全缘叶紫珠化学成分研究
2023-07-17马文杰苏志维马仲辉
马文杰 苏志维 马仲辉
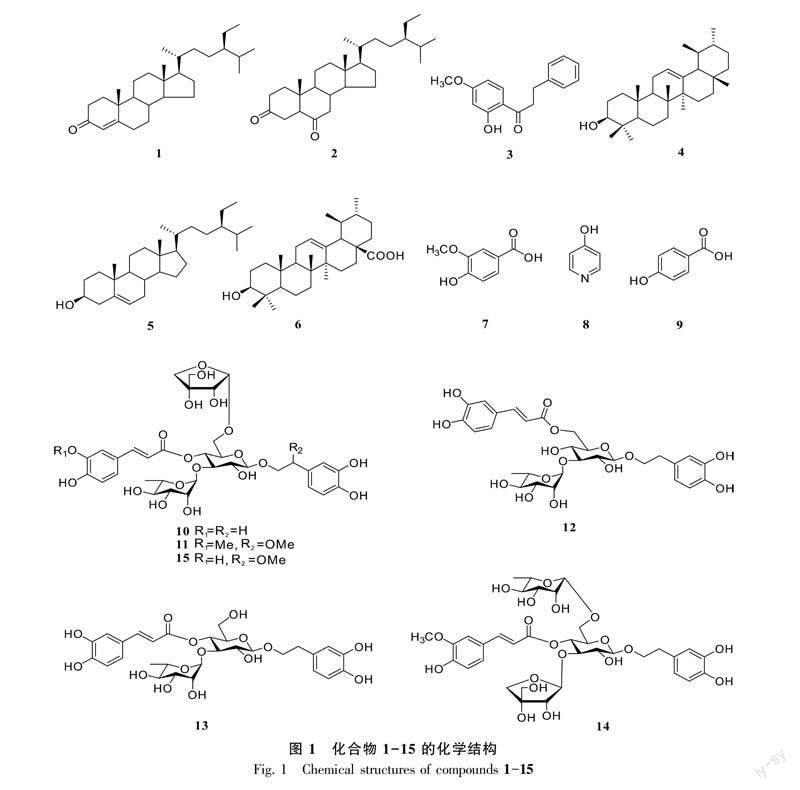
摘 要: 全緣叶紫珠(Callicarpa integerrima)具有祛风散结和治风湿瘰疬的功效,但目前对其化学成分的报道较少。为探究全缘叶紫珠根、茎的化学成分,该研究利用硅胶柱层析色谱、Sephadex LH-20葡聚糖凝胶柱层析色谱、ODS反相硅胶柱层析色谱以及高效液相色谱等现代分离方法对全缘叶紫珠根和茎的95%乙醇提取物进行系统的分离纯化,并运用NMR和ESI-MS等现代波谱技术对化合物进行结构鉴定。结果表明:从全缘叶紫珠根和茎的95%乙醇提取物中共鉴定了15个化合物分别为豆甾烷-4-烯-3-酮 (1)、(24R)-5α-豆甾烷-3,6-二酮 (2)、2′-羟基-4′-甲氧基二氢查尔酮 (3)、α-香树脂醇 (4)、 β-谷甾醇 (5)、熊果酸 (6)、对羟基间甲氧基苯甲酸 (7)、4-羟基吡啶 (8)、对羟基苯甲酸 (9)、连翘酯苷B (10)、nepetifosides D (11)、异毛蕊花苷 (12)、毛蕊花苷 (13)、pedicularioside M (14)、 β-甲氧基连翘酯苷B (15)。除化合物4-6、12和13外,其他化合物均为首次从全缘叶紫珠植物中分离得到,其中化合物1、2、3、8、11和14为首次从紫珠属植物中分离得到。该研究结果丰富了全缘叶紫珠植物的化合物库,为该药用植物的进一步开发利用奠定了科学基础。
关键词: 紫珠属, 全缘叶紫珠, 化学成分, 苯乙醇苷类, 三萜类
中图分类号: Q946文献标识码: A文章编号: 1000-3142(2023)06-1135-10
Chemical constituents of Callicarpa integerrima
MA Wenjie1,2, SU Zhiwei2*, MA Zhonghui1
( 1. College of Agriculture, Guangxi University / Guangxi Key Laboratory of Sugarcane Biology / State Key Laboratory for Conservation and Utilization
of Subtropical Agro-Bioresources / Traditional Chinese Herbal Medicine Resources and Agriculturalization Research Institute, Guangxi University,
Nanning 530004, China; 2. Institute of Marine Drugs, Guangxi University of Chinese Medicine, Nanning 530200, China )
Abstract: Callicarpa integerrima has very good effects in emoving blood stasis and resolving static blood and treatment of rheumatism evil. However, there are few reports on its chemical constituents. In order to explore the chemical constituents from the roots and stems of C. integerrima, the 95% ethanol extracts of C. integerrima roots and stems were isolated and purified by diverse column chromatography, such as silica gel, Sephadex LH-20 gel column, ODS column chromatography, and preparative HPLC. In addition, these compounds were identified on the basis of NMR, ESI-MS as well as other modern spectral techniques. The results were as follows: A total of 15 compounds were isolated from the 95% ethanol extracts of the roots and stems of C. integerrima, which were identified as stigmast-4-en-3-one (1), (24R) -5α-stigmastane-3,6-dione (2), 2′-hydroxy-4′-methoxydihydrochalcone (3), α-amyrin (4), β-sitosterol (5), ursolic acid (6), 4-hydroxy-3-methoxy-benzoic acid (7), 4-pyridinol (8), p-hydroxybenzoic acid (9), forsythoside B (10), nepetifosides D (11), isoacteoside (12), acteoside (13), pedicularioside M (14), β-methoxy forsythoside B (15). All compounds, except for compounds 4-6, 12 and 13, were isolated from C. integerrima for the first time. Furthermore, compounds 1, 2, 3, 8, 11 and 14 were isolated from the genus of Callicarpa for the first time. Therefore, the results of this research has enriched the compound library and afford a scientific foundation for the further rational use of C. integerrima.
