Recent advances in pathophysiology, diagnosis and management of hepatorenal syndrome: A review
2023-07-04CalvinKianiAndreasZori
Calvin Kiani,Andreas G Zori
Abstract
Key Words: Hepatorenal syndrome; Pathophysiology; Diagnosis; Management; Review
INTRODUCTION
Acute kidney injury (AKI) is a common complication in patients with cirrhosis and has been reported in 20%-50% of the hospitalized patients with cirrhosis[1,2].Within the spectrum of AKI in cirrhosis,hepatorenal syndrome (HRS) with AKI (HRS-AKI) has by far the worst prognosis[3].HRS-AKI is a rapidly progressive type of AKI with a median survival of few weeks[3,4].Although most commonly seen in the setting of cirrhosis, HRS-AKI can occur in patients with acute liver injury such as acute liver failure or severe alcoholic hepatitis[3,5].Observations of acute progressive kidney injury without significant preexisting kidney dysfunction, minimal histological abnormalities, and reversible angiographic changes in renal vasculature led to the hypothesis that HRS-AKI is a functional and potentially reversible phenomenon caused by hemodynamic instability, splanchnic vasodilation and renal vasoconstriction[6-10].However, more recent data have shown the pathophysiology is more complex[10-14].
In this review, we examine the current understanding of the mechanisms of kidney injury in patients with cirrhosis, discuss the contemporary classification of AKI in patients with cirrhosis, and review the recent advances in diagnosis and management of HRS-AKI.
PATHOPHYSIOLOGY
HRS-AKI is primarily due to an unbalanced but potentially reversible cirrhosis-induced circulatory dysfunction without structural kidney damage[11-14].This understanding of HRS pathophysiology is supported by clinical findings including the return to normal kidney function occurring commonly after liver transplantation, successful kidney transplant using donors with HRS, and normal postmortem histology and angiography[9,10,15,16].Direct experimental evaluation of the pathophysiology of HRS remains lacking due to lack of a fitting animal model.The primary mechanism of renal injury,splanchnic hypoperfusion, triggers a physiologic response including sodium retention and renal vasoconstriction.These mechanisms generally cannot compensate to maintain perfusion and as the disease progresses, eventually contribute to circulatory dysfunction and thus worsen renal function.The compensatory mechanisms, maladaptive responses, and their role in disease progression will be detailed in the following sections.
Cirrhosis-induced circulatory dysfunction
Cirrhosis causes elevated intrahepatic vascular resistance and a paradoxical splanchnic vasodilatation due to increased production of mediators such as nitric oxide and prostacyclins[17-20].Compensatory hyperdynamic circulation initially preserves the effective intravascular volume in early stages of cirrhosis.As the cirrhosis progresses or during times of acute stress, the hyperdynamic cardiac circulation cannot compensate for the splanchnic vasodilation resulting in activation of other compensatory mechanisms.The renin-angiotensin-aldosterone system (RAAS), the sympathetic nervous system (SNS), and at later stages, the non-osmotic secretion of arginine vasopressin are subsequently activated to maintain effective intravascular volume[21-24].These responses cause vasoconstriction as well as water and sodium retention in an attempt to counteract vasodilation and maintain adequate intravascular volume.However, with further disease progression and in the absence of effective treatment, these compensatory mechanisms not only will fail to adequately counterbalance the vasodilation but begin to contribute to renal dysfunction.Renal vasoconstriction begins to impair renal blood flow and therefore worsens renal function.Volume retention contributes to worsening portal hypertension, which in turn worsens the underlying physiology which initiates and perpetuates HRS.
An interesting and specific finding in HRS-AKI is the sequence and distribution of microvascular changes in the kidney itself.In the early stages of cirrhosis and in the presence of mild portal hypertension, as renal blood flow decreases, resistive indices (RIs) measured by Doppler ultrasound show a gradual increase starting from the main renal artery (hilum) toward the cortical arteries, sparing the outer cortex parenchyma[25-27].By contrast, in later stages and in the presence of severe portal hypertension, the RI gap between hilum and cortex disappears and cortical ischemia occurs[26,27].Therefore, cortical ischemia is considered to be a hallmark feature of HRS-AKI.Another potential contributing factor to HRS-AKI in cirrhosis is abnormal renal vascular response to stimuli and altered autoregulation of kidney blood flow.Despite decreased renal blood flow, the vasoconstrictor effect of angiotensin II on the efferent arterioles and vasodilator effect of nitric oxide on the afferent arterioles
preserve adequate pressure in the glomeruli to keep glomerular filtration rate (GFR) within normal limits[26].However, as cirrhosis advances, GFR starts to decline presumably due to the disruption of nitric oxide production and progressive cortical ischemia caused by the very same compensatory mechanisms explained above.Animal models have also shown a blunted vasodilatory response to bradykinin and an augmented vasoconstrictive response to noradrenaline[28,29].If the decreased renal blood flow is not reversed quickly, then the persistent vasoconstriction and ischemia could lead to acute tubular necrosis (ATN), which may not improve even after the adequate renal blood flow has been restored.The potential for permeant dysfunction as a result of an acute insult from HRS-AKI illustrates the importance of early diagnosis and treatment.
