Neutrophil elastase: From mechanisms to therapeutic potential
2023-05-29WeilinZengYingqiuSongRunzeWngRongHeTinluWng
Weilin Zeng , Yingqiu Song , Runze Wng , Rong He , Tinlu Wng ,*
a Department of Radiotherapy, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang,110000, China
b Department of Surgery, Cancer Hospital of China Medical University, Liaoning Cancer Hospital and Institute, Shenyang,110000, China
Keywords:
Neutrophil elastase
Sivelestat
COVID-19
Pathophysiological mechanisms
Therapeutic potential
ABSTRACT Neutrophil elastase (NE), a major protease in the primary granules of neutrophils, is involved in microbicidal activity.NE is an important factor promoting inflammation, has bactericidal effects, and shortens the inflammatory process.NE also regulates tumor growth by promoting metastasis and tumor microenvironment remodeling.However, NE plays a role in killing tumors under certain conditions and promotes other diseases such as pulmonary ventilation dysfunction.Additionally,it plays a complex role in various physiological processes and mediates several diseases.Sivelestat, a specific NE inhibitor, has strong potential for clinical application,particularly in the treatment of coronavirus disease 2019(COVID-19).This review discusses the pathophysiological processes associated with NE and the potential clinical applications of sivelestat.
1.Introduction
Neutrophil elastase(NE),a serine protease expressed in primary neutrophils, decomposes several substances, including elastin,collagen,and fibronectin.NE hydrolysis accounts for approximately 80% of the total protease hydrolysis activity in the human body,and promotes inflammation, bacterial infection progression, and the hypersecretion of mucus.The levels and activity of NE reflect disease state and severity [1].Although NE is associated with the development of several diseases, it plays a role in cancer [2] and inflammation resistance [3].
In China,sivelestat,a specific inhibitor of NE with several clinical applications, has been approved for using in patients with coronavirus disease 2019 (COVID-19) [4].The pathophysiological processes mediated by NE and the clinical applications of sivelestat remain to be fully investigated.In February 2022, the Chinese Expert Consensus on expert guidance on the clinical application of sivelestat was issued [5].However, more studies are needed to further support the clinical use of sivelestat.
This review presents 10 pathophysiological processes associated with NE, which may contribute to a clearer understanding of NErelated diseases.The potential clinical applications of sivelestat are also presented in the review, to support the developing of the potential of sivelestat to benefit patients in a wider range of clinical applications.
2.Pathophysiological processes associated with NE
2.1.Anti-infection effects
Inflammation, a comprehensive physiological response to pathogens, viruses, and bacteria, is an important part of human biology.It provides protection from harmful environmental factors by strengthening the homeostasis defense and the functional and structural integrity of tissues [6].
As a whole, the inflammatory process is extremely complex.First, in the early initiation stage of inflammation, a variety of signaling molecules such as pro-inflammatory cytokines, chemokines, vasoactive amines, and eicosane are activated.These inflammatory mediators not only lead to increased vascular permeability resulting in bodily damage, but also recruit neutrophils, monocytes, and other immune cells.Locally enriched immune cells have the role of fighting against pathogens and toxic substances produced by the body's overreactive response.Finally,macrophages clear away the immune cells that die after performing their immune function.Many immune cells are involved in the entire inflammatory process, such as vascular endothelial cells(ECs), neutrophils, monocytes, mast cells, T cells, myeloid-derived suppressor cells (MDSCs), basophils, and eosinophils [6].The specific process is discussed below.
2.1.1.Vascular ECs
Vascular ECs have a role in the division of blood vessels from subcutaneous tissue.During inflammation, ECs are activated through the tumor necrosis factor receptor TNFR/interleukin(IL)-1 pathway and nuclear factor-kappa-B (NF-κB) signal transduction.Activated ECs express adhesion molecules (P- and E-selectin,intercellular adhesion molecule (ICAM)-1) and CXC chemokines(CXCL8, CXCL-2, CXCl-2, TNFR/IL-1), as well as complement C5a[7,8].
