The integration of dinitropyrazole and 1,3,4-oxadiazole: A novel hybrid heterocyclic skeleton for balancing energy and stability
2023-07-04XiaoxiaoZhengTingouYanLeiQianHongweiYangGuangbinCheng
Xiao-xiao Zheng, Ting-ou Yan, Lei Qian, Hong-wei Yang, Guang-bin Cheng
School of Chemistry and Chemical Engineering, Nanjing University of Science and Technology, Nanjing, 210094, China
Keywords:Heterocycle Azo-bridged Hybrid skeletons Energetic materials
ABSTRACT
1. Introduction
Energetic Materials (EMs) are defined as the metastable chemicals or their formulations that can perform chemical reactions independently followed by emitting high amounts of chemical energy [1,2]. In recent years, nitrogen-rich heterocycles have been extensively studied in the field of EMs [3—5]. At present, the skeletons of nitrogen-rich heterocycles are mainly divided into fivemembered heterocycles (azoles and oxadiazoles) and sixmembered heterocycles (azines) [6,7]. As an important class of five-membered heterocycles,oxadiazoles have attracted quite wide interest in recent decades due to their oxygen- and nitrogen-rich skeletons [8]. To our knowledge, the only difference in the position occupied by the nitrogen and oxygen in the skeleton of oxadiazole leads to four isomers,i.e.,1,2,3-oxadiazole(unstable),1,2,5-oxadiazole (furazan),1,2,4-oxadiazole and 1,3,4-oxadiazole [9,10].
1,3,4-Oxadiazole derivatives are popular in the fields of agriculture, medicine and optical materials because of their unique biological and optical activities [11]. However, compared with furazan(ΔHf=185 kJ/mol),1,3,4-oxadiazole(ΔHf=51 kJ/mol)was received less attention in the field of EMs due to its low enthalpy[12,13]. To expand the application of 1,3,4-oxadiazole in EMs, the low enthalpy of formation of this skeleton is the top concern. An effective strategy was presented to increase the enthalpy of skeleton by matching multiple nitrogen-rich heterocycles into one molecule [14]. However, the assembly of multiple nitrogen-rich heterocycles may be limited by sophisticated synthesis procedures [15]. Therefore, the development of biheterocycles by hybridizing two heterocyclic skeletons into one molecule is relatively simple and feasible [16—18] (Fig. 1). Hybrid skeletons have prevailed in EMs for a long time because of their significant advantages:(a)strong π-conjugated effect;(b)many modifiable sites for explosophoric groups; (c) good thermal stabilities [19,20]. For example,5,5′-bis(3,5-dinitro-1H-pyrazol-4-yl)-1H,1′H-3,3′-bi(1,2,4-triazole) (BDBT-2) reveals remarkable thermal stability(Td=372◦C)because of the powerful π-conjugation[21].Another example is 3-(3,4-dinitro-1-(trinitromethyl)-1H-pyrazol-5-yl)-4-nitrofurazan, the compound shows high density (1.94 g/cm,298 K) and excellent detonation performance (Dv= 9200 m/s,P= 38.0 GPa) due to the combination of six nitro groups [22]. In general, the installation of —NO2in the pyrazole is an effective measure to intensify the detonation performance of energetic materials [23].

Fig.1. The synthesis strategy of this work.
Considering the points discussed above, 1,3,4-oxadiazole and pyrazole with easily modified sites are sensibly selected as two compositions to construct biheterocyclic skeleton [24—26]. In this study,5-(3,4-dinitro-1H-pyrazol-5-yl)-1,3,4-oxadiazol-2-amine(2)was synthesized. The nitroamino moieties as explosophoric group were brought into the ring of 1,3,4-oxadiazole to glorify the performance of the compound. Three energetic salts were further developed. Among them, dihydroxylammoinium (6) has good comprehensive properties, which had good detonation velocity of 9023 m/s and impact sensitivity of 20 J.In conclusion,the assembly of nitrogen-rich heterocycles and the design of reasonable hybrid skeletons have potential application prospects in the synthesis of polyheterocyclic compounds with good comprehensive properties.