Key words: Callicarpa, Callicarpa integerrima, chemical constituents, phenylethanoids, triterpenoids
紫珠属植物具有悠久的药用历史,首载于《本草拾遗》,民间常用于止血和解毒,该属植物的化学成分主要有萜类、苯乙醇苷类、黄酮类,具有止血、抗菌、抗炎、改善记忆力等药理作用(占丽丽等,2020)。目前,《中国药典》(2020)收录了4种紫珠属植物,即广东紫珠、裸花紫珠、大叶紫珠、杜虹花。以这4种紫珠属植物为主要药效成分研发的临床药物已得到了广泛应用,如以广东紫珠为主要成分的抗宫炎胶囊和抗宫炎片,以裸花紫珠为主要成分的裸花紫珠片、裸花紫珠抑菌凝胶、裸花紫珠颗粒、裸花紫珠胶囊等,以大叶紫珠为主要成分的紫地宁血散和三七血伤凝胶囊,以杜虹花为主要成分的11号止血粉、痔炎消颗粒和妇炎灵胶囊等(莫霞和李瑶,2019;邬秋萍等,2019)。由此可见,紫珠属植物资源的开发利用具有广阔的发展空间。
全缘叶紫珠(Callicarpa integerrima)为唇形科紫珠属罕见的藤本型植物,系我国特有种(王雪芬等,1986),常生长于海拔200~700 m的山坡或谷地林中,主要分布于广西、广东、江西、福建等省(区)(《中国植物志》编辑委员会,1982)。全缘叶紫珠的根和叶入药,具有祛风散结、治风湿瘰疬的作用(柴玲等,2010)。目前已从全缘叶紫珠中发现二萜类、三萜类、黄酮类、挥发油等化学成分(王雪芬等,1986;柴玲等,2010;祝晨蔯等,2012;Di et al., 2021)。数十年来,国内外的研究重点集中在该属小乔木型、灌木型植物,如裸花紫珠、广东紫珠和杜虹花等个别种类的化学及药理活性研究,而对该属罕见的藤本型植物全缘叶紫珠的化学成分研究却很少报道,且其药效物质基础仍不明确。全缘叶紫珠作为我国南方民间常用药,明确其化学成分是其用药安全和资源开发的根本前提。因此,为了更好地了解和应用全缘叶紫珠这一药用植物,阐明其化学成分组成和药理药效基础,进而深入开发利用这一特色资源,该研究从全缘叶紫珠的根、茎提取物中共鉴定了15个化合物(图1),分别鉴定为豆甾烷-4-烯-3-酮 (1)、(24R) -5α-豆甾烷-3,6-二酮 (2)、2′-羟基-4′-甲氧基二氢查尔酮 (3)、α-香树脂醇 (4)、β-谷甾醇 (5)、熊果酸 (6)、对羟基间甲氧基苯甲酸 (7)、4-羟基吡啶 (8)、对羟基苯甲酸 (9)、连翘酯苷B (10)、nepetifosides D (11)、异毛蕊花苷 (12)、毛蕊花苷 (13)、 pedicularioside M (14)、 β-甲氧基连翘酯苷B (15)。除化合物4-6、12、13外,其他化合物均为首次从全缘叶紫珠植物中分离得到,其中化合物1、2、3、8、11、14为首次从紫珠属植物中分离得到。这些化合物的发现,丰富了紫珠属植物的化合物库,为进一步系统地探究全緣叶紫珠的药理活性以及作用机制奠定了基础,也为更加合理地开发和利用紫珠属药用植物资源提供了科学依据。
1 仪器与材料
DHG-9240电热恒温鼓风干燥箱(上海精宏实验设备有限公司);SHB-ⅢA循环水式真空泵(巩义市予华仪器有限责任公司);AR224CN(上海电子分析天平奥豪斯仪器有限公司);CX-1000A粉碎机(上海市晟喜制药机械有限公司);3510E-DTH超声波清洗机(美国必能信公司);KQ-800DE数控超声波清洗器(昆山市超声仪器有限公司);AVIII HD 600 和AVIII 500 型核磁共振波谱仪(瑞士Bruker 公司);岛津 LC-2030C 3D Plus高效液相色谱仪(日本岛津公司);数显恒温水浴锅 HH-S4(常州金坛良友仪器有限公司);郑州长城DLSB-10/20低温冷却循环泵(郑州长城科工贸有限公司);上海EYELA OSB-2200小型旋转蒸发仪(上海爱朗仪器有限公司);WFH-203B暗箱式紫外分析仪(杭州齐威仪器有限公司);MCI GEL CHP20P树脂填料(日本三菱化学公司);ODS反相色谱填料 C18 MB100-40/75 (富士化学有限公司);分析型 HPLC 色谱柱为YMC-Pack ODS-A 色谱柱(250 mm×4.6 mm, 5 μm);半制备型 HPLC 色谱柱为 YMC-Pack ODS-A 色谱柱(250 mm×10 mm, 5 μm);优普UPH-II-20T纯水机(南京优普仪器设备有限公司);柱色谱硅胶(100~200目、200~300 目,烟台江友硅胶开发有限公司);GF254薄层层析硅胶板(青岛谱科分离材料有限公司);显色剂为10%硫酸乙醇溶液,浸湿后加热显色;聚酰胺(浙江省台州市路桥四甲生化塑料厂);Sephadex LH-20葡聚糖凝胶(40~70 μm, GE healthcare)。
石油醚、正丁醇购于天津富宇精细化工有限公司,乙酸乙酯购于天津大茂化学试剂厂,甲醇、二氯甲烷、氯仿购于上海泰坦化学有限公司,上述所用试剂均为分析纯级别;氘代试剂购于Cambridge Isotope Laboratories, Inc.;色谱纯乙腈、甲醇均购于上海星可高纯溶剂有限公司。
本实验所用全缘叶紫珠植物样品于2019年4月采自广东省广州市从化区温泉镇温泉派出所附近,经广西大学农学院马仲辉副教授鉴定为唇形科紫珠属植物全缘叶紫珠 (Callicarpa integerrima)。凭证标本(采集号:20190426)保存于广西大学农学院植物标本室 (GAUA)。
2 提取分离
将阴干的全缘叶紫珠根0.55 kg、茎9.78 kg分别粉碎后,用95%乙醇室温浸泡1周后各提取3次,提取液经减压浓缩得根部醇提物(41.66 g)、茎部醇提物(448.50 g)。将茎部醇提物(448.50 g)加热水混悬后,依次用石油醚、乙酸乙酯和正丁醇进行萃取,减压浓缩后得到石油醚部位(46.26 g)、乙酸乙酯部位(72.90 g)和正丁醇部位(106.56 g)。
取根部乙醇提取物(41.66 g)经聚酰胺柱层析,用甲醇-水(0∶100 ~ 30∶70 ~ 50∶50 ~ 70∶30 ~ 100∶0)梯度洗脱,得到11个流分(Fr.1 ~ Fr.11)。流分 Fr.9(1.00 g)经Sephadex LH-20柱层析(氯仿∶甲醇=1∶1)纯化,在氯仿甲醇混合液中重结晶,得到化合物1(7.10 mg)和化合物2(11.70 mg),经薄层层析硅胶板进行制备,得到化合物3(2.60 mg)。
取茎部石油醚萃取物(46.26 g)经硅胶柱层析,用石油醚-二氯甲烷(98∶2 ~ 5∶5)梯度洗脱,得到15个流分(Fr.1 ~ Fr.15)。流分Fr.10(5.69 g)经Sephadex LH-20柱层析(氯仿∶甲醇=1∶1)、硅胶柱层析(石油醚∶二氯甲烷= 19∶1),ODS反相硅胶柱 (90%甲醇水)反復纯化,得到化合物4(21.20 mg),在氯仿甲醇混合液中重结晶,得到化合物5(10.11 mg)。Fr.14(6.80 g)经MCI柱层析(85%甲醇水)和硅胶柱层析(石油醚∶二氯甲烷∶甲醇= 50∶99∶1)纯化,得到化合物6(11.40 mg)。
取茎部乙酸乙酯萃取物(72.90 g)经硅胶柱层析,二氯甲烷-甲醇(98∶2 ~ 5∶5)梯度洗脱,得到13个流分(Fr.1 ~ Fr.13)。Fr.5(3.64 g)经Sephadex LH-20柱层析(氯仿∶甲醇=1∶1)纯化,再经半制备HPLC,18%乙腈水等度制备,流速2 mL·min-1,得到化合物7(10.00 mg, tR=19 min)和化合物8(4.50 mg, tR=27 min)。Fr.6(2.56 g)经Sephadex LH-20柱层析(氯仿∶甲醇=1∶1)纯化,再经半制备HPLC,20%乙腈水(含0.1% 甲酸)等度制备,流速2 mL·min-1,得到化合物9(5.22 mg, tR=15 min)。Fr.12(56.11 g)经硅胶柱层析(二氯甲烷-甲醇=95∶5 ~ 5∶5),中压ODS反相柱层析(乙腈-水=15% ~ 100%),Sephadex LH-20柱层析(氯仿∶甲醇=1∶1),反复纯化,得到化合物10 ( 69.90 mg )。再经半制备HPLC,21%乙腈水(含0.1%甲酸)等度制备,流速2 mL·min-1,得到化合物11(7.04 mg, tR=52 min);34%甲醇水等度制备,流速2 mL·min-1,得到化合物12(40.00 mg, tR=31 min);35%甲醇水等度制备,流速2 mL·min-1,得到化合物13(94.00 mg, tR=20 min)和化合物14(13.00 mg, tR=27 min);15%乙腈水(含0.1%甲酸)等度制备,流速2 mL·min-1,得到化合物15(10.62 mg, tR=36 min)。
