CB1R拮抗剂对MPTP诱导PD小鼠运动行为影响
2023-08-26张腾元商晓钰谢俊霞徐华敏
张腾元 商晓钰 谢俊霞 徐华敏
[摘要]目的研究大麻素受体1(CB1R)拮抗剂NESS 0327对1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)诱导的帕金森病(PD)小鼠运动行为的影响。方法将24只8周龄C57BL/6J雄性小鼠随机分为对照组、MPTP组和MPTP+NESS 0327组。对照组和MPTP组小鼠均双侧黑质致密部(SNpc)微量注射二甲基亚砜(DMSO)和Tween-80混合溶液,分别腹腔注射生理盐水和MPTP;MPTP+NESS 0327组小鼠则双侧SNpc微量注射NESS 0327,腹腔注射MPTP。小鼠连续5 d给药后进行旷场实验和爬杆实验检测小鼠运动能力的改变。结果旷场实验结果显示,3组小鼠总移动距离比较差异具有统计学意义(F=8.279,P<0.01),其中MPTP组小鼠总移动距离较对照组小鼠显著减少(q=5.705,P<0.01),MPTP+NESS 0327组小鼠总移动距离较MPTP组小鼠显著增加(q=3.504,P<0.05)。爬杆实验结果显示,3组小鼠下杆时间比较差异具有统计学意义(F=12.110,P<0.01),其中MPTP组小鼠下杆时间较对照组小鼠显著增加(q=6.790,P<0.01),MPTP+NESS 0327組小鼠的下杆时间较MPTP组小鼠显著减少(q=4.713,P<0.01)。结论SNpc给予CB1R拮抗剂NESS 0327可改善MPTP诱导的PD小鼠运动功能障碍。
[关键词]大麻素受体拮抗剂;帕金森病;1-甲基-4-苯基-1,2,3,6-四氢吡啶;密部;运动活动;小鼠
[中图分类号]R338.2[文献标志码]A[文章编号]2096-5532(2023)03-0357-04
doi:10.11712/jms.2096-5532.2023.59.088[开放科学(资源服务)标识码(OSID)]
[网络出版]https://kns.cnki.net/kcms2/detail/37.1517.R.20230801.1101.003.html;2023-08-0115:17:12
EFFECTS OF CB1R ANTAGONIST ON MOTOR BEHAVIOR IN A MOUSE MODEL OF MPTP-INDUCED PARKINSONS DISEASE ZHANG Tengyuan, SHANG Xiaoyu, XIE Junxia, XU Huamin (Department of Physiology and Pathophysiology, School of Basic Medicine, Medical College of Qingdao University, Qingdao 266071, China)
[ABSTRACT]ObjectiveTo investigate the effects of a cannabinoid-1 receptor (CB1R) antagonist (NESS 0327) on motor behavior in a mouse model of Parkinsons disease (PD) induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP). MethodsTwenty-four 8-week-old male C57BL/6J mice were randomly divided into control group, MPTP group, and MPTP+NESS 0327 group. The control group and MPTP group received microinjection of a mixture of dimethyl sulfoxide (DMSO) and Tween-80 into bilateral substantia nigra pars compacta (SNpc) and also intraperitoneal injection of saline and MPTP, respectively. The MPTP+NESS 0327 group received microinjection of NESS 0327 into bilateral SNpc and intraperitoneal injection of MPTP. After consecutive five days of treatment, the open field test and pole test were used to assess the change in locomotor activity of mice. ResultsThe open field test showed a significant difference in the total movement distance between the three groups (F=8.279,P<0.01). The total movement distance was significantly shorter in the MPTP group than in the control group (q=5.705,P<0.01), and significantly longer in the MPTP+NESS 0327 group than in the MPTP group (q=3.504,P<0.05). The pole test showed a significant difference in the time of descending the pole between the three groups (F=12.110,P<0.01). Compared with the control group, the MPTP group had a significantly longer time of descending the pole (q=6.790,P<0.01). The time of descending the pole in the MPTP+NESS 0327 group was significantly shorter than that of the MPTP group (q=4.713,P<0.01). ConclusionAdministration of the CB1R antagonist NESS 0327 into the SNpc can ameliorate MPTP-induced motor deficits in PD mice.