2. Experimental part
2.1. Security instructions
All developed products are high-energy substances. Hence,protective measures need to be implemented.The entire procedure ought to be carried out in a fume hood.Dedicated helmets,goggles and lab gloves should always be worn. Attentively, the small dose experiment is necessary. The mechanical stimulation, flame and energization should be avoided during the operation and storage.
2.2. Synthesis
2.2.1. Synthesis of2
The solution of potassium bicarbonate (2.00 g,19.98 mmol) in 15 mL water was added to the suspension of 4,5-dinitro-1H-pyrazole-3-carbohydrazide(3.39 g,15.69 mmol)in 45 mL ethanol and then cyanogen bromide (1.67 g, 15.77 mmol) was added in small portions.The reaction mixture was stirred at room temperature for 24 h. The precipitate was filtered off and then the filtrate was concentrated to about 10 mL under vacuum. Compound2was collected by the second filtration from the concentrated filtrate,washed with cold water,dried in air as a yellow solid2(1.52 g,40%).1H NMR chemical shifts (500 MHz, DMSO‑d6, ppm): δ = 7.77,4.48.13C NMR chemical shifts (125 MHz, DMSO‑d6, ppm):δ=164.78,148.49,148.12,128.65,124.93.IR(KBr,cm-1):ṽ=3417,3359,3147,1687,1664,1518,1500,1469,1364,1331,1045,945,849,817, 738, 572. Elemental analysis (C5H3N7O5, 241.12, %): calculated C, 24.91; H,1.25; N, 40.66. Found C, 24.84; H 1.11; N,40.46.
2.2.2. Synthesis of3
0.42 g KMnO4in 5 mL water was scooped into to 5 mL 37%hydrochloric acid of2(0.50 g,2.07 mmol).The mixture was stirring for 4 h at 50◦C. The terminal solution was treated with 30% H2O2when the reaction was over. And the precipitate was filtered off,washed with water and dried in air to afford3(0.62 g,63%).1H NMR chemical shifts (500 MHz, DMSO‑d6, ppm): δ = 5.32.13C NMR chemical shifts (125 MHz, DMSO‑d6, ppm): δ = 168.22, 157.38,156.93,148.48,127.41. IR (KBr, cm-1):ṽ= 3563, 3432,1560,1540,1504,1424,1358,1331,1237,1204,1055,1016, 976, 953, 848, 813,756, 735, 608, 587. Elemental analysis (C10H2N14O10, 478.20, %):calculated C, 25.12; H, 0.42; N, 41.01. Found C, 25.22; H, 0.31; N,41.46.
2.2.3. Synthesis of5
At a low temperature(<0◦C),2(0.24 g,1.00 mmol)was slowly scooped into 5 mL nitric acid (98%). Then it was warmed to room temperature. TLC (thin-layer chromatography) was used to check the completion of the reaction.Subsequently,the reaction solution was quenched with crushed ices and stirred for several hours at 25◦C.Ethyl acetate was used as the extractant.The upper extracts were collected and further dried with MgSO4. Hereafter, the ethyl acetate was removed by air to obtain4as yellow oil. The nitration product4was dissolved in 5 mL acetonitrile.And a large amount of NH3(g) was passed through under stirring for 2 h at normal temperature. The precipitate5was afforded by suction filtration and placed in the air to dry (0.27 g, 84%).1H NMR chemical shifts(500 MHz, DMSO‑d6, ppm): 7.14 (s, br.).13C NMR chemical shifts(125 MHz,DMSO‑d6,ppm):165.56,153.77,150.11,132.70,126.25.IR(KBr,cm-1):ṽ=3196,3012,1527,1509,1492,1425,1384,1373,1310,1212, 1149, 1114, 1078, 1002, 959, 852, 815, 778, 752, 742, 718.Elemental analysis(C5H8N10O7,320.18,%):calcd C,18.76;H,2.52;N,43.75. Found C,18.52; H, 2.36; N, 43.66.
