短期抗生素暴露对氧化偶氮甲烷诱导小鼠结直肠癌前病变相关指征变化的影响
2023-08-25林嘉玲何夏梦蒲芳芳蒙婷商正云胡雯曾献春
林嘉玲?何夏梦?蒲芳芳?蒙婷?商正云?胡雯?曾献春
摘要:目的 探討短期抗生素暴露对氧化偶氮甲烷(Azoxymethane,AOM)诱导小鼠结直肠癌前病变相关指征变化的影响。方法 40只3~4周龄SPF级雄性ICR小鼠随机分为4组(10只/组):对照组(Control组)、AOM干预组(AOM组)、抗生素+AOM干预组(Antibiotics+Azoxymethane,AbxAOM组)以及抗生素组(Antibiotics,Abx组)。AbxAOM组和Abx组灌胃抗生素溶液(氨苄西林100 mg/kg+新霉素100 mg/kg+甲硝唑100 mg/kg+万古霉素50 mg/kg+两性霉素B 1 mg/kg),Control组和AOM组灌胃等体积纯水,2次/天,连续14 d。灌胃结束后,AOM组和AbxAOM组腹腔注射AOM溶液(10 mg/kg·bw),Control组和Abx组腹腔注射相应体积的无菌0.9% NaCl溶液,1次/周,连续4周。HE染色观察小鼠结直肠组织病理学改变;RT-qPCR法检测结直肠组织中VCAM-1、ICAM-1、Ki-67、IL-1β、IL-6、TNF-α、VEGF-A、TLR4、MyD88、NF-κB p65及COX-2的mRNA表达水平;免疫组化法观察结直肠组织中Ki-67、NF-κB p65蛋白表达。结果 与Control组相比,AOM组小鼠结肠病理组织学评分显著升高(P<0.01),IL-6、TLR4、NF-κB p65及COX-2的mRNA表达显著升高(P<0.05)。与AOM组比较,AbxAOM组异常隐窝灶(Aberrant crypt foci, ACF)数量增多(P<0.05),ICAM-1、IL-1β、NF-κB p65的mRNA表达显著升高(P<0.05)。结论 AOM处理可导致结肠病理损伤及ACF形成,可能与TLR4/MyD88/NF-κB信号通路的激活有关。抗生素暴露在短期内有加剧AOM所致结肠损伤和TLR4/MyD88/NF-κB信号通路进一步激活的趋势,但此变化趋势还需在长期实验基础上进一步探讨。
关键词:结直肠癌;抗生素;氧化偶氮甲烷
中图分类号:R965文献标志码:A
Effects of short-term antibiotic exposure on changes in indicators related to azomethane-induced colorectal precancerous lesions in mice
Lin Jia-ling1, He Xia-meng2, Pu Fang-fang2, Meng Ting2, Shang Zheng-yun3, Hu Wen2, and Zeng Xian-chun1
(1 School of Laboratory Medicine, Chengdu Medical College, Chengdu 610500; 2 West China Hospital, Sichuan University, Chengdu 610041; 3 West China School of Public Health, Sichuan University, Chengdu 610041)
Abstract Objective To investigate the effect of short-term antibiotic exposure on the changes of azoxymethane (AOM)-induced colorectal precancerous lesions in mice. Methods Forty 3- to 4-week-old SPF-grade male ICR mice were randomly divided into four groups (10 mice/group): Control group (Control group), AOM intervention group (AOM group), antibiotic+AOM intervention group (Antibiotics+Azoxymethane, AbxAOM group), and antibiotic group (Antibiotics, Abx group). The AbxAOM group and the Abx group were administered with antibiotic solution (ampicillin 100 mg/kg+neomycin 100 mg/kg+metronidazole 100 mg/kg+vancomycin 50 mg/kg +amphotericin B 1 mg/kg), The control group and the AOM group was given an equal volume of pure water, twice a day, for 14 consecutive days. After gavage, the AOM group and the AbxAOM group were intraperitoneally injected with AOM solution (10 mg/kg.bw), and the control group and the Abx group were intraperitoneally injected with the corresponding volume of sterile 0.9% NaCl solution, once a week for 4 consecutive weeks. HE staining was used to observe the pathological changes of colorectal tissue in mice. The expression of VCAM-1, ICAM-1, Ki-67, IL-1β, IL-6, TNF-α, VEGF-A, TLR4, MyD88, NF-κB p65, and COX-2 mRNA and the expression of Ki-67 and NF-κB p65 protein in colorectal tissues were detected by RT-qPCR and immunohistochemistry, respectively. Results Compared with the control group, mice in the AOM group had significantly higher colonic pathological histological scores (P<0.01) and significantly higher mRNA expression of IL-6, TLR4, NF-κB p65, and COX-2 (P<0.05). Compared with the AOM group, the number of ACF was increased in the AbxAOM group (P<0.05), and the expression of mRNA for ICAM-1, IL-1β, and NF-κB p65 was significantly higher (P<0.05). Conclusion AOM treatment leads to colon pathological damage and ACF formation, which may be related to the activation of TLR4/MyD88/NF-κB signaling pathway. Antibiotic exposure tends to aggravate AOM-induced colon damage and further activate TLR4/MyD88/NF-κB signaling pathway in the short term. However, this trend needs to be further explored on the basis of long-term experiments.
