Treatment of liver fibrosis: Past, current, and future
2023-07-04ChunYeZhangShuaiLiuMingYang
Chun-Ye Zhang, Shuai Liu, Ming Yang
Abstract
Key Words: Liver fibrosis; Molecular mechanism; Therapeutic targets; Treatments; Clinical trials
INTRODUCTION
Liver fibrosis accompanies the progression of chronic liver diseases independent of etiologies[1], such as hepatitis viral infection, alcohol abuse, and metabolic-associated fatty liver disease (MAFLD).It is commonly associated with liver injury, inflammation, and cell death.Abnormal accumulation of extracellular matrix (ECM) components expressed by liver myofibroblasts, such as collagens and alphasmooth actin proteins, are the markers of hepatic fibrogenesis[2].Activated hepatic stellate cells (HSCs)contribute to the major population of myofibroblasts in liver fibrosis[3].Although many drugs have been investigated in clinical trials, there are no Food and Drug Administration (FDA)-approved treatments for liver fibrosis.
The activation of HSC is a complex pathogenesis in liver fibrosis[4].Many factors including intrahepatic and extrahepatic factors can drive HSC activation to induce liver fibrosis.A variety of molecular signaling pathways are involved in the regulation of HSC activation[1,5], such as transforming growth factor-β (TGF-β), Toll-like receptors (TLRs), and epigenetic signals (e.g., microRNAs, or miRNAs).The activation of HSCs can be divided into two phases, the initiation and perpetuation phases.RNA sequencing results have shown that fibrogenic transcriptional programs in the initiation phase are also active in the perpetuation phase; therefore, targeting the initial activation of HSC is also critically important for live fibrosis treatment[6].
In this review, the cellular and molecular mechanisms of liver fibrosis are reviewed.Importantly, the currently available treatments for liver fibrosis are summarized and discussed.Some pros and cons of available treatments are discussed.In addition, the future direction for liver fibrosis therapy is predicted.
INITIATION OF LIVER FIBROSIS: CELLULAR AND MOLECULAR MECHANISMS
Hepatic cell death
Liver cell death and inflammation are the initial events in chronic liver disease independent of etiologies.Many factors can cause liver injury and hepatic cell death and inflammation[7,8], including hepatitis viral infection, alcohol consumption, metabolic liver disease, abnormal bile acid products, and genetic factors.These pathogenic factors cause immune cell inflammation, hepatocyte death,mitochondrial dysfunction, and endoplasmic reticulum stress (Figure 1), resulting in HSC activation and differentiation to myofibroblasts to lead to liver fibrosis[5,9].
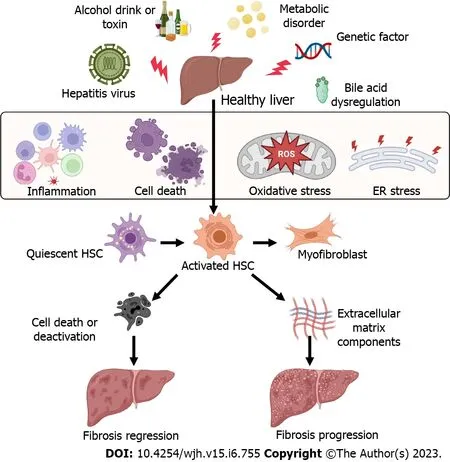
Figure 1 Factors causing the activation of hepatic stellate cells and liver fibrosis.Many factors can cause liver injury and hepatic cell death and inflammation, including hepatitis viral infection, alcohol consumption, metabolic liver disease, abnormal bile acid products, and genetic factors.These pathogenic factors cause immune cell inflammation, hepatocyte death, oxidative stress, and endoplasmic reticulum stress, resulting in hepatic stellate cell activation and differentiation to myofibroblasts to lead to liver fibrosis.All cartoons in this figure were prepared using Biorender (https://biorender.com).ER: Endoplasmic reticulum;HSC: Hepatic stellate cell; ROS: Reactive oxygen species.
In non-alcoholic steatohepatitis (NASH), hepatocyte death results in the infiltration of monocytederived macrophages and upregulation of the expression of inflammatory cytokines[9], such as tumor necrosis factor α (TNF-α), TGF-β1, and interleukin-1β (IL-1β), Hepatocyte death can be classified into programed cell death including pyroptosis, apoptosis, necroptosis, ferroptosis, and autophagymediated cell death (Figure 2), as well as non-programed cell death (necrosis).In chronic liver disease,different types of cell death may be associated with the progression of liver fibrosis and end-stage of liver disease, such as hepatocellular carcinoma (HCC).Single-cell RNA sequencing coupled with spatial mapping approaches has been started to dissect the key cellular and molecular functions in liver disease[10].

Figure 2 Programmed cell death subtypes of hepatic cells, including apoptosis, necroptosis, pyroptosis, ferroptosis, and autophagymediated cell death.Apoptosis, necroptosis, pyroptosis, and ferroptosis are programmed forms of cell death, while necrosis is unprogrammed cell death.Autophagy-mediated cell death should be defined when autophagic flux is raised without the involvement of other types of programmed cell death, and pharmacological or genetic inhibition of autophagy blocks cell death.DAMPs: Danger-associated molecular patterns; FADD: Fas-associated protein with a death domain; FAS: Fas cell surface death receptor; MLKL: Mixed lineage kinase domain-like; NLRP3: Nod-like receptor family, pyrin domain containing 3; PAMPs:Pathogen-associated molecular patterns; RIPK1/3: receptor-interacting protein kinase 1/3; TNF: Tumor necrosis factor; TNFR: Tumor necrosis factor receptor.All cartoons in this figure were prepared using Biorender(https://biorender.com).