2.1.2.Neutrophils
Neutrophils are the largest group of white blood cells in the blood and play an irreplaceable role in fighting pathogen invasion.After inflammation occurs,neutrophils rapidly converge to the site of inflammation through a multi-step adhesion cascade.After trauma, ECs express P-selectin rapidly, which promotes the adhesion of neutrophils to ECs.Adherent neutrophils then interact with vascular EC cadherin platelet EC adhesion molecule-1,ICAM-1,and connection adhesion molecule family members to penetrate the vascular endothelium and migrate to the site of inflammation.Neutrophils arriving at the site of inflammation act against the pathogen through a number of processes, including the involvement of phagocytic microorganisms,production of reactive oxygen species (ROS), secretion of NE, and formation of neutrophilic extracellular traps (NETs).Notably, neutrophils work collaboratively to digest microorganisms by releasing NE, nicotinamide adenine dinucleotide phosphate (NADPH) oxidase, and myeloperoxidase (MPO), as well as ROS.In other words, NE can act as a weapon for neutrophils to destroy pathogenic microorganisms[9,10].
2.1.3.Monocytes
During the progression of inflammation, the expression of macrophage colony-stimulating factor(M-CSF)is increased,which promotes the conversion of monocytes to macrophages.Specialized proresolving mediators have been reported to upregulate microRNAs targeting inflammatory genes in macrophages and inhibit the translation of inflammatory cytokines.Macrophages can express elevated 15-lipoxygenase and transforming growth factor(TGF)-β levels to promote wound repair.Besides, it has been reported that FcγR-mediated severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of monocytes activates inflammation [10].
2.1.4.Mast cells
Mast cells are long-lasting immune cells found in most tissues of the body, such as the skin, airways, and intestines.After inflammation occurs,the number of mast cells increases rapidly.Through microbial interaction with pattern recognition receptors,mast cells are activated to release more histamine,proteases,and members of the IL-1 family [11].
2.1.5.T cells
T cells are activated after inflammation,and the activated T cells differentiate into different T cells, including TH1 cells that can secrete interferon tumour necrosis factor (TNF)-α, IL-2 and interferon (IFN)-γ under the stimulation of TNF-α and IFN-γ; TH2 cells that secrete IL-4, IL-5, IL-9, and IL-13 formed from the stimulation of IL-4 or IL-10; Treg cells that secrete immunosuppressive factors(such as IL-10 and TGF-β) under TGF-β stimulation; CD4+ T cells that play important roles in the production of virus-specific antibodies; CD8+ T cells that produce IFN-γ and TNF-α and kill virusinfected cells.These cells work together to fight off pathogens[12].
2.1.6.MDSCs
MDSCs can accumulate in the inflammatory tissue after inflammation.MDSCs can degrade L-arginine,produce ROS,secrete anti-inflammatory cytokines (including IL-10 and TGF-β),downregulate IL-12 and prostaglandin E2,and inhibit immune cell activity through these pathways [13].Prostaglandins are also involved in inflammation.During inflammation, the level of prostaglandins changes dramatically,especially during the acute phase of inflammation, and before the invasion of neutrophils and other immune cells,prostaglandin production increases significantly.The main effect of prostaglandins involves causing two classic signs of inflammation: redness and pain.Yao et al.[14] found that prostaglandins can regulate neutrophils and other immune cells,and play an anti-inflammatory role.In addition, Robb et al.[15] found that prostaglandins inhibit the production of ROS via neutrophils.Thus,prostaglandins are closely related to neutrophils.Besides, the potential roles that non-steroidal anti-inflammatory drugs (NSAIDs)and prostaglandins may play during SARS-CoV-2 infection and the development and progression of COVID-19 have been reported.
2.1.7.Basophils and eosinophils
Basophils are known to make up a small percentage of the blood, but they play an important role in allergies and inflammation.Studies have shown that with the action of IL-18 and IL-33,basophils release various cytokines and pro-inflammatory mediators including macrophage inflammatory protein (MIP)-1, granulocyte-M-CSF, IL-4, IL-13, IL-6, IL-9, C-C chemokine ligand 5(CCL5),and monocyte chemotactic protein-1,which may affect the inflammatory process.Eosinophils play an important role in fighting parasitic, viral, fungal, and bacterial infections [16].