Key words Colorectal cancer; Antibiotics; Azoxymethane
结直肠癌(colorectal cancer,CRC)是全球常见肿瘤之一。WHO报道每年CRC发病人数超过百万,死亡人数超过50万[1]。2020年,中国CRC新发病例56万,在恶性肿瘤中发病率位居第二[2]。CRC的病因复杂多样,主要包括遗传背景因素和环境危险因素[3]。近年来,大量证据提示,抗生素暴露可以损伤肠黏膜、造成毒性影响和变态反应、刺激肠道收缩和蠕动、改变肠道微生态平衡等,进而引起肠道病变,与结直肠癌的患病风险存在关联[4-9]。异常隐窝灶(aberrant crypt foci,ACF)是CRC癌前病变过程中最早期出现的病理结构,既往文献认为,在肠道肿瘤进展期,ACF数量随组织学改变的严重性增加,并且ACF可作为CRC的独立预测因素[10-13]。此外,一些重要的炎性因子(如白细胞介素-6(interleukin-6,IL-6)、肿瘤坏死因子-α(tumor necrosis factor-alpha,TNF-α)、血管内皮生长因子(vascular endothelial growth factor,VEGF)等)和炎症通路(NF-κB信号通路)被认为是促进肿瘤发生的关键因素,会加速大肠癌的发展,尤其是结肠炎相关CRC[14-17]。但不同种类、剂量或时间的抗生素暴露和受试对象自身差异等因素,可能导致不同的研究结论。因此,需要进一步探索抗生素暴露对结直肠癌前病变相关指征变化的影响。本研究目的旨在研究短期抗生素暴露对氧化偶氮甲烷(azoxymethane,AOM)诱导小鼠CRC癌前病变相关指征变化的影响,并探索其可能的作用机制是否与NF-κB信号通路的激活有关。
1 实验对象与方法
1.1 实验动物
ICR小鼠,SPF级,雄性,3~4周龄,40只,购自北京维通利华实验动物技术有限公司,实验动物生产许可证号:SCXK(京)2016-0006,并饲养于四川大学华西公共卫生学院分析测试中心,动物中心许可证号:SYXK(川)2018-209。饲养温度设定在23~25℃范围,湿度设定在40%~70%范围,昼夜明暗交替周期设定为12 h,饲以SPF级大小鼠维持饲料并自由饮水,饲料和饮水均经无菌处理,正式试验开始前适应性饲养7 d。
1.2 主要试剂与液体的制备
AOM购自Sigma-Aldrich公司,用无菌0.9% NaCl溶液配制,使AOM溶液终浓度为1 mg/mL。
根據参考文献[18-20]报道的抗生素联用方案,确定本研究抗生素种类及剂量方案为:氨苄西林100 mg/kg (Cas: 7177-48-2)、新霉素100 mg/kg(Cas: 1405-10-3)、甲硝唑100 mg/kg(Cas: 443-48-1)、万古霉素50 mg/kg (Cas: 1404-93-9)和两性霉素B 1 mg/kg(Cas: 1397-89-3)。以上抗生素均购自大连美仑生物技术有限公司。