Cirrhotic cardiomyopathy
Cirrhosis-induced cardiac dysfunction or cirrhotic cardiomyopathy is cardiac dysfunction and abnormal response to stimuli in patients with advanced cirrhosis in the absence of structural cardiac disease.This phenomenon is observed in up to 50% of cirrhotic patients with varying degree of severity[30-32].As mentioned earlier, the compensatory response to the splanchnic vasodilation includes both increase in cardiac output and activation of RAAS and SNS.However, chronic activation of RAAS and SNS may result in impaired cardiac response to stress, diastolic dysfunction, electrophysiological abnormalities (e.g., prolonged QT interval), and eventually decreased cardiac output[30-33].Although cardiac compensatory mechanisms may be able to maintain adequate profusion under normal circumstances,they can collapse under physiologic and pathologic stressors such as infections (particularly spontaneous bacterial peritonitis), bleeding, or inappropriate use of medications such as β-blockers,diuretics and angiotensin-converting enzyme inhibitors[31,33,34].Because increased cardiac output is an essential compensatory mechanism to maintain renal profusion in the setting of vasodilation,reduced cardiac output can have significant negative effects on renal profusion and has been associated with development of HRS and poor outcomes in patients with HRS[33].
Inflammation
In recent years, the notion that decompensated cirrhosis is a constant inflammatory state has emerged and a growing body of evidence shows pro-inflammatory cytokines and chemokines such as interleukin 6 (IL-6), IL-8 and tumor necrosis factor alpha (TNF-α) may play a central role in the organ dysfunction in patients with cirrhosis[35-38].The levels of pro-inflammatory cytokines increase with disease progression as a response to sterile (non-infectious) inflammation or infectious inflammation[37,39].Sterile inflammation typically manifests with systemic inflammatory response syndrome and is mainly driven by danger-associated molecular patterns such as high-mobility group protein B1.In patients with cirrhosis infectious, inflammation is mainly driven by pathogen-associated molecular patterns from gutderived bacterial translocation but can also be associated with other sources of infection[38-40].The inflammatory response may have prognostic value in predicting progression of AKI and mortality[40,41].Furthermore, levels of certain inflammatory mediators in the serum (IL-6, TNF-α, vascular adhesion protein-1) and urine (monocyte chemoattractant protein-1, neutrophil gelatinase–associated lipocalin)may help to differentiate AKI-HRS from other causes of AKI such as prerenal azotemia.In addition to changes in the systemic inflammatory environment, patients with decompensated cirrhosis have increased expression of inflammatory receptors such as toll-like receptor 4 (TLR-4) in the kidneys[38,42].These subtle structural changes can lead to exaggerated tubulointerstitial, glomerular, and vascular injuries in response to relatively minor hemodynamic changes or occult infections[43,44].
Other factors
In addition to circulatory dysfunction and inflammation, there is evidence that other factors contribute to the development of HRS.Bile cast nephropathy or cholemic nephropathy in decompensated cirrhosis may contribute to renal dysfunction as patients with higher bilirubin have lower response to therapy in HRS[45,46].However, the causative relationship between bile cast nephropathy and renal dysfunction in HRS has not been clearly established.Relative adrenal insufficiency in cirrhosis, formerly called hepatoadrenal syndrome, is a relatively common phenomenon occurring in 24%-47% of patients with decompensated cirrhosis.The lack of normal adrenal function impairs the compensatory response to hypoperfusion and increases the risk of AKI-HRS[47].Although glucocorticoid supplementation may improve outcomes in patients with relative adrenal insufficiency and septic shock, the effect of supplementation on AKI-HRS outcomes has not been evaluated rigorously.Figure 1 summarizes the pathophysiology of HRS-AKI
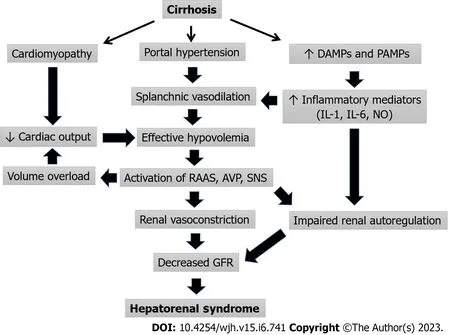
Figure 1 Pathophysiology of hepatorenal syndrome.AVP: Arginine vasopressin; DAMPs: Danger-associated molecular patterns; GFR: Glomerular filtration rate; PAMPs: Pathogen-associated molecular patterns; RAAS: Renin angiotensin aldosterone system; SNS: Sympathetic nervous system.