The above is a general description of the roles of different immune cells in the inflammatory process.NE,as the main character of this review,is considered as an active promoter of inflammation and is present in the airways of patients with various lung diseases.As early as 1994,D¨oring[17]concluded that NE is closely related to inflammation.NE exposure induces airway remodeling and interrupts epithelial repair.In addition, NE directly activates inflammation by increasing cytokine expression and release and indirectly activates inflammation by triggering extracellular trap and exosome release, thereby amplifying protease activity and inflammation in the airway[1].
NE kills pathogenic bacteria through NETs,which are produced by activated neutrophils and exert antimicrobial activity at low concentrations.When the nuclear membrane collapses and chromatin disintegrates, enzymes (including NE) typically present in secreted granules attaching to the scattered chromatin.As the membrane disintegrates, chromatin is scattered into the extracellular matrix.DNA is a major skeleton of NETs;the DNA skeleton of NETs captures and kills pathogens using a high concentration of antibacterial substances and limits the diffusion of inflammation between tissues.NETs also serve as a physical barrier to prevent the spread of bacteria [18].
NE as well protects against degrading toxic substances released by bacteria.Exposure to cytokines, chemokines, or bacterial products can lead to the accumulation of NE and anti-infective factors such as microbicide peptides and ROS produced by NADPH oxidase and MPO at the infection sites.NE and anti-infective factors digest microorganisms,thus playing an important role in microorganism death [19].NE is also involved in the inflammatory response of extracellular matrix remodeling, which generates neutrophil and monocyte chemotaxis by hydrolyzing extracellular matrix fragments.This inflammatory response attracts additional antiinflammatory factors, thereby shortening the process of infection.Furthermore, damaged basement membranes release laminin fragments to promote neutrophil migration and antioxidant factor recruitment,thus accelerating the inflammatory process[3](Fig.1).
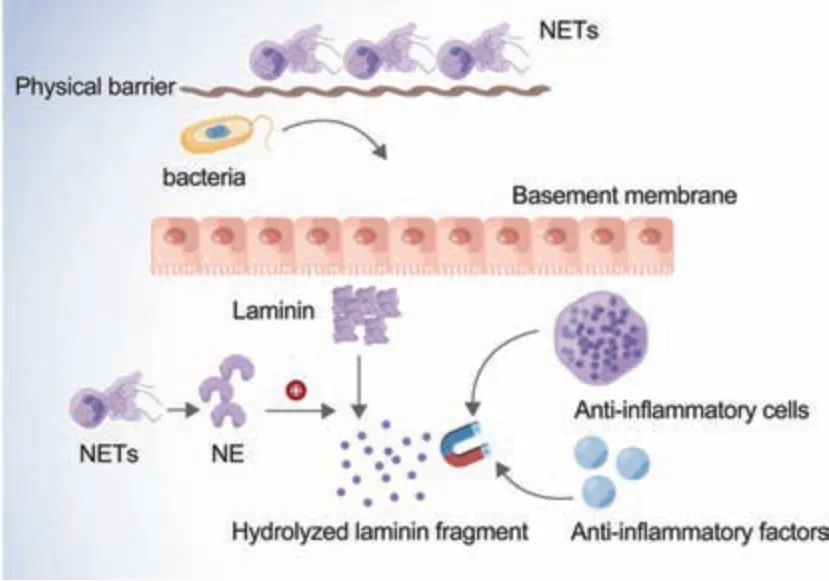
Fig.1 .The specific process of the anti-infection effect of NE.NE: neutrophil elastase.NETs: neutrophilic extracellular traps.
2.2.Role in pulmonary ventilation dysfunction
NE is associated with several lung diseases, including cystic fibrosis (CF), chronic obstructive pulmonary disease (COPD),bronchiectasis, and bronchopulmonary dysplasia (BPD) [1].