1.3 实验方法
1.3.1 动物分组与处理
本实验选取40只雄性ICR小鼠作为实验对象,在实验初始阶段,采用随机数字表法将所有小鼠随机等分为4组,每组10只,分别为:对照组(control组)、AOM干预组(AOM组)、抗生素+AOM干预组(antibiotics+azoxymethane,AbxAOM组)以及抗生素组(antibiotics,Abx组)。各组处理措施如下:AbxAOM组和Abx组灌胃含广谱抗生素的纯水溶液,control组、AOM组则灌胃相应体积的纯水,每天2次,连续14 d。灌胃结束后,AOM组和AbxAOM组腹腔注射AOM溶液(10 mg/kg·bw),control组和Abx组则腹腔注射相应体积的无菌0.9% NaCl溶液,每周1次,连续4周。干预期间,每天监测小鼠生长状况及精神状态,每周测量并记录1次小鼠体重变化,并于处死前1天再进行1次体重称量及粪便采集。实验第11周,处死所有小鼠,采集1 cm结肠组织于4%多聚甲醛中固定48 h后,以备后续病理及免疫组化检测;剩余结肠组织则立即转于-80℃保存,以备后续RT-qPCR检测。
1.3.2 ACF的识别与计数
取多聚甲醛固定后的结肠组织约1 cm,在显微镜下(40~100)观察并计数各病灶的ACF总数。ACF具有以下形态学特征:①隐窝较正常黏膜增大、增高;②隐窝周围间隙增大、管腔不规则[21]。ACF发生率=发生ACF动物数/实验动物数×100%。
1.3.3 结直肠组织苏木精-伊红染色(hematoxylin-eosin staining,HE染色)
将结直肠组织置于4%多聚甲醛(W/V)溶液中固定48 h后,石蜡包埋、切片,随后行常规HE染色,封片后于光学显微镜下观察,每张切片随机选择合格的3处视野,根据参考文献[22]标准进行病理组织学评分。
1.3.4 结直肠组织RNA提取与RT-qPCR分析
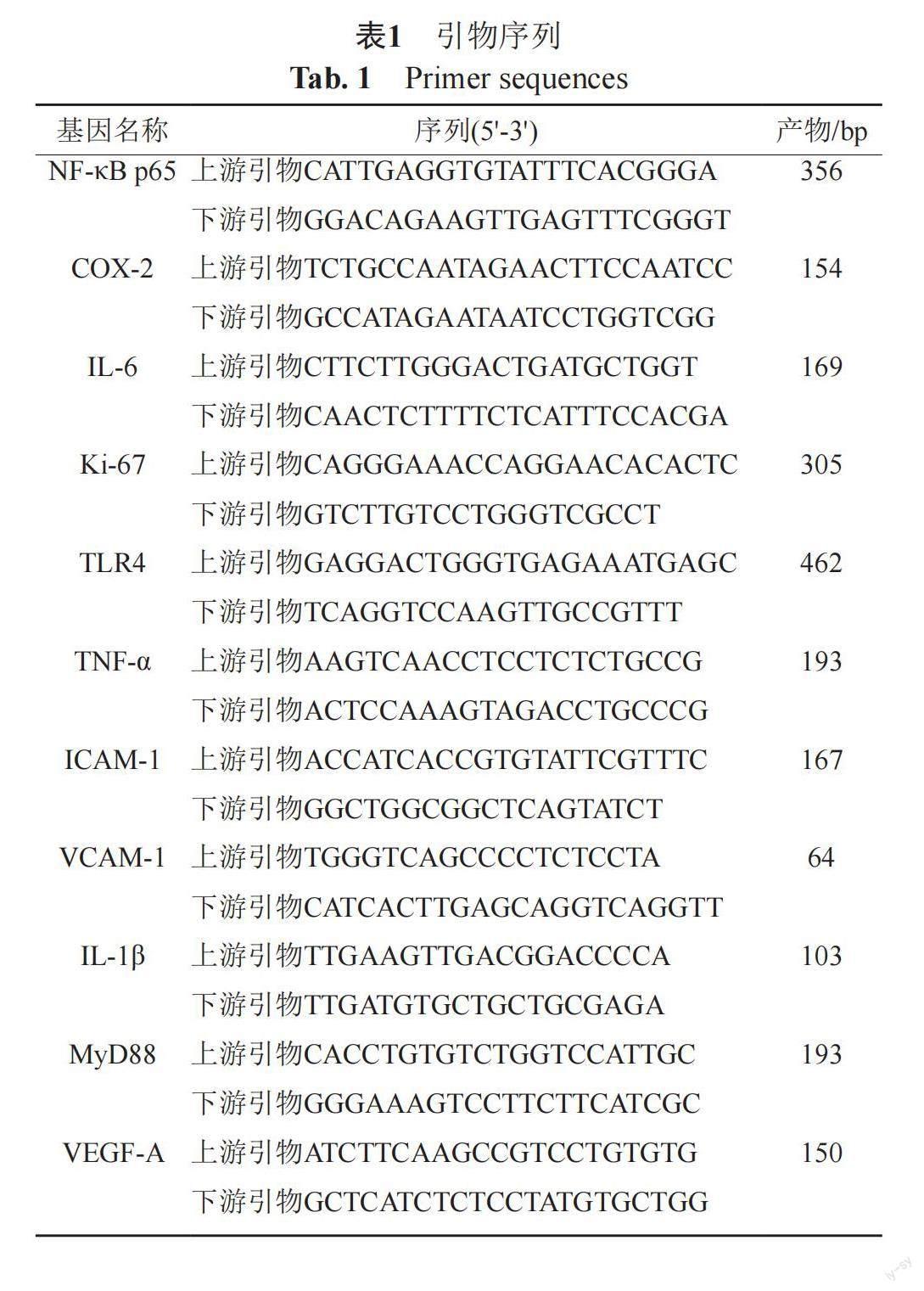
严格按照试剂盒(动物组织总RNA提取试剂盒,成都福际生物科技有限公司)说明书步骤提取RNA。随后,使用逆转录试剂盒(iscript cDNA Synthesis Kit,Bio-rad)从总RNA合成互补DNA。根据NCBI基因数据库提供的基因序列,通过Primer 5软件自行设计本研究所需目的基因和内参的基因序列,退火温度均设置为60℃,如表1所示。采用反转录-定量PCR(quantitative reverse transcription PCR,RT-qPCR)法检测小鼠结直肠组织核因子-κB p65(nuclear factor-kappa B p65,NF-κB p65)、环氧合酶-2(cyclooxygenase-2,COX-2)、IL-6、Ki-67、Toll样受体4(toll-like receptors 