DIAGNOSIS
estimated GFR (eGFR) < 60 mL/min per 1.73 m2for less than 3 months and HRS-chronic kidney disease(HRS-CKD) defined as eGFR < 60 mL/min per 1.73 m2for more than 3 months.Currently, the American Association for the Study of Liver Diseases (AASLD) defines AKI-HRS according to the International Club of Ascites (ICA) criteria as an increase in serum creatinine (SCr) ≥ 0.3 mg/dL within 48 h or increase ≥ 1.5 times from baseline SCr that is known or presumed to have occurred within the preceding 7 d.The other diagnostic criteria for HRS-AKI have remained largely unchanged from HRS type 1 including cirrhosis with ascites, no response after 2 consecutive days of diuretic withdrawal and plasma volume expansion with albumin infusion (1 g/kg body weight per day), absence of shock, no current or recent use of nephrotoxic drugs (non-steroidal anti-inflammatory drugs, aminoglycosides, or iodinated contrast media), and no signs of structural kidney injury.Evidence for structural kidney disease includes proteinuria (> 500 mg per day), microhematuria (> 50 red blood cells per high-power field),and/or abnormal renal ultrasonography.The updated criteria have adopted lower thresholds of creatinine increase and no absolute minimum creatinine necessary for diagnosis primarily to facilitate earlier identification of patients at risk for poor outcomes.Despite improvement in the HRS-AKI criteria, they do not differ for patients with underlying CKD, which can make diagnosis more challenging for these patients.
Novel diagnostic biomarkers
Despite improved understanding of the pathogenesis and diagnostic criteria of HRS-AKI, it remains a diagnosis of exclusion and requires a period of observation after diuretic/nephrotoxic medication withdrawal.Establishing the diagnosis can be difficult due to the similar presentations of other causes of AKI such as prerenal AKI and ATN.In addition, AKI-HRS can result in ATN as the disease progresses, which further complicates distinguishing the two entities[51].Although the ICA criteria and its proposed treatment algorithm try to address this issue, accurate differentiation may not be feasible in a timely fashion especially when patients’condition is rapidly changing.Thus, there is an unmet need for biomarkers to quickly and accurately differentiate HRS-AKI from other causes of AKI and stratify risk.Accurately predicting renal function in patients with cirrhosis is also essential as this would allow for earlier identification of patients with renal dysfunction.However, accurately estimating GFR is challenging as standard SCr and Cr-based equations are unreliable in patients with cirrhosis because sarcopenia, impaired production of Cr (the precursor of SCr), and increased Cr filtration are common and result in the underestimation of renal dysfunction[52,53].A new model, the royal free hospital cirrhosis GFR, which includes sodium, presence of ascites, blood urea nitrogen, and international normalized ratio in the equation, has been suggested to be more accurate for estimating renal function in this population.However external validation in large cohorts has not been completed[54].There are several novel biomarkers under investigation to improve diagnosis and prognostication in AKI-HRS including plasma cystatin C, urinary neutrophil gelatinase-associated lipocalin (uNGAL), interleukin-18(IL-8), kidney injury molecule-1, and liver-type fatty acid-binding protein and albumin[55-60].Among these biomarkers, uNGAL, IL-18 and cystatin C appear to be the most promising biomarkers.IL-18 and uNGAL can differentiate ATN from other types of AKI and predict mortality in cirrhosis[59,60].Specifically, uNGAL is identified in the most recent 2021 AASLD guidelines as the most promising biomarker in distinguishing HRS-AKI from ATN and suggests measuring it on day 3 from onset of renal dysfunction for greatest accuracy[49].Plasma cystatin C (the most commonly used marker besides SCr) predicts HRS and mortality in patients with cirrhosis[59,60].An additional advantage to cystatin C is that it has become more widely performed, can yield results relatively quickly, and can predict GFR more accurately in patients with sarcopenia[59].Using biomarkers including uNGAL, IL-18, liver fatty acid-binding protein (L-FABP), and albumin in a combination panel may improve their ability to differentiate between HRS and ATN as well as predict AKI progression and death[55].Specifically, the biomarker combination of cystatin C and uNGAL and predictive models MELD-cystatin C and MELDNGAL have shown the potential for improving diagnosis and risk stratification, making them attractive topics for future research[58,60].MicroRNAs (e.g., microRNA-122) and metabolomics signature associated with hepatorenal dysfunction (4-acetamidobutanoate, trans-aconitate, 1-methylhistidine,glucuronate, N4-acetylcytidine, 3-ureidopropionate, 3-methoxytyramine sulfate, cytidine, S-adenosylhomocysteine, and myo-inositol) have shown promising results predicting mortality and kidney dysfunction in small studies but need validation in large prospective cohorts[61,62].Tables 1 and 2 summarize commonly used methods of estimating GFR and the novel biomarkers and equations for diagnosis of AKI in cirrhosis.Although these novel markers are promising, they are not all readily available, often do not have standard cut-off values, have values that do not correlate with specific stages of AKI, and have cut-off values that vary by type of AKI.Therefore, standardization and validation are needed in prospective studies.In addition, given the importance of early diagnosis and intervention, biomarkers need to have a rapid turn-around to be clinically useful, which is often not the case in many, especially smaller, institutions.However, they have the potential to allow for earlier more specific diagnosis, which facilitates more aggressive intervention, and when appropriate, evaluation for liver transplant.