NE impairs the innate immune and ciliary function and exacerbates inflammation in the lungs of CF patients via various mechanisms.NE can lead to airway surface liquid (ASL) dehydration and mucus obstruction.ASL,a thin layer of fluid that covers the surface of the respiratory epithelium, is the first barrier between the respiratory epithelium and the environment that plays a critical part in maintaining airway function.NE can activate the apical epithelial sodium channel, which in turn increases sodium uptake from the ASL.Furthermore,NE can lead to a self-perpetuating cycle of neutrophil inflammation in the ASL.The underlying mechanism involves NE upregulating the epithelial expression of CXCL8.The CXCL8 alias IL-8 is a chemokine that increases neutrophils releasing NE.NE can further maintain large-scale proteolytic inflammation by degrading some proteases,such as secretory leukocyte protease inhibitor(SLPI)and tissue inhibitor of metalloprotease-1(TIMP-1),and activating some proteases,such as neutrophil metalloprotease gelatinase.In short,excess NE can degrade antimicrobial proteins in the ASL,thereby cleaving opsonin and its receptors and generating oxidative stress in airway epithelia,ultimately damaging the innate and adaptive immune systems.Also,NE,ROS MPO,and bacteria can activate the release of NETs and increase the viscoelasticity of airway mucus.NETs include several proteins (such as NE) that contribute to the progression of airway inflammation and lung disease in patients with CF [20].
NE plays a role in lung dysfunction in patients with COPD,where it and other proteases co-regulate protease-antiprotease activity in the airways.Studies have found that bacterial infection is an important factor in the progression of COPD.Bacterial products can cause lung tissue damage.As one of the most important means for neutrophils to play an antibacterial role,NE can be activated when a specific signal is transmitted, playing a key role in antibacterial immunity [1].Thulborn et al.[21] applied certain statistical methods in the clinical sputum analysis of COPD patients and found that NE increased in the COPD exacerbation stage.Therefore, they predicted that NE may be a feasible biomarker to identify bacterial exacerbation in COPD patients.
MMP-12 and macrophage elastase have been reported to mediate emphysema.NE activates MMPs and macrophage elastase and degrades the major inhibitor of MMPs, TIMP-1.NE can associate with MPO to activate the release of NETs into the airway environment,thereby increasing the neutrophil granule proteolytic and proinflammatory activities of NE.The abundance of NETs in the airways of patients with COPD is related to reduced neutrophil phagocytosis and pulmonary ventilation dysfunction [22].
Decreased α-1-antitrypsin can weaken the antagonistic effect of NE and disrupt the alveolar matrix and structure [23].Together,these processes accelerate the formation of COPD.
NE also plays a role in BPD pathogenesis,where inflammation is an important trigger and NE is a key mediator[24].A study using a transgenic mouse model of mimic airway inflammation found that NE-induced mouse models had a BPD-like lung phenotype during development,whereas NE-deficient mouse models showed healthy alveolar structure.In this study, NE was elevated in the airways of mice with BPD and other pulmonary diseases, strongly suggesting that excessive NE causes protease hydrolysis, leading to abnormal lung development.In addition,fibroblast cultures of mouse models of BPD and other diseases revealed that NE may downregulate the mRNA expression of elastin assembly genes, suggesting its involvement in the pathophysiological process of BPD.In short,the NE-exposed mice may have an abnormal lung structure,similar to that in emphysema [25] (Fig.2).

Fig.2 .NE mediates pulmonary ventilation dysfunction.NE: neutrophil elastase; ASL:airway surface liquid; CXCL8: chemokine ligand 8 or interleukin 8; SLPI: secretory leukocyte protease inhibitor; MMP-9: matrix metalloproteinase 9; ROS: reactive oxygen species; TIMP-1: tissue inhibitor of metalloproteinase-1.
NE-mediated pulmonary ventilatory dysfunction is not limited to these diseases.NE can damage alveolar and vascular structures and mediate several pathophysiological processes in lung diseases,including COVID-19.