4,TLR4)、TNF-α、细胞间黏附分子-1(intercellular cell adhesion molecule-1,ICAM-1)、血管细胞黏附分子-1(vascular cell adhesion molecule-1,VCAM-1)、白细胞介素-1β(interleukin-1β,IL-1β)、髓样分化因子88(myeloid differentiation factor 88,MyD88)及血管内皮生长因子A(vascular endothelial growth factor A,VEGF-A)的mRNA。RT-qPCR反应严格遵照试剂盒(SsoFast EvaGreen? Supermix,Bio-rad)说明书所示步骤进行。
1.3.5 免疫组化实验
每组随机挑选3只小鼠,采用免疫组化印迹(immunohistochemistry staining,IHC)检测结直肠组织中Ki-67、NF-κB p65蛋白含量。使用Image-Pro Plus 6.0分析软件,统一以像素面积pixel作为标准单位,分别测量每张切片中5个视野阳性的累积光密度值(IOD)以及对应的组织像素面积(Area),并计算出面密度=IOD/Area;分别测量每张照片中阳性细胞数以及对应的总细胞数,计算阳性率(%)=阳性细胞数/总细胞数×100%。
1.4 统计方法
数据统计分析均通过SPSS 26.0进行。不服从正态分布的资料,使用中位数和四分位数间距M(Q1~Q3)进行描述;定量资料条件若满足正态性、方差齐,采取均数±标准差(x±s)的形式进行描述,多组独立样本则采用单因素方差分析(One-way ANOVA),两组间比较采用LSD法。定性资料或不满足参数检验运用规则的定量资料,则采用非参数检验,若差异具有统计学意义,将各组原始数据转换为秩次后,对秩次使用LSD法进行两两比较。病理组织学评分分析用Kruskal-Wallis秩和检验。按检验水准α=0.05,P<0.05或P<0.01则认为差异有统计学意义。
2 结果
2.1 小鼠体重增长趋势及生长情况
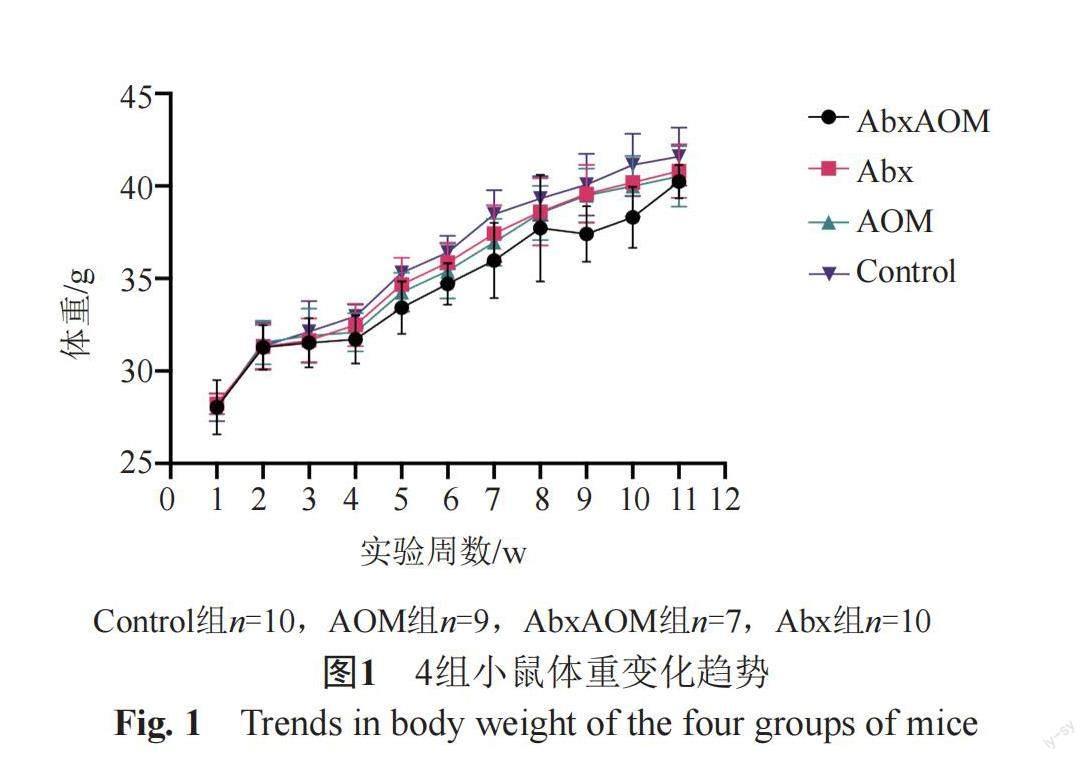
整个实验过程中,AbxAOM组有3只小鼠意外死亡,分别发生在实验第3、4周,AOM组有1只小鼠死亡,发生在实验第3周,解剖未发现特殊病变或肿瘤。如图1所示,在总体趋势上,4组小鼠的体重均呈增加趋势。组间比较差异无统计学意义。
2.2 小鼠结直肠组织病理组织学观察、ACF及肿瘤发生情况
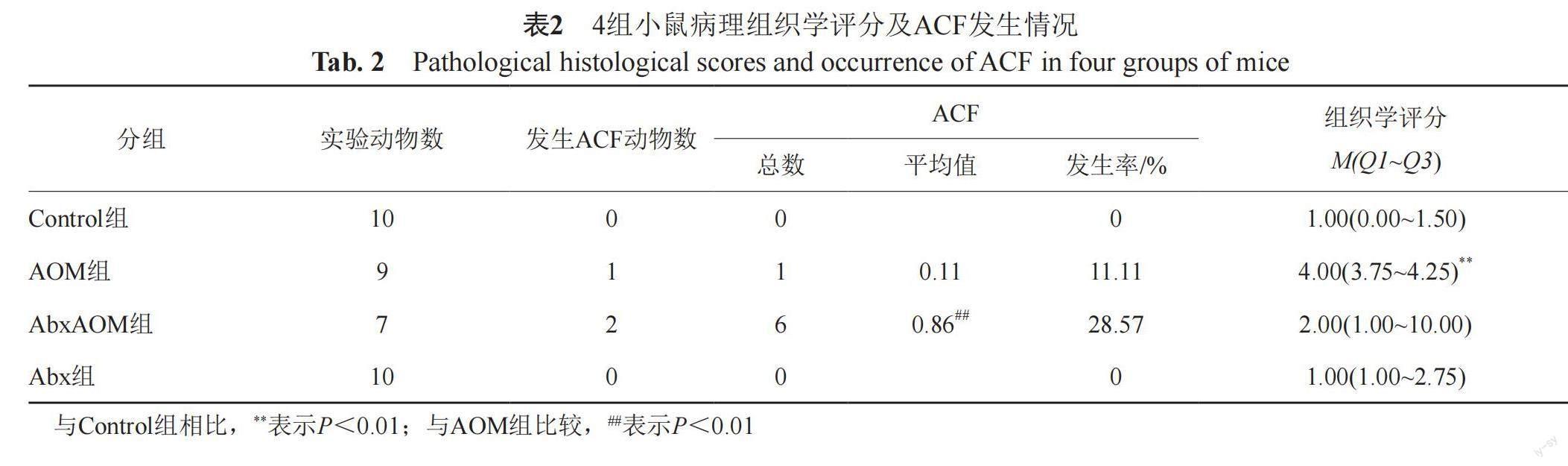
HE染色结果显示:各组小鼠结直肠组织在肉眼及镜下均未观察到肿瘤形成,但有癌前病变ACF形成。AOM组中ACF发生率为11.11%,AbxAOM组中ACF发生率为28.57%,Abx组及Control组中无ACF形成。AOM组与AbxAOM组之间ACF发生率差异无统计学意义。此外,AbxAOM组的小鼠结直肠组织中ACF数量显著高于AOM组(P<0.01),如表2所示。