TREATMENT
Despite the growth of knowledge in pathogenesis and shifts in definition and prognostication, HRS-AKI is still associated with high morbidity and mortality.The mainstay of therapy includes volume expansion and vasoconstrictors.However, there have been changes in the availability and data supporting the use of terlipressin recently.If medical management fails, renal replacement therapy and eventually organ transplant should be considered.
Medical management
In patients with cirrhosis and AKI, when precipitating factors are excluded first patients are generally treated with diuretic withdrawal and a 48-hour volume expansion with albumin (1 g/Kg, 100 g maximum).Historically subsequent treatment has been variable depending on the availability of terlipressin.In places where terlipressin is not available, a combination of midodrine (α1-receptor agonist), octreotide (splanchnic vasoconstrictor), and albumin are typically used outside the intensive care unit (ICU) and low-dose noradrenaline with albumin are used in the ICU.Terlipressin (vasopressin agonist), which can be administered peripherally, has been used for many years outside the United States and has demonstrated a higher response rate than albumin alone or midodrine, octreotide, and albumin combination regimen and comparable to noradrenaline plus albumin[63-73].When coadministered with albumin, terlipressin has better outcomes than terlipressin alone, which may be due to some of the anti-inflammatory and immunomodulatory properties of albumin besides oncotic volume expansion[74,75].Terlipressin has been studied extensively in prospective studies, randomized trials, and meta-analyses and its efficacy in reversal of HRS-AKI (Tables 1 and 2) and as a result has been approved for the treatment of HRS outside the United States for several years[76-80].However,despite mounting evidence for the benefit of terlipressin, including initial data from the United Statesbased CONFRIM trial, the United States Food and Drug Administration (FDA) rejected terlipressin due to safety concerns as recently as 2020.This was controversial at the time not only because of terlipressin’s wide approval outside the United States but also because the FDA subcommittee on Cardiovascular and Renal Drugs Advisory Committee voted 8-7 in favor of approval.After post-hoc analyses of the CONFIRM trial with proposed changes to mitigate the risk of safety events, terlipressin was ultimately approved in the United States for the treatment of HRS-AKI in 2022, although with several warnings.The CONFIRM trial showed terlipressin was more effective than placebo in reversal of HRS (32%vs17%), although there was no statistical difference in death at 90 d.However, the trial also highlighted safety considerations when using terlipressin.Respiratory failure (14%vs5%) and death within 90 d due to respiratory disorders (11%vs2%) was more common in the terlipressin group compared to in the placebo group.As a result, terlipressin is contraindicated in patients with ongoing ischemia (coronary, peripheral, or mesenteric) and hypoxia or worsening respiratory symptoms.For all patients, continuous pulse oximetry is recommended to monitor the development of respiratory failure.The FDA also recommends paying close attention to volume status as this may predispose patients to respiratory failure and consider discontinuing terlipressin in patients who develop fluid overload.Liver transplantation was performed in 29% of the placebo group compared to 23% in the terlipressin group.Because of the possibility that terlipressin treatment resulted in clinical change, which precluded patients from transplant, the FDA added a warning label indicating that terlipressin-induced adverse events may make a patient ineligible for liver transplant and risks of using terlipressin may outweigh benefits in patients with MELD ≥ 35.An additional warning was issued for patients with severe acute on chronic liver failure (ACLF grade 3), because the likelihood of adverse events was higher and the response to treatment diminished[72,81].These findings suggest that renal replacement therapy and liver transplant evaluation should be considered early in patients with high baseline SCr and ACLF grade.Tables 3 and 4 summarize clinical trials and meta-analyses on terlipressin effects on HRS-AKI,although it should be noted that no meta-analyses include the CONFIRM trial.