[KEY WORDS]cannabinoid receptor antagonists; Parkinson disease; 1-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine; pars compacta; motor activity; mice
帕金森病(PD)是中老年人第二大常见的神经退行性疾病,其病理特征为黑质致密部(SNpc)多巴胺能神经元缺失[1-2],最终导致运动功能障碍[3-4],但其发病机制目前尚未完全阐明。近期研究发现,内源性大麻素系统(ECS)可能参与了PD的发病[5]。ECS主要由内源性大麻素(eCBs)、与大麻素相互作用的受体包括大麻素受体1(CB1R)和大麻素受体2、负责合成代谢及降解eCBs的酶所组成[6-7]。其中CB1R主要分布于脑、脊髓和外周神经系统中,高表达于脑内的基底神经核,又称中枢型大麻素受体,广泛参与学习记忆、认知和运动行为的调控[8-10]。有研究结果表明,PD病人脑脊液中eCBs水平升高[11];在1-甲基-4-苯基-1,2,3,6-四氢吡啶(MPTP)处理的猴的大脑中,纹状体CB1R的数量增加[12];在PD大鼠的纹状体中编码CB1R的mRNA水平升高[13]。这些研究结果提示,CB1R可能参与PD的发病。NESS 0327是一种新型的CB1R拮抗剂,对CB1R有极高的选择性,然而其对PD小鼠运动行为的影响目前尚不明确。因此,本研究利用旷场实验和爬杆实验进行行为学观察,旨在探讨黑质给予CB1R拮抗剂NESS 0327对MPTP诱导PD小鼠运动行为的影响。
1材料与方法
1.1实验材料
1.1.1实验动物SPF级8周龄雄性C57BL/6J小鼠购自北京维通利华实验动物公司。小鼠每笼4只,在温度(21±2)℃、湿度(50±5)%、12 h-12 h昼夜循环光照的环境下饲养,可自由摄食饮水,实验前适应环境1周。
1.1.2实验药品MPTP(Sigma-Aldrich),用生理盐水稀释成6 g/L浓度。二甲基亚砜(DMSO)和Tween-80混合溶液,含有体积分数0.05的DMSO、体积分数0.05的Tween-80以及生理盐水。NESS 0327(APExBIO),用DMSO和Tween-80混合溶液溶解成0.05 g/L。
1.2实验方法
1.2.1双侧SNpc套管埋置小鼠放置于麻醉箱内使用异氟烷初步麻醉后,取俯卧位固定于立体定位仪上,实验中使用体积分数0.015~0.020的异氟烷维持小鼠麻醉状态。剃除小鼠头部毛发,涂抹碘附消毒,剪去小鼠颅顶正中皮肤,剥离骨膜,暴露前后囟,调节耳杆和鼻夹,使前后囟处于同一水平面。参考小鼠脑立体定位图谱定位SNpc的位置:前囟后2.92 mm,旁开1.35 mm,颅骨表面下4.00 mm。在该坐标下用颅钻钻孔,将套管置入SNpc上方,并用自凝牙托粉固定。
1.2.2动物分组及处理埋置套管7 d后将小鼠随机分为对照组(A组)、MPTP组(B组)和MPTP+NESS 0327组(C组),每组8只。对照组小鼠连续5 d双侧SNpc微量注射DMSO和Tween-80混合溶液,腹腔注射生理鹽水;MPTP组小鼠双侧SNpc微量注射DMSO和Tween-80混合溶液,腹腔注射MPTP;MPTP+NESS 0327组小鼠双侧SNpc微量注射NESS 0327,腹腔注射MPTP。其中SNpc注射剂量为每侧500 nL,腹腔注射MPTP或生理盐水的剂量为30 mg/kg。
1.2.3旷场实验小鼠连续5 d给药,于第6天将小鼠放在40 cm×40 cm×40 cm大小的上方开放式不透明箱体中,让小鼠自由运动10 min,同时用摄像机捕捉其运动轨迹,并用 Noldus 软件分析小鼠10 min内的总移动距离和运动速度来评估小鼠的运动能力。每只小鼠实验完成后用体积分数0.75的无水乙醇擦拭箱体,以免留有前一只小鼠气味,待乙醇挥发后将下一只小鼠放入箱体内进行实验。
1.2.4爬杆实验小鼠旷场实验1 d后进行爬杆实验,爬杆装置为直径0.8 cm、高50 cm的金属杆,杆顶端有一直径3 cm的圆球,用无纺布胶带将金属杆及圆球包裹起来,保证其表面粗糙,防止小鼠打滑。实验前让小鼠进行爬杆训练1次,实验时将小鼠头朝上置于爬杆顶端,用秒表记录小鼠爬下杆(至四肢落地)的时间,每次检测时间间隔1 min,连续检测5次,取平均值。