2.2.4. Synthesis of6
The synthesis steps were similar to compound5 (40 μL). 50%NH2NH2∙H2O was applied in this synthesis process. The dihydrazinium6: yellow solid, 0.23 g, 66%.1H NMR chemical shifts(500 MHz, DMSO‑d6, ppm): 6.03 (s, br.).13C NMR chemical shifts(125 MHz,DMSO‑d6,ppm):165.58,154.01,150.16,132.86,126.16.IR(KBr, cm-1):ṽ= 3360, 3286, 2646, 1613, 1489, 1471, 1350, 1307,1282,1238,1173,1080, 966,850,816,754,538. Elemental analysis(C5H10N12O7,350.21,%):calculated C,17.15;H,2.88;N,47.99.Found C,17.11; H, 2.86; N,48.16.
2.2.5. Synthesis of7
The synthesis steps were also similar to compound5.(50 μL).50% NH2OH solution was applied in this synthesis process. The dihydroxylammonium7: yellow solid, 0.25 g, 71%.1H NMR chemical shifts (500 MHz, DMSO‑d6, ppm): δ = 8.51 (s, br.).13C NMR chemical shifts (125 MHz, DMSO‑d6, ppm): δ = 165.65, 153.91,150.16,132.82,126.22. IR (KBr, cm-1):ṽ= 3168, 3027,1657,1490,1418,1362,1313,1112,1078,1000,958,920,849,813,741,668,624,612, 545. Elemental analysis (C5H8N10O9, 352.18, %): calculated C,17.15; H, 2.29; N, 39.77. Found C,17.18; H, 2.14; N, 40.06.
3. Results and discussion
3.1. Synthesis
The synthesis processes of all the energetic compounds in this work were present in Scheme 1. In this study, 4,5-dinitro-1H-pyrazole-3-carbohydrazide (1) was synthesized according to the previously reported method [27] The heterocyclization reaction of1with cyanogen bromide in the presence of potassium bicarbonate afforded precursor2.It is worth noting that the previously reported 1,3,4-oxadiazole derivatives obtained from the above heterocyclization reaction were usually collected from the filter cakes.However,in this work,because of the good solubility of the product in this reaction system, precursor2was obtained from the concentrated filtrate instead of the filter cake in high purity.Thereafter, the azo-bridged compound3was prepared smoothly based on the oxidative coupling of C—NH2groups of2. The nitrification reaction of precursor2was performed with the 98%fuming HNO3to improve the detonation performances. Disappointingly,we cannot successfully obtain solid-state product4due to its high hygroscopicity. Thereafter, three commonly used Brønsted bases(ammonia, 50% hydrazine hydrate and 50% hydroxylamine) were selected to synthesize corresponding energetic salts5—7in the yield of 66—84%.

Scheme 1. The synthesis process of newly developed compounds 2—7.
3.2. Single crystal X-ray diffraction
Single crystal cultivations of compounds2,3, and5were conducted to determine their structures and obtain more configuration information. All three crystals were obtained by slow evaporation of their saturated solution, both3∙2H2O and5crystallized from aqueous solution and2∙CH3CN from acetonitrile at room temperature.