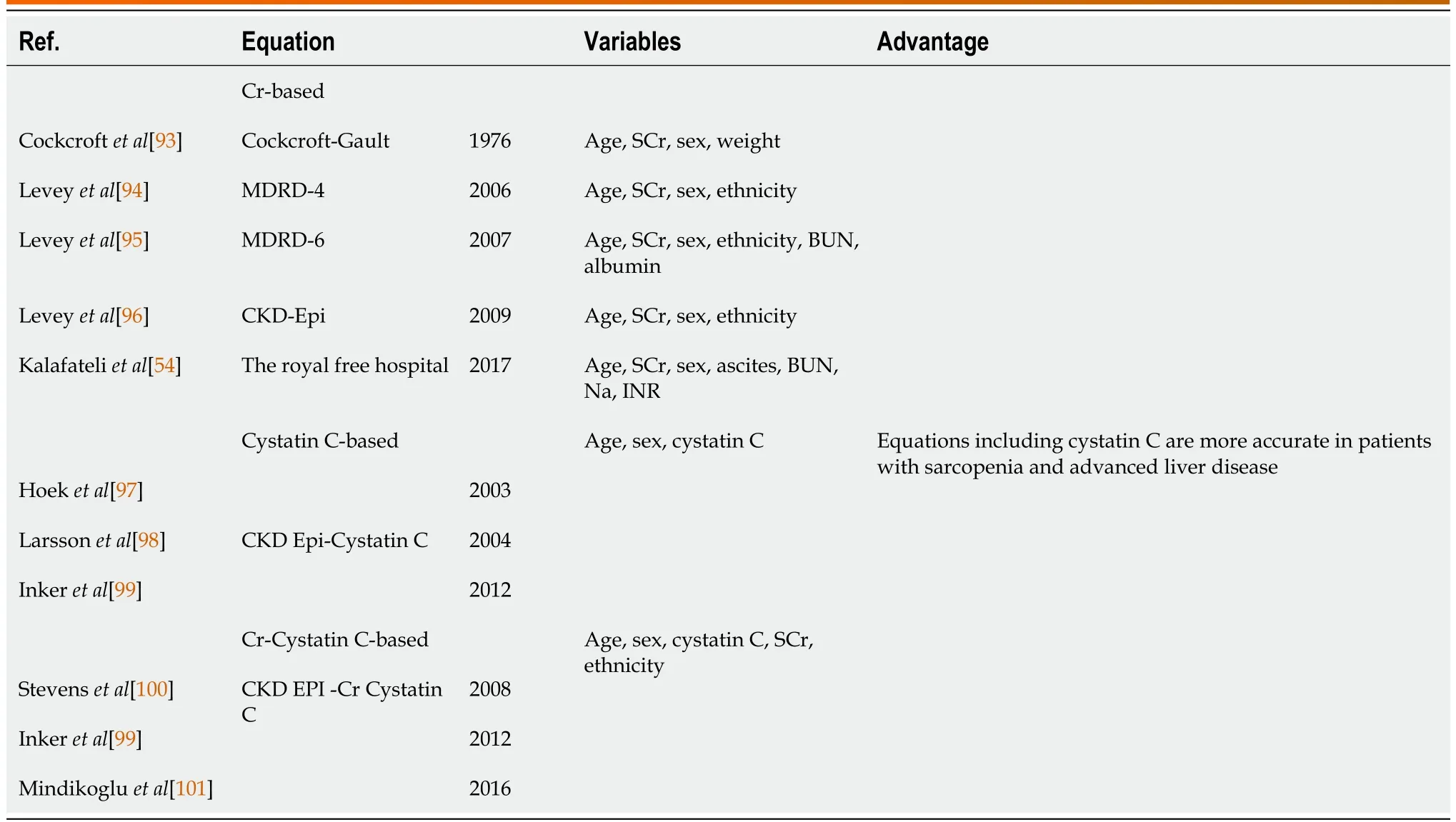
Table 1 Methods of estimating glomerular filtration rate and the novel equations for diagnosis of acute kidney injury in cirrhosis

Table 2 Methods of estimating glomerular filtration rate and the novel biomarkers for diagnosis of acute kidney injury in cirrhosis
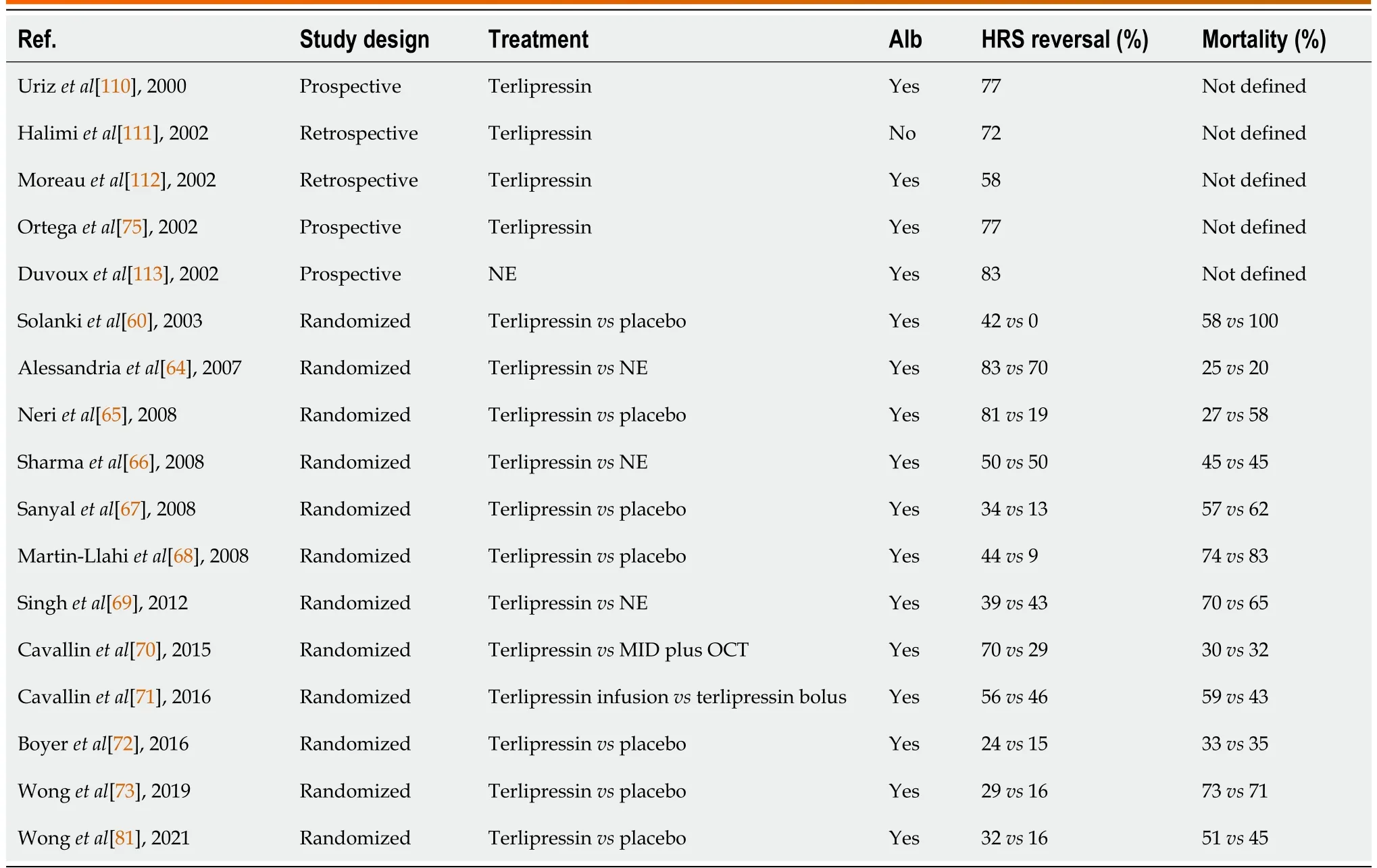
Table 3 Results of studies using vasoconstrictor therapy in patients with hepatorenal syndrome with acute kidney injury
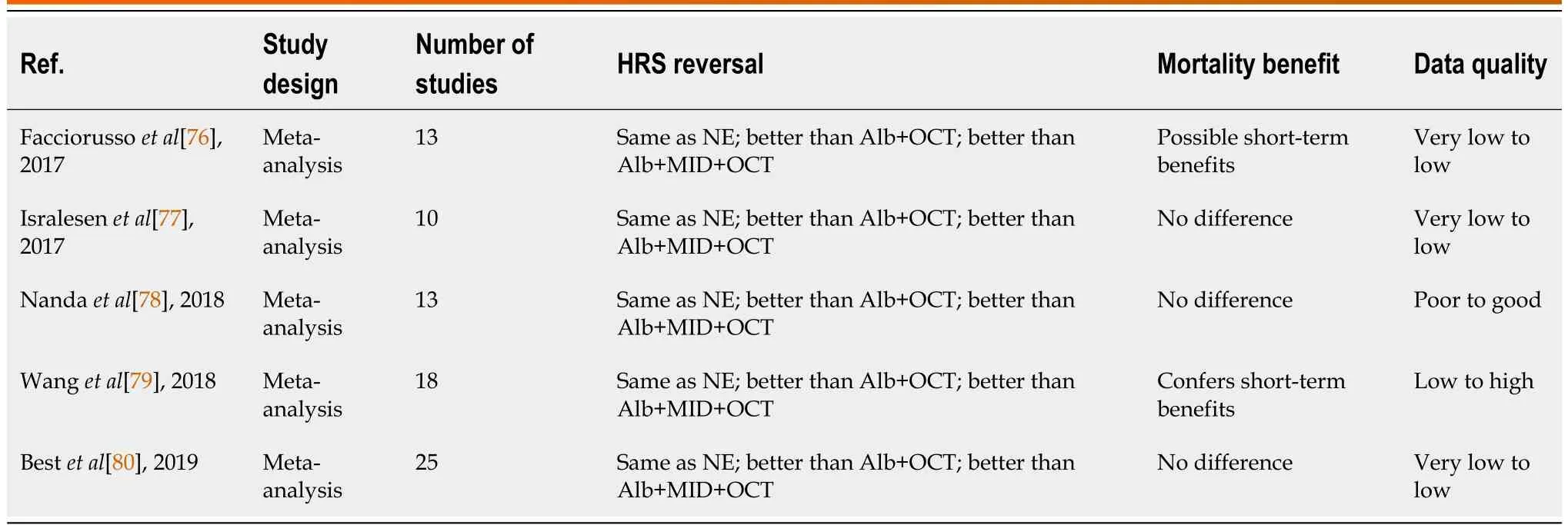
Table 4 Results of recent meta-analyses comparing terlipressin to other vasoconstrictor therapies in hepatorenal syndrome with acute kidney injury
Norepinephrine, although not FDA-approved for the treatment of HRS-AKI, has shown efficacy and is frequently used off label for the treatment of HRS, especially in the United States where terlipressin was not available until recently.The need for central venous administration and close hemodynamic monitoring generally limits its use to the ICU.Comparison of terlipressin and norepinephrine has been limited to single-center open-label studies, but has not shown a clear difference in the reversal of HRS or mortality[81,82].Given the lack of clear