Pyroptosis is an inflammatory cell death, associated with cell membrane rupture by cleaved gasdermin D[11].The cleave of gasdermin D is induced by the activation of caspase-1 or caspase-11/4/5[12].For example, in mice with non-alcoholic fatty liver disease (NAFLD), feeding a high-fat diet can increase the expression of caspase-11 to cause pyroptosis of bone marrow monocyte-derived macrophages by cleave gasdermin D[13].The expression of pyroptosis-related indicators including gasdermin D, IL-1β, and IL-18 has been shown to be increased in human patients with liver fibrosis and mice with CCl4-induced fibrosis[14].In addition, S100 calcium-binding protein A8 plays an essential role in macrophage pyroptosis in liver fibrosis, by inducing the expression of nucleotide-binding domain leucine-rich repeat-receptor, pyrin domain-containing-3 (NLRP3) inflammasome, pro-IL-1β, and pro-IL-18viathe activation of TLR4/nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB)signaling pathway[14].
Apoptosis is a form of programmed cell death that occurs in all liver cell types.In hepatocytes,kindlin-2 deficiency can increase the apoptosis of hepatocytes, resulting in liver fibrosis and accumulation of ECM components by activating the TNF signaling pathway[15].In NAFLD, microRNAs such as miR-22 from adipocyte-derived exosomes can cause hepatocyte apoptosis to increase hepatic inflammation, lipid accumulation, and fibrosis by regulating sirtuin 1 expression[16].In contrast, promoting the apoptosis of HSCs can inhibit and reverse liver fibrosis.For example, treatment with gomisin D can inhibit CCl4-induced HSC proliferation and activation in mice and increase HSC apoptosis to reduce liver fibrosis by regulating the platelet-derived growth factor receptor β signaling pathway[17].
Necroptosis is a regulated cell death that has the features of apoptosis and necrosis.Necroptosis can be induced by TLRs, interferons, and death receptors[18], which is mediated by receptor-interacting protein kinase-3 and its substrate mixed lineage kinase-like[19].In addition, receptor-interacting serine/threonine-protein kinase 1 has an important role in this process (Figure 2).It contributes to hepatocyte death in NASH.Necroptotic hepatocytes cannot be removed by liver macrophages due to the activation of "don't-eat-me" signaling pathway, the CD47/signal regulatory protein alpha axis[20].
Ferroptosis is an intracellular iron-dependent lytic cell death which is different from apoptosis and necrosis[21,22].Excessive iron accumulation and the inhibition of glutathione peroxidase 4 trigger ferroptosis, which causes cell plasma membrane rupture[23].Accumulating research studies have demonstrated that ferroptosis is involved in different liver diseases, including alcoholic liver disease,NASH, cirrhosis, and cancer[24,25].Several molecular signaling pathways are involved in ferroptosis in liver diseases, such as the nuclear factor erythroid 2-related factor 2[26], heat shock protein family A member 8[27], and nuclear receptor coactivator 4[28].Pharmacological regulation of HSC ferroptosis is a therapeutic strategy for liver fibrosis[29].For example, curcumol can induce ferroptosis of activated HSCs to inhibit liver fibrosis by inducing autophagy[28].
Furthermore, some dying cells develop autophagosomes that trigger apoptosis and necroptosis,namely autophagy-mediated cell death (ACD).However, ACD should be defined when some criteria are met[30], including: (1) Cell death happens without the involvement of other types of programmed cell death; (2) autophagic flux is raised; and (3) pharmacological or genetic inhibition of autophagy blocks cell death.Overall, the molecules implicated in the process and signaling pathways of hepatic cell death are potential targets for liver fibrosis.
Hepatic innate and adaptive immunity
Liver resident immune cells and infiltration of myeloid cells or circulating immune cells in the liver during chronic liver injury play key roles in the activation of HSCs.For example, in NASH liver, gut microbiota-diet interplay-resulted metabolite can activate liver macrophages to produce profibrotic factors[31], such as TGF-β1.Treatments such as astaxanthin can suppress the infiltration of monocytederived macrophages to suppress HSC activation, liver oxidative stress response, and hepatocyte death by decreasing the expression of proinflammatory cytokines[32], such as TNF-α, TGF-β1, and IL-1β.Another study also during NAFLD progression, mast cells can increase Western diet-induced biliary and liver damage to the development of microvesicular steatosis through microRNA (miR-144-3p)-targeted signaling pathway[33].
Adaptive immune cells including T and B cells have various roles in liver fibrosis.The imbalance of liver regulatory T cells and T helper (Th) cells, such as Th1 cells and Th17 cells plays an essential role in liver fibrosis, cirrhosis, and cancer[34].The populations of multicytokine-producing CD4 T cells were significantly increased in the livers of patients with NASH compared with patients with NAFLD[35].The cytokines produced by these CD4 T cells include TNF-α, IFN-γ, IL-17A, and IL-10.The phenotype of T cells is also important for their functions.One study showed that the reduction of tissue-resident memory CD69+CD103−CD8 T cells significantly decreased the resolution of fibrosis in NASH liver.These CD8 T cells can induce FasL/Fas-mediated apoptosis of HSCs[36].Therefore, targeting immune cells or their secreted inflammatory cytokines is an optional treatment for liver fibrosis[37].
Furthermore, HSC activation is also associated with vascular aging[38], ischemia/reperfusion injury[39], and angiogenesis[40].
SIGNALING PATHWAYS AND MOLECULAR TARGETS FOR LIVER FIBROSIS TREATMENTS
Bile acid receptors
Farnesoid-X-receptor (FXR) and the G protein-coupled bile acid receptors are two widely studied bile acids regulating receptors, which play important roles in lipid and glucose metabolism, inflammation,fibrosis, and immune responses.Many FXR ligands have been investigated in clinical trials for NASH and liver fibrosis treatments, such as EDP-305[41,42], cilofexor[43,44], and MET409[45,46].Hepatic concentrations of conjugated 12α-hydroxylated bile acids, such as taurodeoxycholate and glycodeoxycholate, were significantly increased in patients with NASH and mouse liver fibrosis models[47].These bile acids contribute to HSC activation and liver fibrosis by regulating the signaling of G proteincoupled bile acid receptor 1, also known as TGR5.