Three crystals (2∙CH3CN,3∙2H2O and5) crystallize in the monoclinic space group P21/n, monoclinic space group P21/c and triclinic space group P-1(2),respectively.The calculated density of2∙CH3CN is 1.719 g/cm3at 170 K,which is lower than that of3∙2H2O(1.808 g/cm3at 170 K) and5(1.817 g/cm3at 167 K). The lowest density of2∙CH3CN among three crystals is blamed on its packing mode and the presence of acetonitrile in the structures. The layer structures of2∙CH3CN and3∙2H2O are both stacked in the “W/M”mode,but the stacking mode of 3∙2H2O is more compact.The layer structures of5are stacked in the layer-by-layer mode. Generally,the face-to-face and the tight packing mode can increase the crystal density [28] (Fig. 2(g)—Fig. 2(i)). The3∙2H2O has a near-planar geometry, which can be further confirmed by the torsion angles of C4—O5—C5—N6 179.2 (3)o, N2—N3—C3—C4 178.9 (3)oand C3—C1—C2—N1 179.0 (3)o. Only the nitro groups of3∙2H2O are distorted out of the molecular plane because of the spatial resistance (Fig. 2(e)). The dihedral angle between pyrazole and oxadiazole of crystal2∙CH3CN is 15.15◦,which is smaller than that of5(20.55◦). In Fig. 2(g)—Fig. 2(l), the vertical distances between the two layers of2∙CH3CN,3∙2H2O and5are 3.78, 3.51 and 3.34 Å,respectively, which are all within the range of the distance of π-π interaction (<4.0 Å) [29]. Additionally, there are a great many intramolecular hydrogen bonds between anions and cations in the molecules of5, resulting in the closest interlayer spacing. In Fig.2(i),each ammonium cation