1.3统计学处理
应用GraphPad Prism 8.0软件进行统计学处理。实验结果以±s形式表示,多组间比较采用单因素方差分析(One way ANOVA检验),并继以Newman-keuls法进行组间两两比较。以P<0.05为差异有统计学意义。
2结果
2.1旷场实验
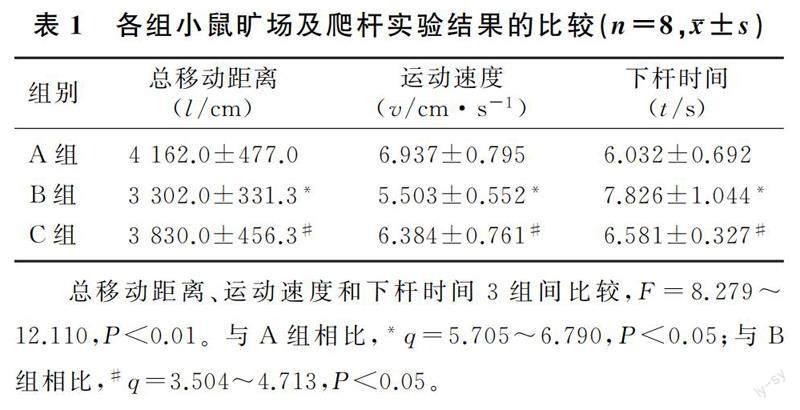
旷场实验结果显示,3组小鼠总移动距离和运动速度差异具有统计学意义(F=8.279、8.280,P<0.01)。组间两两比较,MPTP组小鼠总移动距离和运动速度较对照组显著降低,MPTP+NESS 0327组小鼠总移动距离和运动速度较MPTP组显著升高(q=3.504~5.705,P<0.05)。见表1。
2.2爬杆实验
爬杆实验结果显示,3组小鼠下杆时间差异具有统计学意义(F=12.110,P<0.01)。组间两两比较,MPTP组小鼠下杆时间较对照组显著增加(q=6.790,P<0.01),MPTP+NESS 0327组小鼠的下杆时间较MPTP组显著减少(q=4.713,P<0.01)。见表1。
3讨论
PD是中老年人第二大常见的神经退行性疾病,其病理特征为SNpc多巴胺能神经元缺失,临床表现有静止性震颤、肌强直、运动迟缓、姿势平衡障碍等运动症状和抑郁、焦虑、认知障碍等非运动症状,这些运动症状和非运动症状严重影响了病人的身心健康[14-16]。至今尚无成熟有效的药物或方法能阻止或逆转PD病情的发展。
最近的研究發现,ECS可能参与PD的发病及疾病进展[5,17]。针对ECS的药物开发可能为PD的治疗提供新的策略。ECS主要由eCBs、与eCBs相互作用的受体以及负责合成代谢和降解eCBs的酶所组成。其中CB1R是G蛋白偶联受体,在突触中,CB1R主要位于兴奋性和抑制性神经元的突触前,可被突触后神经元释放的eCBs激活,产生突触前抑制作用[18-19]。越来越多的研究表明,大麻素具有调控运动的作用[10]。eCBs在基底神经核中高表达,并在PD等多种运动障碍疾病中表达失调,从而参与疾病的发生发展[11,20-21]。给予脂肪酰胺水解酶抑制剂URB597可以改善MPTP诱导的PD小鼠的运动损伤[22-23]。因此,调节eCBs水平可能成为改善PD运动症状的新策略。
目前关于CB1R拮抗剂的研究结果表明,PD动物模型苍白球的2-花生四烯酸甘油酯含量升高会导致运动障碍,而给予喹吡罗和CB1R拮抗剂SR 141716A可以改善利血平大鼠的运动障碍[24]。并且在黑质极度损伤阶段,给予低剂量的SR 141716A会显著改善PD动物模型的运动症状[25]。这些结果表明,CB1R拮抗剂可能在改善PD运动障碍中发挥重要的作用。NESS 0327是一种新型的CB1R拮抗剂,对CB1R具有极高的选择性,其对CB1R的亲和力约为SR 141716A的5 000倍,在拮抗CB1R的同时不产生其他的生理学效应[26-27]。但是NESS0327在PD模型中的作用及可能的机制尚不明确。
众所周知,PD病人的病理特征为SNpc多巴胺能神经元缺失,进而释放到纹状体的多巴胺减少。黑质-纹状体是PD中受损的主要脑区,所以本研究选择黑质注射CB1R拮抗剂NESS 0327,观察其对MPTP诱导的PD小鼠运动行为的影响。旷场实验和爬杆实验是评估动物运动行为的有效方法。旷场实验通过比较小鼠在旷场中自由运动10 min的总移动距离和运动速度来反映小鼠的运动能力,总移动距离越长、速度越快表明小鼠的运动能力越好。爬杆实验中小鼠下杆时间越短,表明其运动速度越快、肢体协调性越好。本文结果显示,与MPTP组相比,MPTP+NESS 0327组小鼠的总移动距离显著增加,运动速度显著提高,下杆时间显著缩短,提示CB1R拮抗剂NESS 0327对MPTP诱导的PD小鼠的运动功能障碍具有改善作用。目前研究表明,SNpc多巴胺能神经元胞体及末梢上并不存在CB1R[17],CB1R主要分布于多巴胺能神经元突触前的γ-氨基丁酸和谷氨酸突触的终末,提示CB1R拮抗剂NESS 0327影响小鼠自发活动异常的机制可能是通过影响多巴胺能神经元的抑制性或兴奋性输入而间接发挥作用。
综上所述,抑制黑质CB1R对PD小鼠的运动功能障碍具有明显的改善作用,本研究结果为进一步探讨ECS在PD发病机制中的作用提供了一定的实验依据,为PD的治疗提供了新的靶点。