benefit of terlipressin over norepinephrine, higher cost of terlipressin, warnings issued by the FDA, and established practice patterns using norepinephrine in the United States, it is unclear how quickly and widely terlipressin will be adopted.
Transjugular intrahepatic portosystemic shunt
There is interest in using transjugular intrahepatic portosystemic shunt (TIPS) treatment of HRS-AKI because it can improve portal hypertension and cardiac output, two of the central causes of HRS-AKI.Although data regarding the role of TIPS in HRS generally involves small numbers of patients, a metaanalysis of 128 patients treated with TIPS for HRS showed improvement in renal function in 93% of patients with HRS-AKI.The significance of this finding is difficult to assess as there was no comparison group, significant heterogeneity, and high mortality[83].There is also significant risk associated with TIPS insertion in patients with HRS-AKI including 90-d mortality of 25%-80%[84].However, it is difficult to determine to what degree the high mortality was the result of TIPS.Given the lack of prospective or larger well-conducted retrospective analysis of TIPS for HRS as well as high procedural risks and complications associated with TIPS insertion in patients with HRS-AKI, it remains difficult to accurately identify patients who will benefit.Perhaps the clearest benefit of TIPS in the management of HRS-AKI lies in prevention by ameliorating portal hypertension.This is supported by a lower incidence of HRS in patients with diuretic resistant ascites treated with TIPS compared to those treated with serial paracentesis (9%vs31%)[85].
Renal replacement therapy
Renal replacement therapy (RRT), usually in the form of continuous hemodialysis, is the second-line treatment in patients with HRS-AKI who fail medical management and often regarded as a bridge to organ transplant since it does not address the underlying physiology of HRS.Additionally, RRT does not improve survival in patients with HRS-AKI after failure of medical management[86].Based on current evidence, RRT is best reserved for potential liver transplant candidates or if HRS-AKI is due to a potentially reversible condition such as infection or bleeding.In patients who are not transplant candidates or if the inciting cause is unclear or unlikely to be reversed, palliative care should be considered prior to initiation of RRT.