and the adjoining three dianionic salts form seven H-bonds N9—H9A∙∙∙N5, N9—H9B…O2,N9—H9B…O3, N9—H9C…O7, N9—H9C…N2, N9—H9D∙∙∙O4 and N9—H9D∙∙∙N6(see ESI:Table S2).All the H-bonds range in length from 2.795 (3) to 3.296 (2) Å in5. In particular, the quite strong intramolecular H-bonds in5were N9—H9C…O7 (D∙∙∙A: 2.795 (3)Å; D-H∙∙∙A: 102.3 (14)o) and N9—H9A∙∙∙N5 (D∙∙∙A: 2.915 (2) Å;D-H∙∙∙A: 172 (2)o).
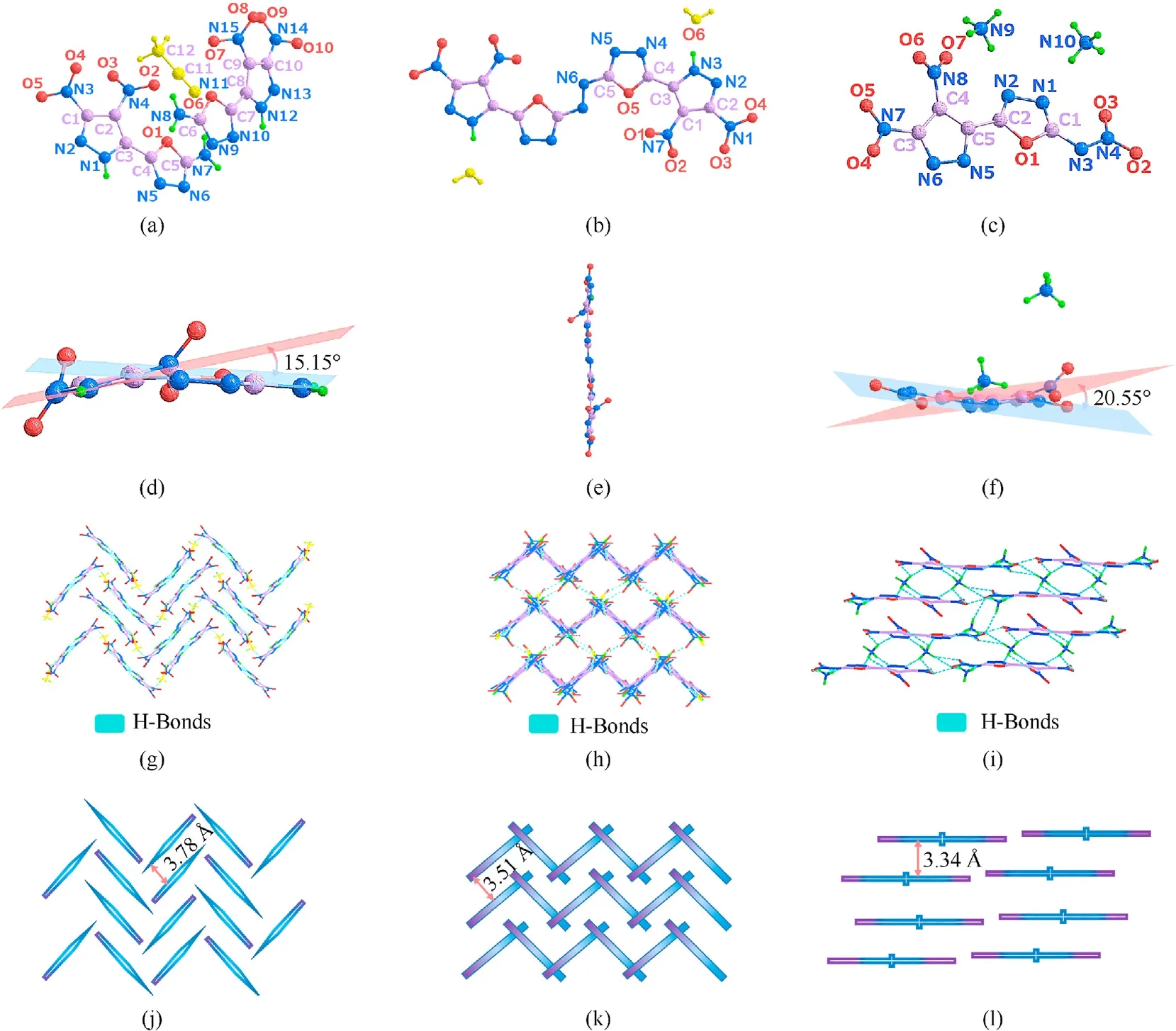
Fig.2. (a)—(c)The X-ray crystal structure of 2∙CH3CN,3∙2H2O and 5,respectively;(d)—(f)The structure planarity of 2∙CH3CN,3∙2H2O and 5,respectively(For clarity,the solvents were removed in 3∙2H2O); (g)—(i) The packing diagram and intermolecular hydrogen bonds (H-bonds) of 2∙CH3CN, 3∙2H2O and 5, respectively; (j)—(l) The simplified stacking diagram of 2∙CH3CN, 3∙2H2O, 5, respectively.
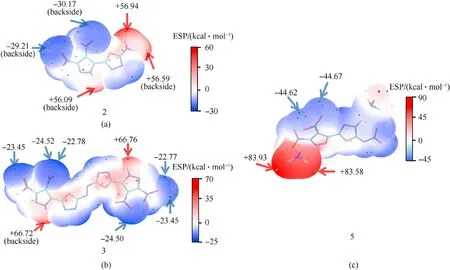
Fig.3. (a)—(c)ESP-mapped molecular van der Waals(vdW)surface of 2,3 and 5.(The positive and negative points are colored by orange and cyan,respectively.Only larger absolute values of ESP were labeled.).
3.3. Physicochemical properties and related quantum calculations
To evaluate the application potential of the newly synthesized compounds, a series of theoretical calculations and practical tests on the performances of these compounds were conducted. All compounds were sufficiently dried at room temperature to remove solvents before tests.The results were summarized in Table 1.
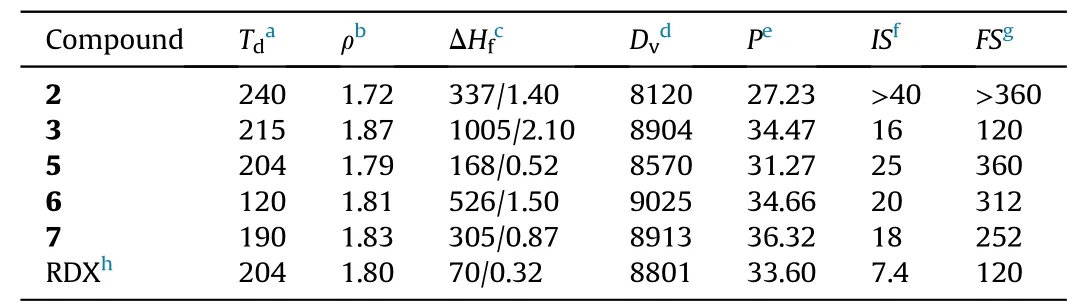
Table 1Physicochemical properties of 2, 3 and 5—7.