[参考文献]
[1]SHARMA V, BEDI O, GUPTA M, et al. A review: traditional herbs and remedies impacting pathogenesis of Parkinsons disease[J]. Naunyn-Schmiedebergs Archives of Pharmacology, 2022,395(5):495-513.
[2]VALENCIA J, FERREIRA M, MERINO-TORRES J F, et al. The potential roles of extracellular vesicles as biomarkers for Parkinsons disease: a systematic review[J]. International Journal of Molecular Sciences, 2022,23(19):11508.
[3]DIRKX M F, BOLOGNA M. The pathophysiology of Parkinsons disease tremor[J]. Journal of the Neurological Sciences, 2022,435:120196.
[4]KRAUSE P, BERKING S, ASTALOSCH M, et al. Motor and non-motor improvements following short-term multidisciplinary day-clinic care in Parkinsons disease[J]. Journal of Neural Transmission, 2022,129(12):1419-1426.
[5]MUHAMMAD F, LIU Y, WANG N B, et al. Neuroprotective effects of cannabidiol on dopaminergic neurodegenerationand α-synuclein accumulation in C. elegans models of Parkin-sons disease[J]. Neurotoxicology, 2022,93:128-139.
[6]LU H C, MACKIE K. An introduction to the endogenous cannabinoid system[J]. Biological Psychiatry, 2016,79(7):516-525.
[7]BHUNIA S, KOLISHETTI N, ARIAS A Y, et al. Cannabidiol for neurodegenerative disorders: a comprehensive review[J]. Frontiers in Pharmacology, 2022,13:989717.
[8]MACKIE K. Distribution of cannabinoid receptors in the central and peripheral nervous system[J]. Handbook of Experimental Pharmacology, 2005(168):299-325.
[9]ZOU S L, KUMAR U. Cannabinoid receptors and the endocannabinoid system: signaling and function in the central nervous system[J]. International Journal of Molecular Sciences, 2018,19(3):833.
[10]KLUGER B, TRIOLO P, JONES W, et al. The therapeutic potential of cannabinoids for movement disorders[J]. Movement Disorders: Official Journal of the Movement Disorder Society, 2015,30(3):313-327.