3 结构鉴定
化合物1 白色针晶状结晶,分子式为C29H48O。ESI-MS m/z: 413.37 [ M + H ]+。1H-NMR (600 MHz, CDCl3) δH: 5.74 (1H, s, H-4), 0.92 (3H, s, H-19), 0.90 (3H, d, J = 7.1 Hz, H-21), 0.86 (3H, t, J = 8.1 Hz, H-29), 0.85 (3H, d, J = 7.1 Hz, H-26), 0.82 (3H, d, J = 6.6 Hz, H-27), 0.70 (3H, s, H-18)。13C-NMR (150 MHz, CDCl3) δC: 198.8 (C-3), 171.6 (C-5), 123.9 (C-4), 56.1 (C-17), 55.9 (C-14), 53.8 (C-9), 45.9 (C-24), 42.3 (C-13), 39.8 (C-10, C-12), 38.7 (C-8), 36.1 (C-20), 35.6 (C-1), 33.9 (C-2), 33.8 (C-22), 32.9 (C-6), 32.0 (C-7), 29.1 (C-25), 28.2 (C-16), 26.0 (C-23), 24.2 (C-15), 23.0 (C-28), 21.0 (C-11), 19.9 (C-27), 19.1 (C-26), 18.8 (C-21), 17.6 (C-19), 12.1 (C-18/29)。以上数据与文献(Abdelhameed et al., 2020)报道的豆甾烷-4-烯-3-酮 (stigmast-4-en-3-one) 的数据基本一致,故鉴定化合物1为豆甾烷-4-烯-3-酮 (stigmast-4-en-3-one)。
化合物2 白色针晶状结晶,分子式为C29H48O2。ESI-MS m/z: 429.37 [ M + H ]+。1H-NMR (600 MHz, CDCl3) δH: 0.95 (3H, s, H-18), 0.92 (3H, d, J = 7.1 Hz, H-21), 0.86 (3H, t, J = 8.1 Hz, H-29), 0.83 (3H, d, J = 6.9 Hz, H-26), 0.81 (3H, d, J = 6.4 Hz, H-27), 0.70 (3H, s, H-19)。13C-NMR (150 MHz, CDCl3) δC: 211.4 (C-6), 209.1 (C-3), 57.5 (C-5), 56.6 (C-17), 56.0 (C-14), 53.5 (C-9), 46.6 (C-7), 45.8 (C-24), 43.0 (C-13), 41.2 (C-10), 39.4 (C-2), 38.1 (C-1), 38.0 (C-8), 37.4 (C-12), 37.0 (C-4), 36.0 (C-20), 33.9 (C-22), 29.1 (C-25), 28.0 (C-16), 26.0 (C-23), 24.0 (C-15), 23.0 (C-28), 21.7 (C-11), 19.9 (C-26), 19.1 (C-27), 18.6 (C-21), 12.7 (C-18), 12.1 (C-19/29)。以上数据与文献(李小军等,2014)报道的(24R)-5α-豆甾烷-3,6-二酮[(24R)-5α-stigmastane-3,6-dione]的数据基本一致,故鉴定化合物2为 (24R)-5α-豆甾烷-3,6-二酮[(24R)-5α-stigmastane-3,6-dione]。
化合物3 白色无定形粉末,分子式为C16H16O3。ESI-MS m/z: 257.11 [ M + H ]+。1H-NMR (600 MHz, CD3OD) δH: 7.53 (1H, d, J=8.5 Hz, H-6′), 7.22 (5H, m, H-2/3/4/5/6), 6.36 (1H, d, J = 1.9 Hz, H-3′), 6.40 (1H, dd, J = 8.5, 2.1 Hz, H-5′), 3.83 (3H, s, H-4′), 3.22 (2H, t, J = 7.6 Hz, H-α), 2.90 (2H, t, J = 7.6 Hz, H-β)。13C-NMR (150 MHz, CD3OD) δC: 201.8 (C=O), 164.8 (C-4′), 163.0 (C-2′), 143.1 (C-1), 133.6 (C-6′), 129.5 (C-2/3/5/6), 127.0 (C-4), 113.3 (C-1′), 108.9 (C-3′), 99.9 (C-5′), 56.0 (4′-OCH3), 46.2 (C-α), 32.2 (C-β)。以上数据与文献(Edyta et al., 2017)报道的2′-羟基-4′-甲氧基二氢查尔酮(2′-hydroxy-4′-methoxydihydrochalcone)的数据基本一致,故鉴定化合物3为2′-羟基-4′-甲氧基二氢查尔酮(2′-hydroxy-4′-methoxydihydrochalcone)。
化合物4 白色针状结晶,分子式为C30H50O。ESI-MS m/z: 427.39 [M + H]+。 1H-NMR (600 MHz, CDCl3) δH: 5.13 (1H, t, J = 3.7 Hz, H-12), 3.22 (1H, dd, J = 11.3, 4.8 Hz, H-3), 1.07 (3H, s, H-27), 1.01 (3H, s, H-26), 1.00 (3H, s, H-23), 0.95 (3H, d, J = 0.8 Hz, H-25), 0.91 (3H, s, H-30), 0.87 (3H, s, H-28), 0.80 (3H, s, H-24), 0.74 (3H, d, J = 11.8 Hz, H-29)。 13C-NMR (150 MHz, CDCl3) δC: 139.6 (C-13), 124.4 (C-12), 79.1 (C-3), 59.1 (C-18), 55.2 (C-5), 47.7 (C-9), 42.1 (C-14), 41.5 (C-22), 40.0 (C-8), 39.7 (C-19), 39.6 (C-20), 38.8 (C-1), 38.8 (C-4), 36.9 (C-10), 33.8 (C-17), 33.0 (C-7), 31.3 (C-21), 28.8 (C-15), 28.1 (C-23), 28.1 (C-28), 27.3 (C-2), 26.6 (C-16), 23.4 (C-11), 23.3 (C-27), 21.4 (C-29), 18.4 (C-6), 17.5 (C-30), 16.9 (C-26), 15.7 (C-25), 15.6 (C-24)。以上數据与文献(莫青胡等,2020)报道的α-香树脂醇(α-amyrin)的数据基本一致,故鉴定化合物4为α-香树脂醇(α-amyrin)。
化合物5 白色粉末,分子式为C29H50O。ESI-MS m/z: 415.39 [M + H]+。 1H-NMR (600 MHz, CDCl3) δH: 5.35 (1H, m, H-6), 3.52 (1H, m, H-3), 1.01 (3H, s, H-19), 0.92 (3H, d, J = 6.5 Hz, H-21), 0.84 (3H, d, J = 2.9 Hz, H-26), 0.83 (3H, m, H-29), 0.81 (3H, m, H-27), 0.69 (3H, s, H-18)。 13C-NMR (150 MHz, CDCl3) δC: 140.8 (C-5), 121.7 (C-6), 71.8 (C-3), 56.8 (C-14), 56.1 (C-17), 50.1 (C-9), 45.9 (C-24), 42.3 (C-4), 42.3 (C-13), 39.8 (C-12), 37.3 (C-1), 36.5 (C-10), 36.2 (C-20), 34.0 (C-22), 31.9 (C-7), 31.9 (C-8), 31.9 (C-2), 29.2 (C-25), 28.3 (C-16), 26.0 (C-23), 24.3 (C-15), 23.0 (C-28), 21.1 (C-11), 19.8 (C-26), 19.4 (C-19), 19.0 (C-27), 18.8 (C-21), 12.0 (C-29), 11.9 (C-18)。以上数据与文献(查显进等,2021)中报道的β-谷甾醇(β-sitosterol)的数据基本一致,故鉴定化合物5为β-谷甾醇(β-sitosterol)。