The two indicators of mechanical and thermal stabilities are commonly discussed in the field of energetic material [30]. In this study,the differential scanning calorimetry under a heating rate of 5◦C/min was used to measure the thermal stability. The decomposition onset temperature of compounds2—7is in the range of 120—240◦C.Among these compounds,the precursor2has the best thermal stability and the highest initial decomposition temperatureof 240◦C. Except for compound6, the initial decomposition temperature of the rest compounds is greater than 190◦C,which meets the need of the actual applications. Sensitivity is a safety factor to be considered for high-energy materials. The sensitivity of impact and friction were measured by using standard BAM procedures.In general,the sensitivities of the newly synthesized compounds(2,3and5—7) are lower than that of the traditional explosive RDX(IS > 7.4 J,FS= 120 N). Among them, compound2(IS> 40 J,FS> 360 N) has the lower sensitivity to external stimuli than compound3(IS= 16 J,FS= 120 N) because of its abundant intermolecular H-bonding.
To further explain the variations of stabilities among the developed compounds, the ESP (electrostatic potential) analysis base on the crystal data of2,3and5were applied to investigate the electrostatic interaction. For all compounds, the maximum electrostatic potential was distributed near the hydrogen atoms,because the electronegativity of hydrogen is less than that of carbon and oxygen. In Fig. 3, the extreme value distribution of electrostatic potential of2(-30.17 to 56.94)is narrower than that of3(-24.52 to 66.76) and5(-44.62 to 85.93), which means that the electrostatic potential distribution of2is more uniform than3and5. Previous works have reported that a compound with more uniform electrostatic potential distribution might have lower sensitivities [31,32]. Thus, compound2has the least sensitivities to external stimuli. Furthermore, compound3has the larger red region (electropositive areas) than that of2, which reveals that the introduction of N-azo bridges might increase the sensitivities.
Density is also one of indicators considered for energetic materials. The density analyzer we utilized is based on the gas expansion method to obtain the true density(ρ)[34].The density of all compounds is between 1.72 g/cm3and 1.87 g/cm3. The azobridged compound3has the highest density of 1.87 g/cm3among all the newly developed compounds.
To explore the differences in crystal densities and interlayer spacing among the three crystals, the Hirshfeld surface and 2D fingerprint of crystals2,3and5were employed to reveal weak interactions in the molecular.Three main features can be obtained from Fig.4.Firstly,the H-bonding information of the three crystals can be seen in Fig.4(a)—4(c),the obvious dark red spots represent the sites where H-bonds are formed. Secondly, in Fig. 4(d)—4(f), a pair of remarkable spikes can be found in the bottom left corner of the maps. Generally, the ability of N atoms as hydrogen bond donors is slightly stronger than that of O atoms,so the peaks formed by N atoms as hydrogen bond donors are sharper. Lastly, the specific ratio data can be obtained in Fig.4(g)—4(i),the strength of Hbonding usually reflected by the percentages of O…H and N…H interactions.And the strength of π-π interactions is guided by C…N and C…O interactions. These main features of2,3and5are consistent with the crystal packing discussed above. For example,the total H-bonding interactions of5are up to 62.5%, which is apparently higher than that of2(44.9%) and3(26.0%), indicating the minimum interlayer spacing and the highest crystal density of5. The proportion of O…N interaction of3accounts for 31.30%,which reflects strong interlayer contacts in the molecules.Thus,the suboptimal interlayer spacing and crystal density of3can be explained [35].