Caspases
Caspases are involved in liver cell inflammation and hepatocyte cell death.Meanwhile, they play an essential role in liver fibrogenesis.For example, caspase 3-deficient mice on a methionine- and cholinedeficient diet had reduced liver collagen production compared to wild-type mice[48].Lipotoxicity can induce caspase-mediated apoptosis of hepatic cells and liver inflammation and injury in NAFLD.Treatment of caspase inhibitors such as emricasan (IDN-6556, a pan-caspase inhibitor) can reduce liver injury in patients with NAFLD[49].
Chemokine receptors
Chemokine receptors are commonly expressed by immune cells or inflammatory cells, which can be recruited during liver inflammation and fibrosis.For example, C-C chemokine receptor 2 (CCR2) and CCR5 are highly expressed by monocytes and subtypes of liver macrophages, which can be targeted to ameliorate liver fibrosis[50].A recent study showed that the roles of CCR2 and CCR5 in liver macrophages are different during liver disease progression in mice with a hepatocyte-specific knock-out of NF-κB essential modulator.CCR2 oversees the recruitment of monocytes during liver injury, whereas CCR5 is needed to promote HSC activation[51].
In addition, many other chemokines and their receptors are involved in the pathogenesis of liver fibrosis, including C-X-C motif ligand 12 (CXCL12) /C-X-C receptor 4[52], chemokine (C-X3-C motif)ligand 1/C-X3-C receptor 1 (CX3CR1)[53], CCL19/CCR7[54], and CXCL12/atypical chemokine receptor 3[55].
Fibroblast growth factors
Fibroblast growth factor 15 (FGF15) is an important endocrine regulator for hepatic bile acid and lipid metabolism, which regulates gut-liver crosstalk in mice[56].A combined treatment using an inhibitor of apical sodium-bile acid transporter (GSK233072) and adeno-associated virus 8-mediated hepatic FGF15 overexpression significantly can improve the therapeutic efficacy against NASH and fibrosis compared to either single treatment[57].Bile acid nuclear receptor FXR plays an important role in the regulation of the expression of FGF15/19, bile acid homeostasis, and lipid metabolism, which is the target for NASH and liver fibrosis[58].The expression of hepatic FXR and plasma FGF19 (the ortholog of mouse FGF15)was decreased in children with NASH compared to their expression in healthy subjects[59].
Galectins
Galectins are carbohydrate-binding proteins and play important roles in liver inflammation, immune response, and fibrosis.Galectin-1 (Gal-1) was shown to be highly expressed in the stroma of HCC by cancer-associated fibroblasts.Silencing Gal-1 in these fibroblasts can suppress inflammation and tumor progression[60].The serum level of Gal-3 was increased in patients with advanced cirrhosis, and liver expression of Gal-3 was also correlated with liver disease severity and inflammation[61].
Lysyl oxidase family members
Lysyl oxidase (LOX) family members are extracellular copper-dependent enzymes, including LOX, lysyl oxidase-like 1 to 4 (LOXL1 to 4) members, which play important roles in the cross-linking of ECM proteins in fibrosis and carcinogenesis.Inhibition of pan-LOX family, LOX, LOXL1, or LOXL2 has been shown to prevent fibrogenesis and accelerate the reversal of liver fibrosis, as well as fibrosis in other organs.However, the roles of LOX family members as therapeutic targets for liver fibrosis need further to be evaluated[62].
NLRP3 inflammasome
Nucleotide-binding oligomerization (NLR) family pyrin domain-containing 3 (NLRP3) plays a pivotal role in liver fibrosis.Activation of NLRP3 can lead to the inflammatory response through the secretion of IL-1β and IL-18 and activation of caspase-1[63], which is involved in liver cell pyroptosis[64].Activation of NLRP3 inflammasome can induce hepatocyte pyroptosis and liver fibrosis, while inhibiting the activation of NLRP3 inflammasome can inhibit the development of NAFLD and NASH in animal models[65,66].Activation of NLRP3 inflammasome in pyroptosis is mediated by canonical caspase-1-mediated signaling pathway and noncanonical caspase-11-mediated signaling pathway[67].
Peroxisome proliferator-activated receptors (PPARs)
PPARs, comprised of three subtypes PPARα, β/δ, and γ, play important roles in liver lipid metabolism,inflammation, and fibrosis[68-70].The expression of liver PPARα was shown to be negatively correlated with NASH severity, visceral fat accumulation, and insulin resistance in human patients[71].Treatment with PPARα/γ dual agonists decreased the concentrations of total cholesterol, triglyceride (TG), and inflammatory cytokine levels in serum, reduced hepatic steatosis, infiltration of inflammatory cells, and decreased the expression of lipogenic gene and NF-κB protein[72].Another study showed that the levels of very low-density lipoprotein receptors (VLDLR) were increased in PPARβ/δ-deficient mice.In patients with hepatic steatosis, the mRNA levels of PPARβ/δ were suppressed and associated with an increase in VLDLR levels[73].A pre-clinical study showed that treatment with pan-PPAR agonist lanifibranor can significantly decrease portal pressure and liver inflammation and induce fibrosis regression[74].
TGF-β/Smad
TGF-β/Smad is the most well-studied signaling pathway in fibrosis.SMAD proteins are essential intracellular effectors of TGF-β and show different roles in liver fibrosis[75], including pro-fibrotic functions (e.g., SMAD3 and SMAD4) and protective functions (e.g., SMAD2 and SMAD7).In addition,many studies have demonstrated that regulating the signaling pathway of TGF-β/Smad can prevent liver fibrosis[76], as well as the protein kinase B (PKB, or AKT)/Forkhead box O3 (FOXO3) signaling pathway.