化合物6 白色粉末,分子式为C30H48O3。ESI-MS m/z: 457.36 [M + H]+。1H-NMR (600 MHz, CD3OD) δH: 5.26 (1H, t, J = 3.7 Hz, H-12), 3.29 (1H, dd, J = 3.3, 1.6 Hz, H-3), 1.32 (3H, m, H-27), 1.30 (3H, s, H-29), 1.27 (3H, s, H-24), 1.16 (3H, s, H-23), 0.90 (3H, m, H-30), 0.83 (3H, s, H-25), 0.75 (3H, s, H-26)。13C-NMR (150 MHz, DMSO) δC: 179.3 (C-28), 138.9 (C-13), 127.1 (C-12), 78.2 (C-3), 55.9 (C-5), 53.6 (C-18), 48.1 (C-17), 48.1 (C-9), 42.6 (C-14), 40.1 (C-8), 39.6 (C-1), 39.5 (C-4), 39.4 (C-19), 39.2 (C-20), 37.5 (C-22), 37.4 (C-10), 33.7 (C-7), 31.1 (C-21), 28.8 (C-23), 28.8 (C-2), 28.2 (C-15), 25 (C-16), 24 (C-11), 23.7 (C-27), 21.4 (C-30), 18.8 (C-6), 17.5 (C-26), 17.5 (C-29), 16.5 (C-24), 15.7 (C-25)。以上数据与文献(陈雪林等,2020)报道的熊果酸(ursolic acid)的数据基本一致,故鉴定化合物6为熊果酸(ursolic acid)。
化合物7 白色粉末,分子式为C8H8O4。ESI-MS m/z: 169.04 [M + H]+。 1H-NMR (600 MHz, CD3OD) δH: 7.56 (1H, d, J = 1.8 Hz, H-2), 7.55 (1H, d, J = 2.0 Hz, H-6), 6.86~6.81 (1H, m, H-5), 3.89 (3H, s, 8-OCH3)。 13C-NMR (150 MHz, CD3OD) δC: 167.6 (C-7), 152.7 (C-4), 148.8 (C-3), 125.3 (C-6), 123.5 (C-1), 115.9 (C-2), 114.1 (C-5), 56.6 (8-OCH3)。以上数据与文献(李药兰等,2006)报道的对羟基间甲氧基苯甲酸(4-hydroxy-3-methoxy-benzoic acid)的数据基本一致,故鉴定化合物7为对羟基间甲氧基苯甲酸(4-hydroxy-3-methoxy-benzoic acid)。
化合物8 白色粉末,分子式为C5H5NO。ESI-MS m/z: 96.04 [M + H]+。 1H-NMR (600 MHz, DMSO-d6) δH: 9.76 (1H, s, 4-OH), 7.74 (2H, d, J = 8.6 Hz, H-2/6), 6.91 (2H, d, J = 8.2 Hz, H-3/5)。 13C-NMR (150 MHz, DMSO-d6) δC: 160.7 (C-4), 149.8 (C-2/6), 115.9 (C-3/5)。以上数据与文献 (王伟忠,2008)报道的4-羟基吡啶(4-pyridinol)的数据基本一致,故鉴定化合物8为4-羟基吡啶(4-pyridinol)。
化合物9 白色粉末,分子式為C7H6O3。ESI-MS m/z: 139.03 [M + H]+。 1H-NMR (600 MHz, CD3OD) δH: 7.87 (2H, d, J = 8.7 Hz, H-2/6), 6.80 (2H, d, J = 8.7 Hz, H-3/5)。 13C-NMR (150 MHz, CD3OD) δC: 171.6 (C-7), 163.1 (C-4), 133.0 (C-2/6), 124.1 (C-1), 116.0 (C-3/5)。以上数据与文献(李艳茸等,2014)报道的对羟基苯甲酸(p-hydroxybenzoic acid)的数据基本一致,故鉴定化合物9为对羟基苯甲酸(p-hydroxybenzoic acid)。
化合物10 红棕色固体,分子式为C34H44O19。ESI-MS m/z: 779.25 [M+Na]+。 1H-NMR (600 MHz, CD3OD) δH: 7.62 (1H, d, J = 15.8 Hz, Caf H-7″), 7.10 (1H, d, J = 2.0 Hz, Caf H-2″), 6.99 (1H, dd, J = 8.2, 2.1 Hz, Caf H-6″), 6.82 (1H, d, J = 8.2 Hz, Caf H-5″), 6.75~6.71 (2H, m, Agl H-2/5), 6.60 (1H, dd, J = 8.1, 2.1 Hz, Agl H-6), 6.31 (1H, d, J = 15.9 Hz, Caf H-8″), 5.21 (1H, d, J = 1.8 Hz, Rha H-1), 4.98 (1H, m, Glu H-4′), 4.94 (1H, d, J = 2.3 Hz, Api H-1′), 4.40 (1H, d, J = 7.9 Hz, Glu H-1′), 4.02 (1H, m, Agl H-8), 3.96~3.90 (2H, m, Rha H-2, Api H-4′a), 3.83 (1H, m, Glu H-3′), 3.80~3.69 (4H, m, Glu H-5′/6′, Api H-2′/4′b), 3.64~3.60 (1H, m, Rha H-3), 3.58 (2H, s, Api H-5′), 3.51 (1H, dd, J = 11.2, 5.7 Hz, Glu H-2′), 3.35~3.30 (2H, m, Rha H-4/5), 2.82 (2H, m, Agl H-7), 1.11 (3H, d, J = 6.2 Hz, Rha H-6)。 13C-NMR (150 MHz, CD3OD) δC: 168.3 (Caf C-9″), 149.8 (Caf C-4″), 148.2 (Caf C-7″), 146.8 (Caf C-3″), 146.1 (Agl C-3), 144.7 (Agl C-4), 131.6 (Agl C-1), 127.7 (Caf C-1″), 123.4 (Caf C-6″), 121.5 (Agl C-6), 117.3 (Agl C-2), 116.7 (Caf C-5″), 116.5 (Agl C-5), 115.4 (Caf C-2″), 114.8 (Caf C-8″), 111.1 (Api C-1′), 104.2 (Glu C-1′), 103.1 (Rha C-1), 81.8 (Glu C-3′), 80.7 (Api C-3′), 78.3 (Api C-2′), 76.2 (Glu C-2′), 75.2 (Api C-4′), 74.6 (Glu C-5′), 73.8 (Rha C-4), 72.4 (Rha C-2), 72.4 (Agl C-8), 72.1 (Rha C-3), 70.9 (Glu C-4′), 70.5 (Rha C-5), 68.5 (Glu C-6′), 65.7 (Api C-5′), 36.7 (Agl C-7), 18.5 (Rha C-6)。以上数据与文献(Yamasaki et al., 2007)报道的连翘酯苷B (forsythoside B) 的数据基本一致,故鉴定化合物10为连翘酯苷B (forsythoside B)。