图2所示,AOM组可见异常隐窝,肠黏膜腺体轻度异型性(可见腺腔增大),轻度不典型增生,可见肠黏膜不同程度炎细胞浸润,与Control组相比,AOM组病理评分极显著升高(P<0.01);AbxAOM组结直肠组织病理学改变与AOM组类似,两者病理组织学评分不具有统计学差异。Control组和Abx组小鼠肠道病理损伤明显减轻,结直肠组织少见或未见肠黏膜轻度炎细胞浸润,上皮无明显缺损,无隐窝萎缩,无ACF、肿瘤等改变。
2.3 AOM和抗生素干预对CRC小鼠結直肠组织相关基因mRNA表达的影响
小鼠结直肠组织相关炎症因子基因的mRNA表达量如图3A所示,与Control组相比,AOM组的IL-6 mRNA(P<0.05)表达显著升高;IL-1β mRNA、TNF-α mRNA、VEGF-A mRNA呈升高趋势。抗生素干预后,与AOM组相比,AbxAOM组IL-1β mRNA(P<0.01)表达水平显著增加;IL-6 mRNA、TNF-α mRNA、VEGF-A mRNA表达呈升高趋势。
小鼠结直肠组织NF-κB信号通路基因mRNA表达量如图3B所示,与Control组相比,AOM组的TLR4 mRNA(P<0.05)、NF-κB p65mRNA(P<0.05)、COX-2 mRNA(P<0.01)表达均显著升高;MyD88 mRNA表达呈升高趋势。抗生素干预后,与AOM组相比,AbxAOM组的NF-κB p65 mRNA(P<0.05)表达水平显著增加;TLR4 mRNA、MyD88 mRNA表达呈升高趋势。
小鼠结直肠组织黏附分子基因的mRNA表达量如图3C所示,与Control组相比,AOM组的VCAM-1 mRNA、ICAM-1 mRNA呈升高趋势。抗生素干预后,与AOM组相比,AbxAOM组的ICAM-1 mRNA(P<0.01)表达水平显著增加;VCAM-1 mRNA表达呈升高趋势。
小鼠结直肠组织增殖相关抗原基因的mRNA表达量如图3D所示,与Control组相比,AOM组的Ki-67 mRNA呈升高趋势。抗生素干预后,与AOM组相比,AbxAOM组的ki-67 mRNA表达呈升高趋势。
2.4 AOM和抗生素干预对CRC小鼠NF-κB p65、Ki-67信号通路基因蛋白表达的影响
如图4所示,与Control组相比,AOM组NF-κB p65蛋白表达呈上升趋势;与AOM组相比,AbxAOM组NF-κB p65蛋白表达呈进一步增高趋势,但差异均无统计学意义。Ki-67蛋白表达各组间差异无统计学意义。
3 讨论