Wnt/β-catenin
Proteins-derived from human amniotic mesenchymal stem cells, including insulin-like growth factor binding protein-3, Dickkopf-1, and DKK-3, can inhibit HSC activation by suppressing Wnt/β-catenin signaling pathwayin vitro[77].In vivo study also showed that treatment of niclosamide in rats can prevent CCl4-induced liver fibrosis by inhibiting the Wnt/β-catenin pathway and glutaminolysis[78].Another study also showed that Wnt3a can upregulate the expression of protein regulator of cytokinesis 1 to active β-catenin signaling to promote liver fibrosis[79].The interaction of β-catenin/transcription factor 4 (TCF4) has been shown to increase during liver fibrosis in mice with bile duct ligation (BDL)[80].Treatment with ICG-001, an inhibitor of the interaction between cyclic adenosine monophosphate response element binding protein binding protein and β-catenin, together with LF3, a small molecule antagonist that inhibits β-catenin/TCF4 transcriptional activity, can reduce liver fibrosis[80].
Yes-associated protein (YAP)
YAP plays a pivotal role in the sensitivity of HSCs to ferroptosis, apoptosis, and senescence in fibrotic livers.Selective depletion of YAP in myofibroblastic HSCs or activated HSCs can promote their senescence or apoptosis to reduce liver injury and fibrosis[81].Taurocholic acid can induce the activation of HSCs through the sphingosine-1-phosphate receptor 2/YAP/p38 mitogen-activated protein kinase (p38 MAPK)[82].
CURRENT DIAGNOSIS FOR LIVER FIBROSIS
The golden standard method for liver fibrosis diagnosis is liver biopsy.Histological or histochemical staining can be used to stain the cells or the extracellular matrix proteins to identify liver fibrosis.Common histological staining methods for liver fibrosis evaluation are hematoxylin-eosin staining with Masson's trichrome or Sirius Red staining[83].Due to the pain and the risk of potential complications of liver biopsy, non-invasive techniques (e.g., elastography scanning) and biomarkers (e.g., aminotransferase to platelet ratio (APRI): The aminotransferase/platelet ratio index) can be applied for diagnosing liver fibrosis[84].Many available scoring systems can be applied for liver fibrosis diagnosis and evaluation, including fibrosis-4 index (FIB-4), APRI, and NAFLD fibrosis score (NFS)[85].
Imaging methods are commonly applied in the clinic to evaluate the progression of liver fibrosis.For example, ultrasound elastography techniques can be applied to characterize liver fibrosis and its stage in adult patients, such as vibration-controlled transient elastography, the most utilized and validated elastography method[86].A meta-analysis study also showed that magnetic resonance elastography(MRE) and point-shear wave elastography (pSWE) can be applied for liver fibrosis diagnostic, and MRE is a more accurate imaging technique than pSWE[87].The pooled sensitivities and specificities for MRE and pSWE were 0.94 (95% confidence level/CI: 0.89-0.97) and 0.95 (95%CI: 0.89-0.98), and 0.86 (95%CI:0.80-0.90) and 0.88 (95%CI: 0.85-0.91), respectively.Their pooled summary receiver operating characteristic curves showed that the area under the curve (AUC) for MRE was 0.98 (95%CI: 0.96-0.99),whereas the AUC for pSWE was 0.93 (95%CI: 0.90-0.95).Another review paper has updated the conventional and molecular imaging diagnostic methods for liver fibrosis[88].In addition, artificial intelligence models have been applied for the diagnosis of liver fibrosis[89-91].For example, the clinical features and imaging data collected from a patient can be analyzed for liver fibrosis diagnosis using a machine learning model.
Recently, studies also have shown that miRNAs, the single-stranded, non-coding RNAs containing 21 to 23 nucleotides, are involved in the pathogenesis of liver fibrosis, which are potential biomarkers for diagnosing liver fibrosis and therapeutic targets for liver fibrosis treatment[92].The methods for liver fibrosis diagnosis have been reviewed in some recent publications[93-96].Here, we will not discuss more details and will focus on the treatment options for liver fibrosis.
CURRENT TREATMENT OPTIONS FOR LIVER FIBROSIS
In this section, we review some different treatment options for liver fibrosis, such as biological intervention, anti-fibrotic drugs, and other treatment strategies.These treatments either target causing factors of liver fibrosis to accelerate the recovery of liver injury, or induce the balance of liver metabolism, such as anti-hepatitis viral infection, anti-cell death treatment, and regulators of lipid metabolism.
Biological intervention
Inhibition of LOXL2 in the fibrotic tumor microenvironment can synergistically increase the efficacy of sorafenib and 5-fluorouracil for liver cancer cells[97].However, some treatments in clinical trials did not show promising results.For example, simtuzumab is a monoclonal antibody against LOXL2.In two phase 2b clinical trials, intravenous infusions of simtuzumab (200 or 700 mg) every other day for 48 wk and 96 wk did not show promising effects to decrease liver fibrosis and the progression of cirrhosis in patients with bridging fibrosis[98].In a pilot clinical trial, intravenous treatment of simtuzumab (700 mg) every 2 wk for 22 wk did not improve liver biopsy fibrosis score for patients with advanced liver fibrosis[99].
Drug treatment
Aramchol, a partial inhibitor of hepatic stearoyl-CoA desaturase, has been shown to improve NASH and liver fibrosis in rodents and decrease liver triglycerides and fibrosis clinical trials[100].
Anti-hepatitis viral infection drugs:Inhibition of hepatitis viral infection can suppress liver inflammation and hepatocyte death to decrease liver injury, resulting in suppression of liver fibrosis.Drugs such as faldaprevir (also known as BI 201335)[101], ribavirin (HCV treatment)[102], and peginterferon alfa-2a (HBV treatment)[103,104], have been tested in clinical trials for the treatment of liver fibrosis.In addition, many other drugs have been evaluated or are under clinical trial evaluation against hepatitis viral infection[105-107], such as simeprevir, daclatasvir, and sofosbuvir.