2.3.Acceleration of the progression of chronic liver disease and cirrhosis
Portal hypertension(PHTN)is a common sequela of chronic liver disease and the main cause of liver transplantation and death in patients with cirrhosis.The most significant feature of PHTN pathophysiology is that the liver vascular structures distort chronic liver congestion.Sinus thrombosis and mechanical forces are two key factors of chronic congestion driving liver fibrosis.In addition,sinus thrombosis is related to the progression of chronic liver disease [26].
To understand the specific mechanism underlying PHTN and chronic liver cirrhosis, several biological concepts should first be clarified, namely, tissue-specific ECs, integrins, and Piezo protein.Tissue-specific ECs secrete angiogenic factors that are key regulators of metabolism,regeneration,and organ homeostasis,of which liver sinusoidal ECs (LSECs)are one type[27].Integrins, which are transmembrane proteins,are involved in sensing and responding to biochemical and mechanical signals through linking cellular cytoskeleton and extracellular matrix molecules[28].Piezo protein is a component of mechanically sensitive non-selective ion channels that participate in the regulation of vascular tension.Integrin interacts with the Notch pathway,a mechanically sensitive signaling pathway that is important for hepatic vasculature and endothelial function.Furthermore, the Notch 1 receptor modulates sinus structure[29].LSECs can secrete mechanically sensitive angiocrine signals to promote PHTN by promoting the aggregation of neutrophils and the formation of NETs and microthrombi in LSECs.The mechanical factors involved in hepatic sinusoidal capillaries upregulate the expression of neutrophil chemokine CXCL1 and recruit neutrophils into hepatic sinuses, thereby forming neutrophil-platelet complexes and NETs.
NETs and NE bound to NETs promote hepatic sinus microthrombosis and fibrosis.NETs can be regarded as prothrombotic structures prior to deep vein thrombosis.Neutrophils are involved in thrombosis formation and transmission.PHTN is driven by microvascular thrombosis and fibrin through sinus pressure and spatial effects associated with NE and NETs [26].
Neutrophils accumulate during the early stage of thrombosis to promote the propagation of the coagulation cascade and aggregate with platelets or other thrombogenic mediators[26].Thrombosis is promoted by neutrophil interactions with platelets through NET formation,while coagulation is initiated or propagated by a variety of cellular components of neutrophils and NETs such as histones,granular proteins, as well as NE.
2.4.Activation of dormant cancer cells
The main cause of death among patients with cancer is metastases to distant tissues.Tumor cells are in a dormant state,and not detectable, until metastasis occurs (although dormant cancer cells are present in the blood or bone marrow).Circulating tumor cells(CTCs) are in the bloodstream, while disseminated tumor cells(DTCs)are in the bone marrow,both of which may remain dormant prior to proliferation.When cancer cells begin to proliferate,T and NK cells eliminate DTCs and CTCs, thereby preventing them from reaching detectable levels.Previous findings have indicated that increased extracellular matrix deposition and angiogenesis may contribute to the metastasis of tumor cells [30-32].
It has been reported that elevated plasma C-reactive protein levels are correlated with reduced disease-free survival in patients with breast cancer, suggesting that inflammation may promote tumor awakening [33].Smoking is significantly related to an increased risk of recurrence and death of patients with breast cancer [34,35].In one study, exposing mice to tobacco smoke resulted in a two-fold increase in lung metastasis [36].
Inflammation is commonly mediated by neutrophils and other inflammatory cells,which are critical for awakening DTCs and CTCs in experimental models [37].NETs are formed after systemic bacterial infection or surgical stress, thereby promoting cancer metastasis [38,39].The molecular pathway of NET production involves the citrullination of histones,de-condensation of chromatin,and disintegration of the nuclear membrane.When the plasma membrane ruptures,the protease-associated chromatin fibers and some proteases from neutrophils, including MMP-9, cathepsin G,and NE, are released into the extracellular space, which awakens dormant tumor cells [40].