化合物11 黄绿色固体,分子式为C36H48O20。ESI-MS m/z: 823.27 [M +Na]+。 1H-NMR (600 MHz, DMSO-d6) δH: 7.54 (1H, d, J = 15.8 Hz, Acyl H-7″), 7.29 (1H, d, J = 2.0 Hz, Acyl H-2″), 7.09 (1H, dd, J = 8.2, 2.0 Hz, Acyl H-6″), 6.79 (1H, d, J = 8.1 Hz, Acyl H-5″), 6.70 (2H, d, J = 8.1 Hz, Agl H-2/5), 6.58 (1H, dd, J = 8.1, 2.1 Hz, Agl H-6), 6.42 (1H, d, J = 15.9 Hz, Acyl H-8″), 5.03 (1H, d, J = 1.7 Hz, Rha H-1), 4.76 (1H, d, J = 2.9 Hz, Api H-1′), 4.68 (1H, t, J = 9.7 Hz, Glu H-4′), 4.42 (1H, d, J = 7.9 Hz, Glu H-1′), 4.25 (1H, dd, J = 7.9, 3.8 Hz, Agl H-7), 3.80 (3H, s, 3″-OCH3), 3.78 (1H, s, Agl H-8a), 3.69 (3H, m, Glu H-5′, Rha H-2, Api H-2′), 3.62 (3H, m, Api H-3′/4′), 3.57~3.48 (3H, m, Agl H-8b, Glu H-2′/3′), 3.40~3.30 (2H, m, Glu H-6′, Rha H-5), 3.26 (3H, m, Rha H-3, Api H-5′), 3.13 (3H, s,7-OCH3), 3.09 (1H, d, J = 9.4 Hz, Rha H-4), 0.98 (3H, d, J = 6.2 Hz, Rha H-6)。 13C-NMR (150 MHz, DMSO-d6) δC: 165.8 (Acyl C-9″), 149.5 (Acyl C-3″), 147.9 (Acyl C-4″), 145.8 (Agl C-4), 145.2 (Acyl C-7″), 145.0 (Agl C-3), 129.7 (Agl C-1), 125.5 (Acyl C-1″), 123.3 (Acyl C-6″), 118.1 (Agl C-6), 115.5 (Agl C-5, Acyl C-5″), 114.1 (Agl C-2), 113.9 (Acyl C-8″), 111.1 (Acyl C-2″), 109.1 (Api C-1′), 102.8 (Glu C-1′), 101.3 (Rha C-1), 82.3 (Agl C-7), 78.9 (Api C-3′), 78.8 (Glu C-3′), 75.9 (Api C-2′), 74.3 (Glu C-2′), 73.7 (Agl C-8), 73.4 (Api C-4′), 72.8 (Glu C-5′), 71.7 (Rha C-4), 70.5 (Rha C-2), 70.4 (Rha C-3), 69.3 (Glu C-4′), 68.8 (Rha C-5), 67.1 (Glu C-6′), 63.2 (Api C-5′), 56.0 (7-OCH3), 55.6 (3″-OCH3), 18.1 (Rha C-6)。以上数据与文献(Xu et al., 2019)报道的 nepetifosides D的数据基本一致,故鉴定化合物11为 nepetifosides D。
化合物12 橙红色膏体,分子式为C29H36O15。ESI-MS m/z: 647.21 [M+Na]+。 1H-NMR (600 MHz, CD3OD) δH: 7.57 (1H, d, J = 15.8 Hz, Caf H-7″), 7.05 (1H, d, J = 2.1 Hz, Caf H-2″), 6.89 (1H, dd, J = 8.2, 2.1 Hz, Caf H-6″), 6.78 (1H, d, J = 8.2 Hz, Caf H-5″), 6.69 (1H, d, J = 2.0 Hz, Agl H-2), 6.65 (1H, d, J = 8.0 Hz, Agl H-5), 6.54 (1H, dd, J = 8.0, 2.0 Hz, Agl H-6), 6.30 (1H, d, J = 15.9 Hz, Caf H-8″), 5.20 (1H, d, J = 1.7 Hz, Rha H-1), 4.51 (1H, dd, J = 11.9, 2.2 Hz, Glu H-6a′), 4.39~4.36 (1H, m, Glu H-6b′), 4.34 (1H, d, J = 7.9 Hz, Glu H-1′), 4.04~3.93 (3H, m, Agl H-8b, Rha H-2/ 4), 3.75~3.69 (2H, m, Agl H-8a, Rha H-3), 3.58~3.55 (1H, m, Glu H-5′), 3.54 (1H, d, J = 8.9 Hz, Glu H-3′), 3.42 (1H, m, Glu H-4′), 3.35~3.30 (1H, m, Glu H-2′), 2.83~2.73 (2H, m, Agl H-7), 1.26 (3H, d, J = 6.2 Hz, Rha H-6)。13C-NMR (150 MHz, DMSO-d6) δC: 169.2 (Caf C-9″), 149.5 (Caf C-4″), 147.2 (Caf C-7″), 146.7 (Caf C-3″), 146.0 (Agl C-3), 144.6 (Agl C-4), 131.4 (Agl C-1), 127.6 (Caf C-1″), 123.2 (Caf C-6″), 121.3 (Agl C-6), 117.1 (Agl C-2), 116.5 (Agl C-5), 116.4 (Caf C-5″), 115.1 (Caf C-2″), 114.8 (Caf C-8″), 104.3 (Glu C-1′), 102.6 (Rha C-1), 83.9 (Glu C-3′), 75.6 (Glu C-2′), 75.3 (Glu C-5′), 73.9 (Rha C-4), 72.4 (Agl C-8), 72.3 (Rha C-3), 72.2 (Rha C-2), 70.3 (Glu C-4′), 70.0 (Rha C-5), 64.6 (Glu C-6′), 36.6 (Agl C-7), 17.9 (Rha C-6)。以上數据与文献(Saimaru & Orihara, 2010)报道的异毛蕊花苷 (isoacteoside)的数据基本一致,故鉴定化合物12为异毛蕊花苷(isoacteoside)。