Cenicriviroc:C-C chemokine receptors 2 and 5 dual antagonist, has been shown to improve liver fibrosis without worsening NASH compared to the placebo in phase 2 clinical trial (Clinicaltrials.gov,NCT02217475)[108].A phase 3 clinical trial has been designed to confirm the efficacy and safety of cenicriviroc for liver fibrosis treatment in adults with NASH[109].
Cholangitis treatment:Obeticholic acid and ursodeoxycholic acid are the only two FDA-approved medicines for the treatment of primary biliary cholangitis[110], which have the potential to cholangitisinduced liver fibrosis.
Cyclophilin inhibitors:CRV431, a pan-cyclophilin inhibitor, can decrease liver fibrosis in mice treated with CCl4for 6 wk and mice with diet-induced NASH[111].Another cyclophilin inhibitor NV556 also displays an antifibrotic effect in two mouse NASH models, the STAM model (streptozotocin plus a high-fat diet) and methionine- and choline-deficient diet-induced NASH model[112].In addition,NV556 can also inhibit TGF-β1-induced activation of HSCsin vitro.
FGF regulators or analogues:Treatment of pegbelfermin (BMS-986036, 10 mg or 20 mg daily), a PEGylated human FGF21 analogue, can significantly decrease liver fat accumulation in patients with NASH without treatment-related severe adverse effects, and it can also improve liver fibrosis in patients with obesity and type 2 diabetes[113].
FXR agonists:Treatment of obeticholic acid (INT-747), a potent and orally active FXR agonist, can significantly ameliorate liver fibrosis and the histological and biological markers of NASH in patients with NASH[114].
Gal-3 inhibitors:GB1211, an inhibitor of Gal-3, can inhibit the differentiation of epithelial cells into myofibroblasts and macrophage or myofibroblast-induced fibrosis in the liver[115].GR-MD-02(belapectin), a galectin-3 inhibitor, has been shown to inhibit liver fibrosis and portal hypertension in rat fibrosis mode, which is safe and well-tolerated in a phase 1 clinical trial.However, a phase 2b clinical trial showed that treatment of GR-MD-02 did not significantly improve liver fibrosis and reduce portal hypertension (hepatic venous pressure gradient) in patients with NASH[116].Further studies are required to evaluate these treatments for liver fibrosis.
Glucagon-like peptide-1 (GLP-1) receptor agonist:GLP-1 analogues have been shown to have the effects to reduce liver fat accumulation, liver injury, and insulin resistance in mice with fatty liver disease.Clinical trial (ClinicalTrials.gov, NCT01237119) showed that treatment of GLP-1 analogue liraglutide was well tolerated and suppressed liver fibrosis progression in patients with NASH[117].Another trial also showed that treatment of liraglutide markedly reduced liver fat content and body weight in patients with uncontrolled type 2 diabetes[118].
Pan-caspase inhibitor:Emricasan (IDN-6556), a pan-caspase inhibitor, can decrease liver cell apoptosis and inflammation and improve portal pressure in rats with CCl4-induced cirrhosis[119].However, a clinical trial (Clinicaltrials.gov, NCT03205345) did not show the efficacy of emaricasan against liver fibrosis, but it was safe and well-tolerated.
PPAR agonists:In rats with BDL-induced liver fibrosis, treatment of PPAR-γ agonist thiazolidinedione inhibited HSC activation and liver fibrosis by regulating fibrogenic factors, such as TGF-β1, plateletderived growth factor, and connective tissue growth factor[120].Farglitazar (GI262570), an agonist of peroxisome proliferator-activated receptor-gamma (PPARγ), can inhibit HSC activation.
Tropifexor:A non-bile acid FXR agonist, can potently inhibit cholestatic liver injury and fibrosis by enhancing the expression of FGF19 in the ileum and the expression of small heterodimer partner in the livers of piglets but inhibit cholesterol 7α-hydroxylase.In addition, tropifexor can increase the abundance of bile acid-biotransforming bacteria and later the amino acid composition in the intestine and decrease intestinal barrier injury in piglets with BLD[121].Clinical trial (Clinicaltrials.gov,NCT02855164) also showed that treatment of tropifexor (10-90 μg) once daily for 12 weeks was safe and decreased the levels of alanine aminotransferase (ALT) and hepatic fat fraction (HFF) compared to baseline in a dose-dependent manner.The decrease of ALT and HFF can be sustained for up to 48 wk at high doses of tropifexor (140 μg and 200 μg once daily)[122].
Natural products or herbal medicines
Natural products or herbal medicines display diverse roles in the treatment of liver fibrosis.For example, a classical Traditional Chinese Medicine formula Yinchenhao decoction has been shown to ameliorate dimethylnitrosamine-induced liver fibrosis in rats and suppress liver cell apoptosis[123].Another study showed that Xiaoyaosan decoction significantly reduced CCl4-induced liver fibrosis in rats by regulating both TGF-β1/Smad and AKT/FOXO3 signaling pathways[76].The major components of these herbal medicines such as Tanshinone IIA extracted from the traditional herbal medicine Salvia miltiorrhiza display broad biological activities, such as anti-inflammatory, antioxidant, antiangiogenic,and anticancer functions[124].Furthermore, clinical trials also illustrate that these traditional medicine formula such as Fuzheng Huayu display therapeutic effects against hepatitis-B-caused cirrhosis in patients[125].
Dietary regulation or supplementation
Consumption of polyunsaturated fatty acids:The endogenous metabolites of n-3 polyunsaturated fatty acids such as 19,20-epoxy docosapentaenoic acid show a protective effect against liver fibrosis in mouse NASH models[126].G protein-coupled receptors can be regulated by polyunsaturated fatty acids to reduce liver inflammation and fibrosis[127].For example, supplementation of docosahexaenoic acid, an omega-3 fatty acid, can reduce liver inflammation and prevent liver fibrosis in diet-induced liver fibrosis modelviaG protein-coupled receptor 120 (GPR120) signaling, also known as free fatty acid receptor 4[128].