本实验研究终点时未观察到各组小鼠体重的组间差异,这或许受实验时间所限。而肠道损伤是CRC进展过程中的重要环节,本研究结果显示出AOM所致的结肠黏膜损伤,与Control组相比,AOM组病理评分显著升高,可见ACF形成、肠黏膜腺体异型性(部分可见肠腔增大),以及轻度不典型增生,而ACF被认为是CRC发生发展过程中在光镜下可观察到的最早期、最小的肠黏膜上皮的癌前病变,与腺瘤、腺癌的发生密切相关[23]。有研究显示,ACF的发生率以及数量在正常组、腺瘤组、CRC组呈逐步上升趋势,CRC组ACF发生率高达95%[24]。本实验研究结果显示,AOM组、AbxAOM组均有ACF出现,与AOM组比,不论是ACF发生率还是均数,AbxAOM组均呈增长趋势,提示短期抗生素暴露可能加速AOM诱导CRC癌前病变的发生发展。
先前研究揭示了微生物群在结直肠癌Toll样受体(Toll-liked receptors,TLRs)依赖性识别中的作用[25]。一旦肠道屏障被微生物破坏,TLRs将识别这些微生物并诱导某些细胞因子的表达,最终激活免疫应答[26]。例如,革兰阴性细菌脂多糖作用于TLR4使其活化后,在细胞质膜表面诱导MyD88的形成,并导致肠黏膜中NF-κB的激活,并诱导许多促炎因子(如细胞因子和黏附分子)的表达,从而放大炎症级联反应[27-28]。NF-κB的持续激活可以促进VEGF的转录表达,促进恶性肿瘤血管生成[29]。Wang等[30-31]发现,在大肠癌患者中,高水平的TLR4和MyD88与肝转移风险增加和生存率降低相关。作为TLR4蛋白的下游因子,NF-κB是一种重要的调节因子,与炎症和癌症在多个水平上相关[32-33]。NF-κB p65在CRC组织中的过度表达与肿瘤分期增加和总生存率低相关[34-36]。COX-2是NF-κB的靶基因,COX-2在CRC中过表达,与CRC的发生有直接相关[37-40]。有研究显示全身炎性细胞因子与TLR4通路关键蛋白(即TLR4、MyD88、NF-κB p65)表达呈正相关[41]。类似地,本研究发现TLR4/MyD88/NF-κB信号通路的激活,TNF-α、IL-6、IL-1β、VEGF-A、ICAM-1、VCAM-1在AOM处理后也有升高趋势。抗生素处理后通路有进一步激活促炎因子和黏附分子有进一步升高的趋势,提示短期抗生素暴露可能会加速AOM诱导CRC。
綜上所述,AOM引起结肠损伤,并导致ACF等癌前病变的产生,其部分机制可能为引起TLR4/MyD88/NF-κB信号通路的激活,并导致下游COX-2、炎症因子、黏附分子以及增殖相关基因表达升高。而在短期内抗生素暴露有加剧AOM所致结肠损伤和进一步激活TLR4/MyD88/NF-κB信号通路的可能。然而,肿瘤形成是长期、慢性、多因素影响的过程,本研究不足之处为样本量较小、抗生素暴露时间较短,研究抗生素暴露对CRC发生发展的影响还需设计更长干预时间、更全面的实验予以论证。
参 考 文 献
Siegel R L, Miller K D, Goding Sauer A, et al. Colorectal cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70(3): 145-164.