化合物13 橙红色膏体,分子式为C29H36O15。ESI-MS m/z: 647.21 [M+Na]+。 1H-NMR (600 MHz, DMSO-d6) δH: 7.46 (1H, d, J = 15.8 Hz, Caf H-7″), 7.03 (1H, d, J = 2.0 Hz, Caf H-2″), 6.97 (1H, dd, J = 8.2, 2.1 Hz, Caf H-6″), 6.76 (1H, d, J = 8.1 Hz, Caf H-5″), 6.65~6.61 (2H, m, Agl H-2/5), 6.49 (1H, dd, J = 8.1, 2.1 Hz, Agl H-6), 6.20 (1H, d, J = 15.9 Hz, Caf H-8″), 4.72 (1H, m, Glu H-4′), 4.35 (1H, d, J = 7.9 Hz, Glu H-1′), 3.88 (1H, dd, J = 9.2, 6.4 Hz, Rha H-2), 3.72~3.07 (10H, m, Agl H-8, Rha/Glu-H), 2.76~2.63 (2H, m, Agl H-7), 0.96 (3H, d, J = 6.1 Hz, Rha H-6)。 13C-NMR (150 MHz, DMSO-d6) δC: 166.0 (Caf C-9″), 148.8 (Caf C-3″), 145.8 (Caf C-7″), 145.2 (Agl C-3, Caf C-4″), 143.8 (Agl C-4), 129.4 (Agl C-1), 125.7 (Caf C-1″), 121.7 (Caf C-6″), 119.8 (Agl C-6), 116.5 (Caf C-5″), 116.0 (Agl C-2), 115.7 (Agl C-5), 114.9 (Caf C-8″), 113.8 (Caf C-2″), 102.5 (Glu C-1′), 101.5 (Rha C-1), 79.3 (Glu C-3′), 74.7 (Glu C-5′), 74.7 (Glu C-2′), 71.9 (Rha C-4), 70.8 (Rha C-2), 70.6 (Rha C-3), 70.5 (Agl C-8), 69.4 (Rha C-5), 69.0 (Glu C-4′), 61.0 (Glu C-6′), 35.2 (Agl C-7), 18.4 (Rha C-6)。以上数据与文献(Lan et al., 2018)报道的毛蕊花苷(acteoside)的数据基本一致, 故鉴定化合物13为毛蕊花苷(acteoside)。
化合物14 黄色固体,分子式为C35H46O19。ESI-MS m/z: 793.26 [M+Na]+。 1H-NMR (600 MHz, DMSO-d6) δH: 7.54 (1H, d, J = 15.8 Hz, Acyl H-7″), 7.29 (1H, d, J = 2.0 Hz, Acyl H-2″), 7.09 (1H, dd, J = 8.3, 2.0 Hz, Acyl H-6″), 6.79 (1H, d, J = 8.1 Hz, Acyl H-5″), 6.59 (2H, m, Agl H-2/5), 6.50 (1H, dd, J = 8.1, 2.1 Hz, Agl H-6), 6.41 (1H, d, J = 15.9 Hz, Acyl H-8″), 5.03 (1H, d, J = 1.7 Hz, Rha H-1), 4.78 (1H, d, J = 2.9 Hz, Api H-1′), 4.64 (1H, t, J = 9.7 Hz, Glu H-4′), 4.38 (1H, d, J = 7.9 Hz, Glu H-1′), 3.80 (3H, s, 3″-OCH3), 3.76~3.63 (7H, m, Glu H-3′/5′, Api H-2′/3′/4′, Rha H-2), 3.57~3.48 (2H, m, Agl H-8), 3.32~3.18 (6H, m, Api H-5′, Glu H-2′/6′, Rha H-3″/5) , 3.10 (1H, t, J = 9.4 Hz, Rha H-4), 2.80~2.65 (2H, m, Agl H-7), 0.98 (3H, d, J = 6.2 Hz, Rha H-6)。 13C-NMR (150 MHz, DMSO-d6) δC: 166.3 (Acyl C-9″), 150.0 (Acyl C-3″), 148.4 (Acyl C-4″), 146.2 (Acyl C-7″), 145.5 (Agl C-3), 144.0 (Agl C-4), 129.6 (Agl C-1), 126.0 (Acyl C-1″), 123.7 (Acyl C-6″), 120.0 (Agl C-6), 116.8 (Agl C-2), 116.0 (Agl C-5), 115.7 (Acyl C-8″), 114.3 (Acyl C-5″), 111.5 (Acyl C-2″), 109.6 (Api C-1′), 102.7 (Glu C-1′), 101.7 (Rha C-1), 79.3 (Glu C-3′, Api C-3′), 76.4 (Api C-2′), 74.8 (Glu C-2′), 73.9 (Api C-4′), 73.3 (Glu C-5′), 72.1 (Rha C-4), 71.0 (Rha C-2), 70.8 (Rha C-3), 70.8 (Agl C-8), 69.9 (Glu C-4′), 69.2 (Rha C-5), 67.6 (Glu C-6′), 63.6 (Api C-5′), 56.1 (3″-OCH3), 35.5 (Agl C-7), 18.6 (Rha C-6)。以上數据与文献(Jia & Gao, 1993)报道的 pedicularioside M的数据基本一致,故鉴定化合物14为 pedicularioside M。
化合物15 黄绿色固体,分子式为C35H46O20。ESI-MS m/z: 809.26 [M+Na]+。 1H-NMR (500 MHz, DMSO-d6) δH: 7.47 (1H, d, J = 15.8 Hz, Caf H-7″), 7.03 (1H, d, J = 2.1 Hz, Caf H-2″), 6.98 (1H, dd, J = 8.3, 2.1 Hz, Caf H-6″), 6.76 (1H, d, J = 8.1 Hz, Caf H-5″), 6.71~6.68 (2H, m, Agl H-2/5), 6.58 (1H, dd, J = 8.0, 2.0 Hz, Agl H-6), 6.20 (1H, d, J = 15.9 Hz, Caf H-8″), 5.02 (1H, d, J = 1.5 Hz, Rha H-1), 4.76 (1H, d, J = 2.8 Hz, Api H-1′), 4.68 (1H, t, J = 9.7 Hz, Glu H-4′), 4.42 (1H, d, J = 7.8 Hz, Glu H-1′), 4.25 (1H, dd, J = 7.8, 3.9 Hz, Agl H-7), 3.79 (1H, d, J = 9.3 Hz, Agl H-8a), 3.72~3.57 (7H, m, Glu H-3′/5′, Api H-2′/3′/4′, Rha H-2), 3.57~3.46 (2H, m, Agl H-8b, Glu H-6′a), 3.36~3.17 (6H, m, Api H-5′, Glu H-2′/6′, Rha H-3/5), 3.13 (3H, d, J = 6.1 Hz,7-OCH3), 3.09 (1H, d, J = 9.4 Hz, Rha H-4), 0.96 (3H, d, J = 6.2 Hz, Rha H-6)。 13C-NMR (125 MHz, DMSO-d6) δC: 165.7 (Caf C-9″), 148.5 (Caf C-4″), 145.8 (Caf C-7″), 145.6 (Caf C-3″), 145.2 (Agl C-4), 145.0 (Agl C-3), 129.7 (Agl C-1), 125.5 (Caf C-1″), 121.4 (Caf C-6″), 118.1 (Agl C-6), 115.8 (Caf C-5″), 115.4 (Agl C-5), 114.8 (Caf C-2″), 114.0 (Agl C-2), 113.4 (Caf C-8″), 109.1 (Api C-1′), 102.8 (Glc C-1′), 101.2 (Rha C-1), 82.2 (Agl C-7), 78.9 (Glc C-3′), 78.8 (Api C-3′), 75.9 (Api C-2′), 74.3 (Glc C-2′), 73.7 (Agl C-8), 73.4 (Api C-4′), 72.7 (Glc C-5′), 71.6 (Rha C-4), 70.5 (Rha C-5), 70.4 (Rha C-3), 69.3 (Glc C-4′), 68.7 (Rha C-5), 67.0 (Glc C-6′), 63.2 (Api C-5′), 56.0 (7-OCH3), 18.1 (Rha C-6)。分析质谱和NMR数据,发现化合物15与连翘酯苷B的结构相近(Wang et al., 2005),主要区别在于化合物15多了1个甲氧基结构片段信号 [δH 3.13 (3H, d, J = 6.1 Hz,7-OCH3),δC 56.0 (7-OCH3)],以及由甲氧基引起的C-7位次甲基CH的化学位移向低场位移的信号δC 73.4 (Agl C-8),82.2 (Agl C-7),129.7 (Agl C-1), HMBC谱上可观测δH 3.13 ( 3H, d, J = 6.1 Hz,7-OCH3 )与δC 82.2( Agl C-7 )远程相关的信号,进一步确定甲氧基连在苯乙醇结构片段Agl C-7位,故鉴定化合物15为 β-甲氧基连翘酯苷B (β-methoxy forsythoside B)。