Artificial liver support systems
Liver support systems, including molecular adsorbent recirculating system and extracorporeal liver assist device, are forms of albumin dialysis where albumin recirculate as a scavenger of bacterial products and inflammatory cytokines have been considered for HRS-AKI.Thus far, there are no clear benefits in AKI-HRS and studies have shown mixed results regarding improving renal blood flow and survival[48].Thus, further studies are needed before its use can be officially recommended in HRS-AKI but it may be considered as a bridge to transplant in selected patients.
Organ transplant
Liver transplant is considered definitive treatment for HRS-AKI because it reverses the underlying pathophysiology causing renal impairment.This is evidenced by renal recovery in up to 75% of patients with HRS after liver transplant alone (LTA)[87,88].The strongest predictor of non-recovery of HRS-AKI is the duration of pretransplant dialysis, with each additional day of pretransplant dialysis increasing the risk of non-recovery by 6%[89].Other pre-transplant factors associated with lack of renal recovery after LTA are older age, higher baseline SCr, prolonged ischemia during transplant, exposure to nephrotoxic agents, diabetes, and development of ATN[87-91].Unfortunately, 6%-10% of patients with HRS who have LTA will develop end-stage renal disease by 1-year post-transplant[87-89].Therefore, itis essential to consider the likelihood of renal recovery after LTA and if the patient would benefit more from simultaneous liver-kidney transplant (SLKT).Currently, the main indications for SLKT are AKI requiring RRT or GFR < 25 mg/dL for more than 4-6 wk (guidelines vary) and CKD, commonly defined as GFR < 30 mg/dL at the time of listing with a GFR persistently < 60 mg/dL for at least 90 d[48,89].The decision regarding LTA or SLKT must also weigh the potential benefit for the individual patient with consideration of the principles of just and equitable organ allocation.In an attempt to balance these factors agencies responsible for organ allocation have set specific guidelines in their respective countries[92].
CONCLUSION
Although the primary cause of HRS remains circulatory dysfunction resulting in impaired renal profusion, there is now an improved understanding of the role of other factors including the inflammatory environment and cardiac dysfunction.This has contributed to the development of better biomarkers for earlier and more accurate diagnosis of HRS.Despite not being widely available the offer promise that effective treatment can be applied during the critical early stages of the disease where there is the greatest potential for benefit.Medical treatment remains primarily vasoactive medications and albumin, and have not yet been able to exploit the improved understanding of pathophysiology.However, the approval of terlipressin in the United States and clearer delineation of patients most likely to benefit from this therapy offers hope for improved medical management in the future.Despite advances in medical treatment, liver transplantation remains the most definitive treatment and should be considered early in the disease course as delay can increase the risk of incomplete renal recovery after transplant.
FOOTNOTES
Author contributions:Kiani C drafted the initial manuscript; Zori AG contributed to, edited, and drafted the final manuscript.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
14Wadei HM, Mai ML, Ahsan N, Gonwa TA.Hepatorenal syndrome: pathophysiology and management.Clin J Am Soc Nephrol2006; 1: 1066-1079 [PMID: 17699328 DOI: 10.2215/CJN.01340406]
23Saló J, Ginès A, Quer JC, Fernández-Esparrach G, Guevara M, Ginès P, Bataller R, Planas R, Jiménez W, Arroyo V,Rodés J.Renal and neurohormonal changes following simultaneous administration of systemic vasoconstrictors and dopamine or prostacyclin in cirrhotic patients with hepatorenal syndrome.J Hepatol1996; 25: 916-923 [PMID: 9007721DOI:10.1016/s0168-8278(96)80297-2]
47Acevedo J, Fernández J, Prado V, Silva A, Castro M, Pavesi M, Roca D, Jimenez W, Ginès P, Arroyo V.Relative adrenal insufficiency in decompensated cirrhosis: Relationship to short-term risk of severe sepsis, hepatorenal syndrome, and death.Hepatology2013; 58: 1757-1765 [PMID: 23728792 DOI: 10.1002/hep.26535]
杂志排行
World Journal of Hepatology的其它文章
- Ductular reaction in non-alcoholic fatty liver disease: When Macbeth is perverted
- Treatment of liver fibrosis: Past, current, and future
- Tumor budding as a potential prognostic marker in determining the behavior of primary liver cancers
- Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value
- Acute pancreatitis in liver transplant hospitalizations: Identifying national trends, clinical outcomes and healthcare burden in the United States
- Lower alanine aminotransferase levels are associated with increased all-cause and cardiovascular mortality in nonalcoholic fatty liver patients