The NCI(noncovalent interaction)analysis is beneficial for us to thoroughly understand the intra- and intermolecular interactions.Generally,the blue flakes represent H-bond interactions,the green flakes represent vdW interactions (π-π interactions) and the red flakes represent steric effect. As can be seen in Fig. 5, the green flakes in compound3are larger than that in2, indicating the stronger π-π interactions of3[36,37],which is consistent with the above analysis in Fig.4.In addition,5shows stronger steric effects than2, which might lead to the increase in the dihedral angle between the pyrazole and oxadiazole rings.
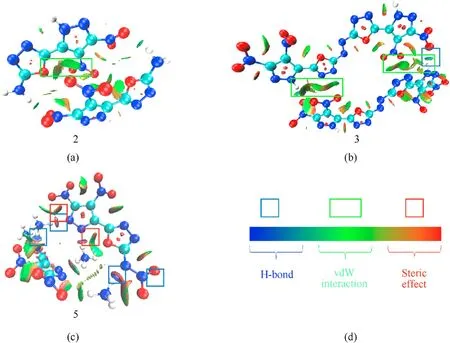
Fig. 5. (a)—(c) The NCI plots of gradient isosurfaces of 2, 3 and 5, respectively; d The legend of NCI plots. The blue flakes, H-bonds; The green flakes, vdW interactions (π-π interaction) and the red flakes, steric effect.
All compounds exhibit positive enthalpy of formation (ΔHf),ranging from 0.52 to 2.10 kJ/g.Compared with RDX,the enthalpy of formation of these compounds exceed more than 0.2 kJ/g at least.The high enthalpy of formation has a positive effect on the detonation performance.In the study,the compound3has the highest the enthalpy of formation (2.10 kJ/g), which is much greater than that of RDX (0.32 kJ/g).
With the main parameters (enthalpy of formation, density)required for the calculation of the detonation performances in hand, the detonation properties can be gained from EXPLO5 v6.01 program.It is worth mentioning that the detonation performances of azo-bridged compound3(Dv= 8904 m/s,P= 34.47 GPa) are higher than that of RDX(Dv=8801 m/s,P=33.60 GPa).Among the three energetic ionic compounds, the detonation performances of the compound6(Dv=9025 m/s,P=34.66 GPa) and compound7(Dv= 8913 m/s,P= 36.32) are both superior than that of RDX.
4. Conclusions
The neutral bi-heterocyclic compounds2, azo-bridged compound3and salts5—7were synthesized and fully characterized.In this study,All the compounds show good detonation performances(Dv:ranging from 8120 m/s to 9025 m/s,P:ranging from 27.23 GPa to 36.32 GPa), moderate onset decomposition temperature(ranging from 120 to 240◦C) and low sensitivities to both friction and impact stimuli. Additionally, the azo-bridged compound3showed good comprehensive performance, which is superior to RDX. The structure-property relationships among the compounds were further demonstrated by ESP, Hirshfeld surfaces, fingerprint plots and NCI based on the single-crystal data of2,3and5.To sum up,the contradiction between energy and safety can be effectively balanced by hybridizing two heterocyclic skeletons. The assembly of nitrogen-rich heterocycles and the design of reasonable hybrid skeletons provide a significant strategy for synthesis of the promising polyheterocyclic energetic materials.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This work was completed with support from the National Natural Science Foundation of China[No.22075143,21875110],as well as the Science Challenge Project [TZ2018004]. H. Yang thanks the Qing Lan Project for the grant.
Appendix A. Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2022.03.003.
杂志排行
Defence Technology的其它文章
- Defence Technology
- Joint target assignment and power allocation in the netted C-MIMO radar when tracking multi-targets in the presence of self-defense blanket jamming
- Design and dynamic analysis of a scissors hoop-rib truss deployable antenna mechanism
- Structural design and modal behaviors analysis of a new swept baffled inflatable wing
- Cooperative trajectory optimization of UAVs in approaching stage using feedback guidance methods
- An improved four-dimensional variation source term inversion model with observation error regularization