Sung H, Ferlay J, Siegel R L, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2021, 71(3): 209-249.
Gao R, Gao Z, Huang L, et al. Gut microbiota and colorectal cancer[J]. Eur J Clin Microbiol Infect Dis, 2017, 36(5): 757-769.
Wang T T, Cai G C, Y P, et al. Structural segregation of gut microbiota between colorectal cancer patients and healthy volunteers[J]. ISME J, 2012, 6(2): 320-329.
Lu S S M, Mohammed Z, H?ggstr?m C, et al. Antibiotics use and subsequent risk of colorectal cancer: A Swedish nationwide population-based study[J]. J Natl Cancer Inst, 2022, 114(1): 38-46.
Zhang W, Zhu B, Xu J, et al. Bacteroides fragilis protects against antibiotic-associated diarrhea in rats by modulating intestinal defenses[J]. Front Immunol, 2018, 9: 1040.
Xue M, Ji X, Liang H, et al. The effect of fucoidan on intestinal flora and intestinal barrier function in rats with breast cancer[J]. Food Funct, 2018, 9(2): 1214-1223.
陈思敏, 毛杰, 陈志茹, 等. 益生菌联合水溶性膳食纤维对老年抗生素相关性腹泻的防治效果[J]. 天津医药, 2018, 46(3): 284-287.
Gianluca I, Herbert T, Antonio G. Antibiotics as deep modulators of gut microbiota: Between good and evil[J]. Gut, 2016, 65(11): 1906-1915.
Cheng L, Lai M D. Aberrant crypt foci as microscopic precursors of colorectal cancer[J]. World J Gastroenterol, 2003, 9(12): 2642.
Rudolph R E, Dominitz J A, Lampe J W, et al. Risk factors for colorectal cancer in relation to number and size of aberrant crypt foci in humans[J]. Cancer Epidemiol Biomarkers Prev, 2005, 14(3): 605-608.
Hurlstone D P, Karajeh M, Sanders D S, et al. Rectal aberrant crypt foci identified using high-magnification-chromoscopic colonoscopy: Biomarkers for flat and depressed neoplasia[J]. Am J Gastroenterol, 2005, 100(6): 1283-1289.
Sakai E, Takahashi H, Kato S, et al. Investigation of the prevalence and number of aberrant crypt foci associated with human colorectal neoplasmACF and colorectal carcinogenesis[J]. Cancer Epidemiol Biomarkers Prev, 2011, 20(9): 1918-1924.
Merga Y J, O'Hara A, Burkitt M D, et al. Importance of the alternative NF-κB activation pathway in inflammation-associated gastrointestinal carcinogenesis[J]. Am J Physiol Gastrointest Liver Physiol, 2016, 310(11): G1081-G1090.
Landskron G, De la Fuente M, Thuwajit P, et al. Chronic inflammation and cytokines in the tumor microenvironment[J]. J Immunol Res, 2014: 149185.
Huang L C, Merchea A. Dysplasia and cancer in inflammatory bowel disease[J]. Surg Clin, 2017, 97(3): 627-639.
Koliaraki V, Pasparakis M, Kollias G. IKKβ in intestinal mesenchymal cells promotes initiation of colitis-associated cancer[J]. J Exp Med, 2015, 212(13): 2235-2251.
Ijssennagger N, Belzer C, Hooiveld G J, et al. Gut microbiota facilitates dietary heme-induced epithelial hyperproliferation by opening the mucus barrier in colon[J]. Proc Natl Acad Sci U S A, 2015, 112(32): 10038-10043.
Wong S H, Zhao L, Zhang X, et al. Gavage of fecal samples from patients with colorectal cancer promotes intestinal carcinogenesis in germ-free and conventional mice[J]. Gastroenterology, 2017, 153(6): 1621-1633. e6.