Probiotics:Treatment with probioticLactobacillus rhamnosusGG can significantly decrease liver inflammation and fibrosis by reducing the production of hepatic bile acids in mice with BLD[129].
Vitamins:The serum levels of vitamin C have been shown to be negatively associated with the odds of liver fibrosis in patients with NAFLD in United States adults[130].Another study showed that a decreased serum level of vitamin B12 is associated with an increased risk of liver fibrosis in patients with NAFLD[131].Treatment of Vitamin D3can alleviate liver injury and the expression of ECM proteins such as TGF-β and α-SMA in thioacetamide-induced hepatic fibrosis rat model[132].The data from the National Health and Nutrition Examination Survey (2017-2018) also showed that levels of 25-Hydroxyvitamin D were inversely associated with liver fibrosis during NAFLD development and progression[133].
Antioxidant and anti-inflammatory agents:Supplementation of natural products with antioxidant and anti-inflammatory components can also ameliorate chronic liver disease to improve liver fibrosis and inhibit cancer development[9], such as β-sitosterol and silymarin.
Bariatric surgery
Studies have shown that bariatric surgery (BS) can provide long-term benefits for the resolution of liver fibrosis.The two most common procedures of BS are laparoscopic Roux-en-Y-gastric and laparoscopic sleeve gastrectomy.For example, one study showed that NASH was resolved in 84% of patients (95%CI:73.1%-92.2%) at year 5 post-BS treatment, while fibrosis was decreased in samples from 70.2% of patients (95%CI: 56.6%-81.6%) compared with baseline and fibrosis was disappeared in samples from 56% of all patients (95%CI: 42.4%-69.3%)[134].BS has been shown to induce NASH disappearance in nearly 85% (95%CI: 75.8%-92.2%) of patients and to decrease fibrosis in 33.8% of patients (95%CI: 23.6%-45.2%) with NASH at 1 year after surgery[135].Another clinical study showed that excessive weight loss shown in patients with cirrhosis with 73% (33%–167%), 85% (33%–190%), and 73% (29%–107%) after 1,2, and 3 years of BS[136], respectively.Among 27 patients with cirrhosis, 3 patients had significant improvement in liver function and did not need liver transplantation, whereas 2 out of 27 patients had deleterious liver function post-BS treatment[136].
Genetic intervention
Gene therapy is a critical tool for disease treatment, including liver fibrosis and cancer.Noncoding RNAs, such as miRNAs and long noncoding RNAs, small interference RNAs, and circular RNAs are important.For example, the treatment of siRNA silencing CCR2 can regulate liver immune to inhibit the infiltration of profibrotic macrophages and neutrophils in murine fibrotic livers[137].Another study showed that circRNA ASPH regulated liver fibrosis by binding miR-139-5p by regulating neurogenic locus notch homolog protein 1 (Notch 1) expression[138].
Transplantation of stem cells
Transplantation of umbilical cord Wharton's Jelly-derived mesenchymal stem cells to rats with CCl4-induced hepatic fibrosis improved liver function, inflammation, and fibrosisviaa paracrine mechanism possibly by targeting TGF-β1 signaling pathway[139].Another study showed that transplantation of human umbilical cord blood mesenchymal stem cells substantially improved liver fibrosis in histopathological evaluation compared to that in the untreated group[140].Infusions of hematopoietic stem cells into mice with methionine-choline-deficient diet- or CCl4-induced liver fibrosis can reduce hepatic collagen production and the expression of α-smooth muscle actin[141,142].
Overall, there are several potent preventive and therapeutic treatments for liver fibrosis, including physical activity (e.g., running), dietary change (e.g., avoid of high-fat and high-sugar diet), dietary supplementation (e.g., vitamin C), biological treatment (e.g., simtuzumab), bariatric surgery (e.g., Rouxen-Y-gastric procedure), drug (e.g., pegbelfermin), change of gut microbiota (e.g., probiotics),nanoparticles (e.g., BMS-986263), genetic regulation (e.g., non-coding RNAs), and transplantation of stem cells (e.g., hematopoietic stem cells) (Figure 3).