By inhibiting the production of NETs and the degradation of NET-DNA skeletons,previous studies have confirmed that NETs are the most important factors that induce dormant cancer cells and activate DTCs and CTCs via extracellular matrix remodeling.The NET-DNA skeleton has a high affinity for laminin,thereby attracting NE, MMP-9, and other elastases [40].NE and MMP-9 hydrolyze laminin-111 in the extracellular matrix, exposing the new antigen and activating the integrin signaling pathway in DTCs and CTCs.Activated integrin signals can alter the signaling pathways mediated by p38α (p38α-MSK1-GATA3/FOXA1 and p38α-NR2F-SOX)that typically maintain dormancy in DTCs and CTCs.As one of the most important pathways of mitogen-activated protein kinase(MAPK)signaling pathways,the p38 MAPK pathway plays a critical part in controlling cell proliferation, differentiation, and survival.Remarkably,tumor cells can change the p38 MAPK pathway state to achieve their own proliferation and invasion.Signal integration by p38 MAPK pathways as well as c-Jun N-terminal kinase (JNK) occurs in cancer development.p38α, a protein encoded by the MAPK14 gene and found in high levels in most cells, has many functions.Studies have shown that p38α can downregulate cell cycles and induce apoptosis, which may be achieved through the downregulation of cyclin,upregulation of cyclin dependent kinase(CDK) inhibitors, and regulation of tumor suppressor p53.At the molecular level, the inhibitory effect of p38α on proliferation may be related to the activity levels of different kinases and interactions between different signaling pathways[41,42].In addition,p38α has been shown to play a critical part in the resistance to proliferation at the beginning of differentiation.Although p38α is usually related to antiproliferative functions, it has also been shown to promote proliferation in specific cells, such as hematopoietic cells and several specific tumor cells [43].
Therefore,when the integrin signaling pathway is activated,the state of p38α will be changed.The related signaling pathway will also be changed, causing the rapid proliferation of DTCs and CTCs,and leading to tumor recurrence [44,45] (Fig.3).

Fig.3 .NE activates dormant cancer cells.NE: neutrophil elastase; NETs: neutrophilic extracellular traps; MMP-9: matrix metalloproteinase 9.
2.5.NE promotes tumor cell proliferation
Neutrophils can not only infiltrate tumors but also modify tumor growth and invasiveness [46-48].For example, lung cancer cells can drive neutrophil recruitment by elaborating CXC chemokines [49].Thus,in some cases, tumor-associated neutrophils may not represent the defense of the host.Furthermore, neutrophil infiltration in tumor cells may be related to poor clinical outcomes[50].
During inflammation, neutrophils secrete more NE, which are directed to specific sites to promote the proliferation of cells at those sites.The discovery that proteases secreted by one kind of cells can alter the proliferative state of another expands the list of potential substrates and functions of proteases [51].To determine whether NE also has a proliferative effect on tumor cells, researchers have used mouse models to conduct a series of experiments and shown that NE can directly enter the endosomal compartment of tumor cells, where it degrades insulin receptor substrate-1(IRS-1),eventually leading to the proliferation of tumor cells[52].Such immune precipitation studies have shown that the interaction between phosphatidylinositol 3-kinase (PI3K) and potent mitogen platelet-derived growth factor receptor increases,as NE degrades IRS-1,while the PI3K axis is skewed toward tumor cell proliferation.In addition, this study identified IRS-1 as a key regulator of PI3K in tumor cells, and that NE reduces IRS-1.When IRS-1 is depleted by NE-mediated degradation or its biological activity is altered, cancer susceptibility may increase.In addition, a recent paper reported that cancer-associated fibroblasts drive NETosis to support cancer progression,identifying amyloid β as the protagonist and potential therapeutic target[53].
2.6.Promotion of cancer metastasis
During invasion and metastasis, cancer cells encounter several obstacles, for example, basement membranes and surrounding tissue stromal matrices composed of elastin, collagen, as well as proteoglycan.The hydrolysis of these barrier agents is necessary for invasion and metastasis [54].