Reikvam D H, Erofeev A, Sandvik A, et al. Depletion of murine intestinal microbiota: Effects on gut mucosa and epithelial gene expression[J]. PLoS One, 2011, 6(3): e17996.
Xiao H, Hao X, Simi B, et al. Green tea polyphenols inhibit colorectal aberrant crypt foci (ACF) formation and prevent oncogenic changes in dysplastic ACF in azoxymethane-treated F344 rats[J]. Carcinogenesis, 2008, 29(1): 113-119.
Meira L B, Bugni J M, Green S L, et al. DNA damage induced by chronic inflammation contributes to colon carcinogenesis in mice[J]. J Clin Invest, 2008, 118(7): 2516-2525.
Corpet D E, Tache S. Most Effective colon cancer chemopreventive agents in rats: A systematic review of aberrant crypt foci and tumor data, ranked by potency[J]. Nutr Cancer, 2002, 43(1): 1-21.
Bouzourene H, Chaubert P, Seelentag W, et al. Aberrant crypt foci in patients with neoplastic and nonneoplastic colonic disease[J]. Hum Pathol, 1999, 30(1): 66-71.
O'neill L A J, Golenbock D, Bowie A G. The history of Toll-like receptors-redefining innate immunity[J]. Nat Rev Immunol, 2013, 13(6): 453-460.
Zou S, Fang L, Lee M H. Dysbiosis of gut microbiota in promoting the development of colorectal cancer[J]. Gastroenterol Rep (Oxf), 2018, 6(1): 1-12.
Kawai T, Akira S. Signaling to NF-κB by Toll-like receptors[J]. Trends Mol Med, 2007, 13(11): 460-469.
Li Q, Withoff S, Verma I M. Inflammation-associated cancer: NF-κB is the lynchpin[J]. Trends Immunol, 2005, 26(6): 318-325.
于錦超,于敏,莫炜.NF-κB信号通路在肿瘤发生和炎症反应中的作用[J]. 药物生物技术,2016, 23(1): 82-85.
Wang A C, Su Q B,Wu F X, et al. Role of TLR4 for paclitaxel chemotherapy in human epithelial ovarian cancer cells[J]. Eur J Clin Invest, 2009, 39(2): 157-164.
Wang E L, Qian Z R, Nakasono M, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlates with poor prognosis in colorectal cancer[J]. Br J Cancer, 2010, 102(5): 908-915.
Koji T, Michael K. NF-κB, inflammation, immunity and cancer: Coming of age[J]. Nat Rev Immunol, 2018, 18(5): 309-324.
Didonato J A, Mercurio F, Karin M. NF‐κB and the link between inflammation and cancer[J]. Immunol Rev, 2012, 246(1): 379-400.
Masayuki K, Takashi M, Nobuhiko S, et al. Increased nuclear factor-κB activation in human colorectal carcinoma and its correlation with tumor progression[J]. Anticancer Res, 2004, 24(2B): 675-682.
Moorchung N, Kunwar S, Ahmed K. An evaluation of nuclear factor kappa B expression in colorectal carcinoma: An analysis of 50 cases[J]. J Cancer Res Ther, 2014, 10(3): 631.
Berardi R, Maccaroni E, Mandolesi A, et al. Nuclear factor-κB predicts outcome in locally advanced rectal cancer patients receiving neoadjuvant radio-chemotherapy[J]. Dig Liver Dis, 2012, 44(7): 617-622.
Schmedtje Jr J F, Ji Y S, Liu W L, et al. Hypoxia induces cyclooxygenase-2 via the NF-kappaB p65 transcription factor in human vascular endothelial cells[J]. J Biol Chem, 1997, 272(1): 601-608.
Eberhart C E, Coffey R J, Radhika A, et al. Up-regulation of cyclooxygenase 2 gene expression in human colorectal adenomas and adenocarcinomas[J]. WB Saunders, 1994, 107(4): 1183-1188.
Stern J, Miller G, Li X, et al. Virome and bacteriome: Two sides of the same coin[J]. Curr Opin Virol, 2019, 37: 37-43.
Jeong J W, Park C, Cha H J, et al. Cordycepin inhibits lipopolysaccharide-induced cell migration and invasion in human colorectal carcinoma HCT-116 cells through down-regulation of prostaglandin E2 receptor EP4[J]. BMB Rep, 2018, 51(10): 532.
Ji C L, Deng Y S, Yang A C, et al. Rhubarb enema improved colon mucosal barrier injury in 5/6 nephrectomy rats may associate with gut microbiota modification[J]. Front Pharmacol, 2020, 11(29): 1092.