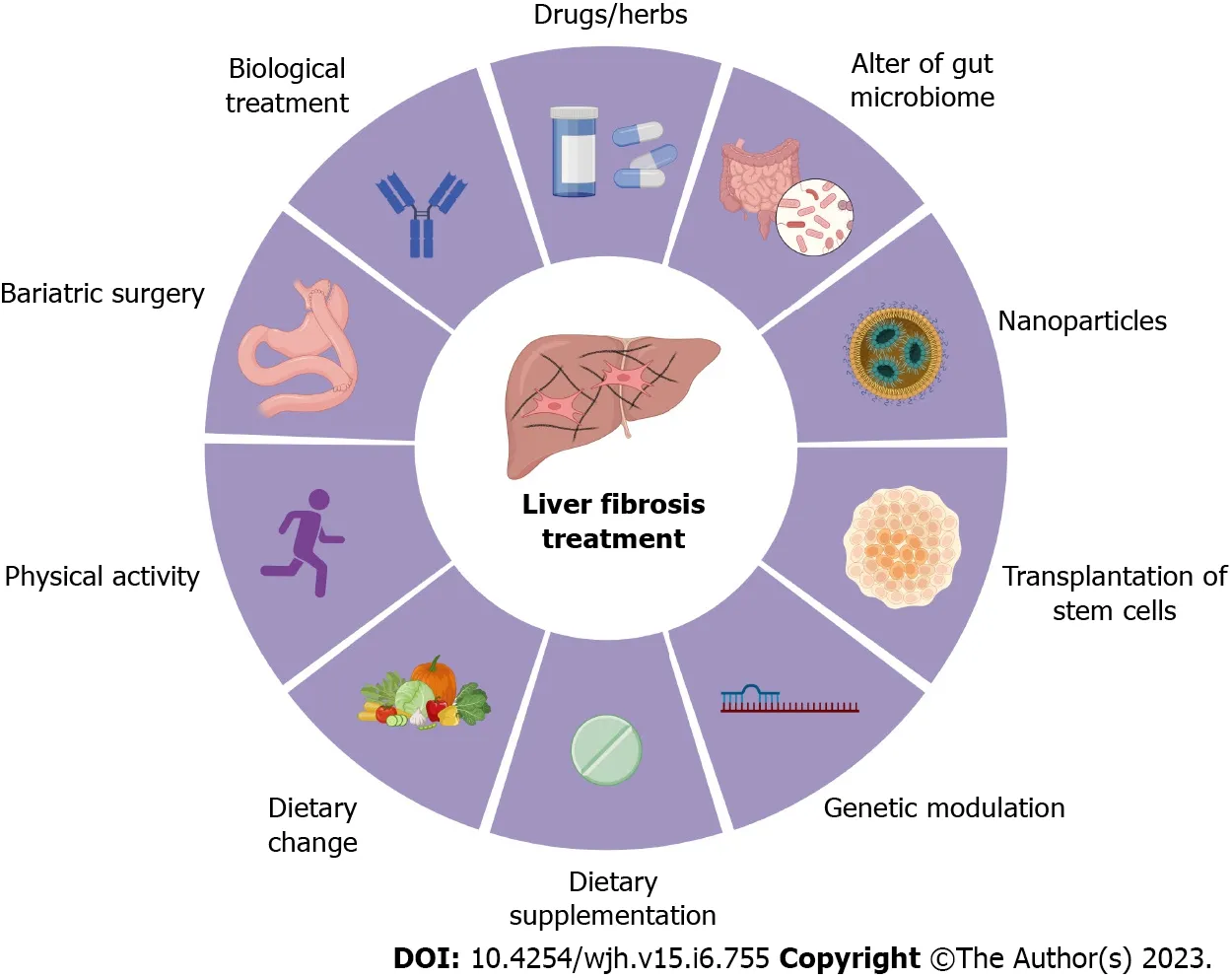
Figure 3 Treatment options for liver fibrosis.Currently, the preventive and therapeutic treatments for liver fibrosis include physical activity (e.g., running),dietary change (e.g., avoid of high-fat and high-sugar diet), dietary supplementation (e.g., vitamin C), biological treatment (e.g., simtuzumab), bariatric surgery (e.g.,Roux-en-Y-gastric procedure), drugs and herb medicines (e.g., pegbelfermin), change of gut microbiota (e.g., probiotics), nanoparticles (e.g., BMS-986263), genetic regulation (e.g., non-coding RNAs), and transplantation of stem cells (e.g., hematopoietic stem cells).All cartoons in this figure were prepared using Biorender(https://biorender.com).
CLINICAL TRIALS
In this section, we first review some completed clinical trials (Clinicaltrials.gov, Table 1).These treatments including biological treatment, drugs, dietary supplementation, and infusion of stem cells.
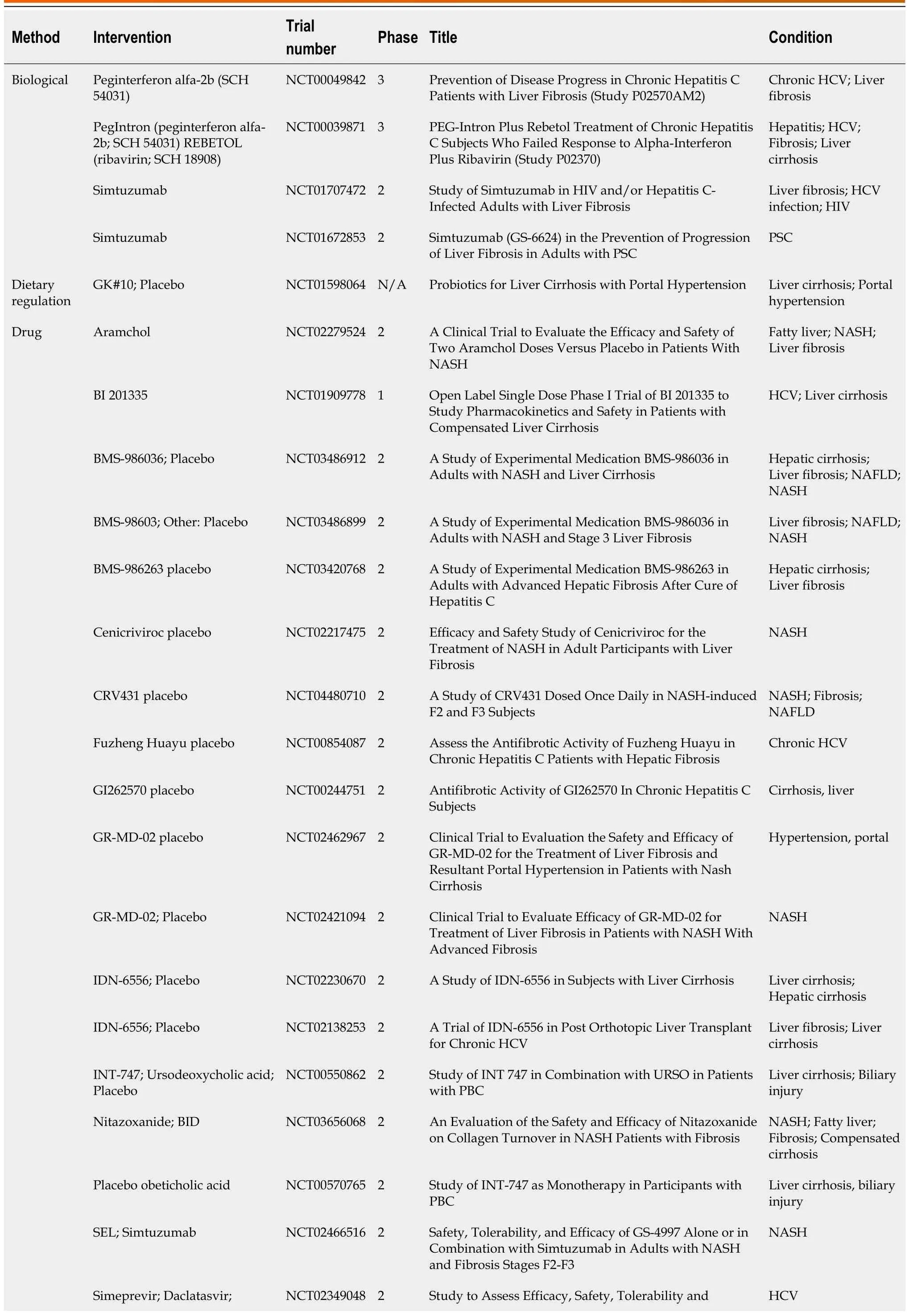
Table 1 Completed clinical trials (Clinicaltrials.gov, accession date: March 1, 2023)
Future treatments
There is an unmet need for treatments for liver fibrosis due to the efficacy of available treatments.Some drugs with potent anti-fibrotic effects in pre-clinical models are now waiting to be further evaluated in clinical trials (Table 2).The promising preventive and therapeutic treatments for liver fibrosis, including treatment of hepatitis viral infection (e.g., Peginterferon Alfa 2a), transplantation of mesenchymal stem cells, bariatric surgery for patients with obesity and NAFLD, dietary modification (e.g., Mediterranean diet or Calorie-restricted diet).
