.com
(2022–06–08)
(2023–07–20)