[11]PISANI A, FEZZA F, GALATI S, et al. High endogenous cannabinoid levels in the cerebrospinal fluid of untreated Parkinsons disease patients[J]. Annals of Neurology, 2005,57(5):777-779.
[12]LASTRES-BECKER I, CEBEIRA M, DE CEBALLOS M L, et al. Increased cannabinoid CB1 receptor binding and activation of GTP-binding proteins in the basal ganglia of patients with Parkinsons syndrome and of MPTP-treated marmosets[J]. The European Journal of Neuroscience, 2001,14(11):1827-1832.
[13]MAILLEUX P, VANDERHAEGHEN J J. Dopaminergic re-gulation of cannabinoid receptor mRNA levels in the rat caudate-putamen: an in situ hybridization study[J]. Journal of Neurochemistry, 1993,61(5):1705-1712.
[14]BALESTRINO R, SCHAPIRA A H V. Parkinson disease[J]. European Journal of Neurology, 2020,27(1):27-42.
[15]TAGUCHI T, IKUNO M, YAMAKADO H, et al. Animal model for prodromal Parkinsons disease[J]. International Journal of Molecular Sciences, 2020,21(6):1961.
[16]RYMAN S G, POSTON K L. MRI biomarkers of motor and non-motor symptoms in Parkinsons disease[J]. Parkinsonism & Related Disorders, 2020,73:85-93.
[17]STAMPANONI BASSI M, SANCESARIO A, MORACE R, et al. Cannabinoids in Parkinsons disease[J]. Cannabis and Cannabinoid Research, 2017,2(1):21-29.
[18]LOVINGER D M. Presynaptic modulation by endocannabinoids[J]. Handbook of Experimental Pharmacology, 2008(184):435-477.
[19]BASAVARAJAPPA B S, SHIVAKUMAR M, JOSHI V, et al. Endocannabinoid system in neurodegenerative disorders[J]. Journal of Neurochemistry, 2017,142(5):624-648.
[20]CRISTINO L, BISOGNO T, MARZO V D. Cannabinoids and the expanded endocannabinoid system in neurological disorders[J]. Nature Reviews Neurology, 2020,16(1):9-29.
[21]GUBELLINI P, PICCONI B, BARI M, et al. Experimental Parkinsonism alters endocannabinoid degradation: implications for striatal glutamatergic transmission[J]. The Journal of Neuroscience: the Official Journal of the Society for Neuroscience, 2002,22(16):6900-6907.
[22]CELORRIO M, FERNNDEZ-SUREZ D, ROJO-BUSTAMANTE E, et al. Fatty acid amide hydrolase inhibition for the symptomatic relief of Parkinsons disease[J]. Brain, Beha-vior, and Immunity, 2016,57:94-105.
[23]VIVEROS-PAREDES J M, GONZALEZ-CASTAEDA R E, ESCALANTE-CASTAEDA A, et al. Effect of inhibition of fatty acid amide hydrolase on MPTP-induced dopaminergic neuronal damage[J]. Neurologia, 2019,34(3):143-152.
[24]DI MARZO V, HILL M P, BISOGNO T, et al. Enhanced levels of endogenous cannabinoids in the globus pallidus are associated with a reduction in movement in an animal model of Parkinsons disease[J]. FASEB Journal: Official Publication of the Federation of American Societies for Experimental Bio-logy, 2000,14(10):1432-1438.
[25]GONZLEZ S, SCORTICATI C, GARCA-ARENCIBIA M, et al. Effects of rimonabant, a selective cannabinoid CB1 receptor antagonist, in a rat model of Parkinsons disease[J]. Brain Research, 2006,1073-1074:209-219.
[26]RUIU S, PINNA G A, MARCHESE G, et al. Synthesis and characterization of NESS 0327: a novel putative antagonist of the CB1 cannabinoid receptor[J]. The Journal of Pharmacology and Experimental Therapeutics, 2003,306(1):363-370.
[27]YE L Y, CAO Z, WANG W W, et al. New insights in cannabinoid receptor structure and signaling[J]. Current Molecular Pharmacology, 2019,12(3):239-248.
(本文編辑马伟平)