4 讨论与结论
本研究采用多种现代色谱分离技术和波谱鉴定手段从我国特有植物全缘叶紫珠的根、茎提取物中分离鉴定了15个化合物,包括3个甾体类(1、2、5),2个三萜类(4、6),6个苯乙醇苷类(10-15),1个黄酮类(3),2个苯甲酸类(7、9)和1个生物碱(8)。本研究结果表明,苯乙醇苷类化合物是全缘叶紫珠的特征性物质成分,该类化合物是由苯乙醇苷元、咖啡酰基、葡萄糖/鼠李糖/芹菜糖等糖基取代衍生而成的天然水溶性糖苷(Tian et al., 2021)。現代药理研究表明,苯乙醇苷类物质具有抗氧化、改善记忆、保肝、神经保护、抗肿瘤等多种药理作用,在治疗阿尔茨海默病、改善记忆力等方面的发展潜力尤为突出(Lee et al., 2006)。紫珠属植物作为常见的民间药,其最早便是被用于止血。余婧等(2015)研究表明广东紫珠发挥止血作用的主要成分为苯乙醇苷类,本研究发现的苯乙醇苷类化合物大多为首次从全缘叶紫珠中得到,其中化合物11和化合物14为首次从紫珠属中报道。因此,本研究不仅丰富了全缘叶紫珠植物的次生代谢化合物库,也为进一步挖掘全缘叶紫珠中结构新颖的止血活性成分及其药理机制研究奠定了物质基础。
本研究结果发现,全缘叶紫珠的石油醚萃取部位的化学成分主要为甾体、萜类等小极性物质,结合文献研究发现:α-香树脂醇(化合物4)对白背飞虱雌虫具有较好的杀灭效果;β-谷甾醇(化合物5)不仅对Aedes aegypti和 Culex quinquefasciatus 2种蚊虫具有较好的杀灭效果(Chenniappan & Kadarkari, 2012; Angajala & Subashini, 2018),还对烟曲霉菌、黑曲霉菌、根霉菌的生长有一定的抑制作用(Prince et al., 2019);熊果酸(化合物6)可通过减少浮游细菌在表面的附着力和破坏其生物膜的形成达到抑菌作用(Sycz et al., 2022);对羟基苯甲酸(化合物9)通过促进氧自由基的产生,抑制铜绿微囊藻的生长(张庭廷等,2008)。因此,我们推测全缘叶紫珠脂溶性物质在植物病害防控方面具有一定的应用前景,为进一步开发和利用该植物提供了一定的科学依据。
2020版《中国药典》收录的紫珠属物种包括广东紫珠、裸花紫珠、大叶紫珠、杜虹花,它们均为常见的乔木型或灌木型类群,这些类群往往喜欢生长在路旁、林缘等易受外界干扰的向阳环境,而全缘叶紫珠作为紫珠属仅有的几个藤本型类群之一,往往生活在林下环境,通常靠攀缘其他植物得以获得阳光和生长空间。这些特定的林下环境胁迫是否影响全缘叶紫珠次生代谢活动?本研究表明全缘叶紫珠中的15个化合物中就有6个化合物为紫珠属首次报道,这些意外的发现将激励着我们进一步加深对全缘叶紫珠天然产物成分的挖掘和药理活性研究。
参考文献:
ABDELHAMEED RFA, NAFIE MS, IBRAHIM AK, et al., 2020. Cytotoxic, apoptosis-inducing activities, and molecular docking of a new sterol from bamboo shoot skin Phyllostachys heterocycla var. pubescens [J]. Molecules (Basel, Switzerland), 25(23): 5650.
ANGAJALA G, SUBASHINI B, 2018. Evaluation of larvicidal potential of β-sitosterol isolated from indigenous Aegle marmelos Correa crude leaf extracts against blood feeding parasites and its binding affinity studies towards sterol carrier protein [J]. Biocatal Agric Biotechnol, 16: 586-593.
CHAI L, LIN ZZ, ZHU CC, et al., 2010. Analysis of the chemical constituents of essential oil from the leaves of Callicarpa integerrima [J]. J Chin Med Mat, 33(3): 382-385. [柴玲, 林朝展, 祝晨蔯, 等, 2010. 全緣叶紫珠叶挥发油化学成分分析 [J]. 中药材, 33(3): 382-385.]
CHA XJ, SHI Q, SHAO F, et al., 2021. Chemical constituents of Euphorbia helioscopia [J]. Chin Trad Herb Drugs, 52(2): 341-348. [查显进, 石强, 邵峰, 等, 2021. 泽漆化学成分研究 [J]. 中草药, 52(2): 341-348.]
CHENNIAPPAN K, KADARKARI M, 2012. Adult mortality and blood feeding behavioral effects of α-amyrin acetate, a novel bioactive compound on in vivo exposed females of Anopheles stephensi Liston (Diptera: Culicidae) [J]. Parasitol Res, 110(6): 2117-2124.
CHEN XL, LU ZY, GONGPAN PC, et al., 2020. Study on the chemical constituents from Trevesia palmate and their cytotoxicity [J]. Nat Prod Res Dev, 32(11): 1882-1888. [陈雪林, 卢志远, 贡潘偏抽, 等, 2020. 刺通草的化学成分及其肿瘤细胞毒活性研究 [J]. 天然产物研究与开发, 32(11): 1882-1888.]
DI QQ, ZHAO XB, ZHANG RH, et al., 2021. Novel clerodane-type diterpenoid Cintelactone A suppresses lipopolysaccharide-induced inflammation by promoting ubiquitination, proteasomal degradation of TRAF6 [J]. Pharmacol Res, 164: 105386.
Editorial Committee of Flora of China, 1982. Flora Reipublicae Popularis Sinicae [M]. Beijing: Science Press, 65(1): 25-79. [《中国植物志》编辑委员会, 1982. 中国植物志 [M]. 北京: 科学出版社, 65(1): 25-79.]
EDYTA KS, MONIKA D, URSZULA G, et al., 2017. Stenotrophomonas maltophilia: a gram-negative bacterium useful for transformations of flavanone and chalcone [J]. Multidiscip Dig Publ Inst, 22(11): 1830.