Furthermore, deliver system can be applied to increase the efficiency of anti-fibrotic treatments.For example, BMS-986263, a lipid nanoparticle, has been applied to deliver small interfering RNA to degrade mRNA of heat shock protein 47, a key collagen chaperone involved in the pathogenesis of fibrosis.Treatment of MS-986263 in patients with HCV infection and sustained virologic response improved the Ishak score, the histology activity index score for levels of liver fibrosis[143].Many other types of nanoparticles have been applied to treat liver fibrosis or its causing chronic liver disease, such as Fibroblast growth factor 2 conjugated superparamagnetic iron oxide nanoparticles[144], cerium oxide nanoparticles[145], and silymarin-conjugated gold nanoparticles[146].
CONCLUSION
Liver fibrosis accompanies the progression of chronic liver diseases independent of their etiologies.The initiation and progression of liver fibrosis are mainly driven by liver inflammation and hepatocyte or cholangiocyte injury and damage, resulting in the activation of HSCs and their differentiation into ECM protein-producing myofibroblasts.Thus, current therapeutic options for liver fibrosis are to prevent the initial causing factors for liver inflammation, hepatocyte cell death and oxidative stress.Unfortunately,the reverse of liver fibrosis is slowly and frequently impossible for advanced fibrosis or cirrhosis.Liver transplantation is the only therapeutic option for the late stage of liver cirrhosis and cancer.To avoid the life-threatening stage of advanced liver fibrosis and cirrhosis, anti-fibrotic treatments including biological, medicines, dietary change, and behavior prevention are needed.Currently, promising treatments for liver fibrosis are still the preventive strategies, such as treatment of hepatitis viral infection (e.g., Peginterferon Alfa 2a), inhibition of the progression of MAFLD and obesity (e.g., bariatric surgery), dietary modification (e.g., Mediterranean diet or Calorie-restricted diet).In addition, nanodelivery systems have been applied to improve the treatment efficacy and specifically deliver the treatments.Pre-clinical and clinical evaluations for new treatments of liver fibrosis are required while we still lack currently effective strategies for liver fibrosis treatment.The treatment efficacy can be evaluated by histological staining methods, imaging methods, and serum biomarkers, as well as fibrosis scoring systems, such as FIB-4, APRI, and NFS.Although many anti-fibrotic candidate agents have shown robust effects in experimental animal models, their anti-fibrotic effects in clinical trials are less clear.The development of patient-derived organoid models for liver fibrosis may advance the development of compounds with anti-fibrotic properties in the future.In addition, new delivery systems can improve the efficacy of potent treatments and reduce the side effects of therapy.Meanwhile, additional clinical studies are required to confirm the efficacy and safety of treatments.
FOOTNOTES
Author contributions:Zhang CY, Liu S, and Yang M designed and collected data, wrote, revised, and finalized the manuscript; all authors contributed equally and shared the first authorship.
Conflict-of-interest statement:The authors declare no conflicts of interest.
Open-Access:This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers.It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BYNC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is noncommercial.See: https://creativecommons.org/Licenses/by-nc/4.0/
Country/Territory of origin:United States
ORCID number:Chun-Ye Zhang 0000-0003-2567-029X; Shuai Liu 0000-0001-9695-2492; Ming Yang 0000-0002-4895-5864.
S-Editor:Ma YJ
L-Editor:A
P-Editor:Yuan YY
5Hou W, Syn WK.Role of Metabolism in Hepatic Stellate Cell Activation and Fibrogenesis.Front Cell Dev Biol2018; 6:150 [PMID: 30483502 DOI: 10.3389/fcell.2018.00150]
杂志排行
World Journal of Hepatology的其它文章
- Ductular reaction in non-alcoholic fatty liver disease: When Macbeth is perverted
- Recent advances in pathophysiology, diagnosis and management of hepatorenal syndrome: A review
- Tumor budding as a potential prognostic marker in determining the behavior of primary liver cancers
- Role of vascular endothelial growth factor B in nonalcoholic fatty liver disease and its potential value
- Acute pancreatitis in liver transplant hospitalizations: Identifying national trends, clinical outcomes and healthcare burden in the United States
- Lower alanine aminotransferase levels are associated with increased all-cause and cardiovascular mortality in nonalcoholic fatty liver patients