JIA ZJ, GAO JJ, 1993. Phenylpropanoid glycosides from Pedicularis striata pall ssp. arachnoidea [J]. Phytochemistry, 34(4): 1188-1190.
LAN YH, CHI XF, ZHOU GY, et al., 2018. Antioxidants from Pedicularis longiflora var. tubiformis (Klotzsch) P.C. Tsoong [J]. Rec Nat Prod, 12(4): 332-339.
LEE KY, JEONG EJ, LEE HS, et al., 2006. Acteoside of Callicarpa dichotoma attenuates scopolamine-induced memory impairments [J]. Biol Pharm Bull, 29(1): 71-74.
LI XJ, YUAN Y, LI Z, et al., 2014. Chemical constituents from cane of Pileostegia viburnoides [J]. Chin Trad Herb Drugs, 45(8): 1052-1055. [李小军, 袁燕, 李芝, 等, 2014. 苗药冠盖藤的化学成分研究 [J]. 中草药, 45(8): 1052-1055.]
LI YL, SU MX, CEN YZ, et al., 2006. Study on the chemical constituents of Ardisia chinensis [J]. J Chin Med Mat, 29(4): 331-333. [李药兰, 苏妙贤, 岑颖洲, 等, 2006. 小紫金牛的化学成分研究 [J]. 中药材, 29(4): 331-333.]
LI YR, LI C, WANG ZM, et al., 2014. Chemical constituents from whole plants of Aconitum tanguticum (Ⅲ) [J]. Chin J Chin Mat Med, 39(7): 1163-1167. [李艳茸, 李春, 王智民, 等, 2014. 藏药甘青乌头化学成分研究(Ⅲ) [J]. 中国中药杂志, 39(7): 1163-1167.]
MO QH, ZHOU XL, ZHOU Y, et al., 2020. Chemical constituents from the leaves of Rhodomyrtus tomentosa [J]. J Chin Med Mat, 43(3): 587-590. [莫青胡, 周先麗, 周云, 等, 2020. 桃金娘叶的化学成分研究 [J]. 中药材, 43(3): 587-590.]
MO X, LI Y, 2019. Analysis on the clincal prescription of Luohuazizhu tablet [J]. Chin Med J Res Prac, 33(3): 65-69. [莫霞, 李瑶, 2019. 裸花紫珠片临床应用处方分析 [J]. 现代中药研究与实践, 33(3): 65-69.]
PRINCE JN, TERRUMUN AT, EMMANUEL M, 2019. Stigmasterol and β-sitosterol from the root of Mangifera indica and their biological activities against some pathogens [J]. J Compl Altern Med Res, 7(4): 1-10.
SAIMARU H, ORIHARA Y, 2010. Biosynthesis of acteoside in cultured cells of Olea europaea [J]. J Nat Med, 64(2): 139-145.
SYCZ Z, TICHACZEK GD, WOJNICZ D, 2022. Anti-planktonic and anti-biofilm properties of pentacyclic triterpenes — asiatic acid and ursolic acid as promising antibacterial future pharmaceuticals [J]. Biomolecules, 12(1): 98.
TIAN XY, LI MX, LIN T, et al., 2021. A review on the structure and pharmacological activity of phenylethanoid glycosides [J]. Eur J Med Chem, 209: 112563.
WANG RD, SUN LN, TAO ZY, et al., 2005. Chemical constituents of Lamiophlomis rotata [J]. Acad J Sec Mil Med Univ, 16(10): 1171-1173. [王瑞冬, 孙连娜, 陶朝阳, 等, 2005. 独一味化学成分的研究 [J]. 第二军医大学学报, 16(10): 1171-1173.]
WANG WZ, 2008. Study on synthesis of 4-pyridinol [D]. Nanjing: Nanjing University of Science and Technology. [王伟忠, 2008. 4-羟基吡啶的合成研究 [D]. 南京: 南京理工大学.]
WANG XF, WEI RF, LU WJ, et al., 1986. Study on chemical constituents of Callicarpa integerrima [J]. Chin Trad Herb Drugs, 17(3): 12. [王雪芬, 韦荣芳, 卢文杰, 等, 1986. 全缘叶紫珠化学成分的研究 [J]. 中草药, 17(3): 12.]
WU QP, XU Y, LUO YH, et al., 2019. Discussion on a novel model for quality evaluation of Kanggongyan tablets based on reference drug [J]. Chin J Pharm Anal, 39(10): 1762-1770. [鄔秋萍, 许妍, 罗跃华, 等, 2019. 基于对照制剂的抗宫炎片质量评价新模式的探讨 [J]. 药物分析杂志, 39(10): 1762-1770.]
XU HT, ZHANG CG, HE YQ, et al., 2019. Phenylethanoid glycosides from the Schnabelia nepetifolia (Benth.) P.D. Cantino promote the proliferation of osteoblasts [J]. Phytochemistry, 164(5): 111-121.
YAMASAKI T, MASUOKA C, NOHARA T, et al., 2007. A new phenylethanoid glycoside from the fruits of Callicarpa japonica Thunb. var. luxurians Rehd. [J]. J Nat Med, 61(3): 318-322.
YU J, YANG YF, HU X, et al., 2015. Spectrum-effect correlation for blood coagulation activity of Callicarpa kwangtungensis Chun [J]. Chin J Pharm, 46(5): 467-472. [余婧, 杨义芳, 胡晓, 等, 2015. 广东紫珠止血谱效相关模式的研究 [J]. 中国医药工业杂志, 46(5): 467-472.]
ZHAN LL, YE XW, ZHANG M, et al., 2020. Progress on chemical composition and pharmacological activity of Callicarpa [J]. Jiangxi J Trad Chin Med, 51(8): 66-73. [占丽丽, 叶先文, 张敏, 等, 2020. 紫珠属植物化学成分及药理活性研究进展 [J]. 江西中医药, 51(8): 66-73.]
ZHANG TT, HE M, WU AP, et al., 2008. Allelopathic inhibition of p-hydroxybenzoic acid on Microcystis aeruginosa Kueitz with no toxicological effects on Cyprinus carpio Linnaeus [J]. Acta Sci Circum, 28(9): 1887-1893. [张庭廷, 何梅, 吴安平, 等, 2008. 对羟基苯甲酸对铜绿微囊藻的化感效应以及对鲤鱼的毒性作用 [J]. 环境科学学报, 28(9): 1887-1893.]
ZHU CC, GAO L, ZHAO ZX, et al., 2012. Triterpenes from Callicarpa integerrima Champ [J]. Acta Pharm Sin, 47(1): 77-83. [祝晨蔯, 高丽, 赵钟祥, 等, 2012. 全缘叶紫珠三萜类成分研究(英文) [J]. 药学学报, 47(1): 77-83.]
(责任编辑 邓斯丽 周翠鸣)
收稿日期: 2022-05-03
基金项目: 国家自然科学基金(31760045,31970220); 广西自然科学基金(2018GXNSFAA281132,2018GXNSFAA281146); 广西甘蔗生物学重点实验室开放课题项目(GXKLSCB-202004)。
第一作者: 马文杰(1996-),硕士研究生,主要从事药用植物天然产物化学研究,(E-mail)mawenjie1018@163.com。
*通信作者: 苏志维,博士,研究员,硕士研究生导师,主要从事天然产物化学等研究,(E-mail)suzw@gxtcmu.edu.cn。