The hydrolysis of NE is strong and can hydrolyze fibronectin,proteoglycans, type IV collagen, and other extra-matrix proteins[48].It has been reported that cancer cells may produce NE to hydrolyze basement membranes and surrounding tissues.The production of NE in tumor cells is associated with metastasis and the invasion of adjacent tissues[55].Similar to neutrophils,tumor cells enhance their invasiveness by increasing their proteolytic activity.
In the case of specific clinical trials, it was found that epithelial breast and lung cancer cells produce NE.Indeed, the prognosis of patients with breast cancer worsens as NE levels increase [56].Inflammatory cell infiltration is also related to poor prognosis in patients with breast cancer [57].These data suggest NE's role as a biomarker for the identification of patients with more aggressive breast cancer and support the hypothesis that NE plays an active role in tumor progression in human breast cancer,leading to tumor metastasis regardless of cellular origin [58].
2.7.Remodeling of the tumor microenvironment
The tumor microenvironment is complex,with several types of cells and materials associated with tumor growth.Circulating neutrophils and MDSCs are associated with patient survival and can secrete NE and NETs, thereby remodeling the tumor microenvironment [59], although NE may promote tumor growth in the tumor microenvironment.
There are several mechanisms to explain the tumor-promoting activity of NE.Firstly, NE may directly stimulate proliferative pathways and induce MAPK signaling through the extracellular transactivation of membrane receptors, such as epidermal growth factor receptor(EGFR)and toll-like receptor 4(TLR4).In breast and prostate cancer cells,NE acts through MAPK to induce extracellular regulated protein kinases (ERK) phosphorylation and the transcription of ERK-dependent genes.Therefore, the preconditioning of MEK inhibitors may eliminate NE-induced proliferation [60,61].Secondly, NE may cleave and release multiple membrane-bound ligands, including EGF-like ligands, TGF-α, and platelet-derived growth factor to transactivate EGFR and promote tumor cell proliferation [62-64].Preconditioning with TGF-α-neutralizing antibodies has been shown to eliminate the downstream proliferative effects of NE in keratinocytes [65].Thirdly, NE activates the PI3KAkt proliferative pathway in lung cancer cells through the internalization and degradation of insulin receptor substrate (IRS)-1[66,67].
NE may also regulate other important growth-promoting processes in the tumor microenvironment.For example,NETs,possibly via NE,promote EC proliferation,motility,and vascularization[68].The formation of vasculature systems is crucial for tumor cell expansion and metastasis.In addition, NE may stimulate the release of vascular endothelial growth factor(VEGF)on the surface of tumor cells, which may subsequently activate tumor-associated EC proliferation [64,66].In a model of NE-related gene knockout mice, angiogenesis markers, including VEGF and CD31, were significantly reduced [69].Other neutrophil-derived proteases,such as MMP-9, may act cooperatively with NE to regulate tumor angiogenesis [70,71].NE can also affect the activity of several members of the MMP family via direct action on the proenzymes and inactivation of the activity regulators[72].
Studies have shown that neutrophils are closely involved in angiogenesis.Neutrophils can produce angiogenic cytokines(including VEGF and IL-8) and the effects of angiogenic enzymes(MMP-9,gelatinase B).In addition,further studies have shown that NE may regulate the biological functions of many molecules related to angiogenesis, and can regulate the activation of specific cell surface receptors.Wada et al.[64] found that NE phosphorylates EGFR rapidly,triggering the extracellular signal-regulated kinase 1 and 2 signaling pathways.It was also found that NE can increase platelet-derived growth factor-AA(PDGF-AA),PDGF-BB,and VEGF.VEGF is known to be a key regulator of tumor angiogenesis [73].Besides,it has been reported that there is a functional link between NETs and inflammatory angiogenesis [68].In summary, NE is closely related to angiogenesis in the tumor microenvironment,which plays an important role in cancer cell growth, thereby affecting tumor growth and metastasis.
2.8.Role in radiation-induced lung injuries
Radiation-induced lung injury is a well-known side effect in patients receiving thoracic radiation.The radiotherapy dose for chest malignancies is typically limited by the sensitivity of the healthy lung parenchyma since the lungs are extremely sensitive to the harmful effects of ionizing radiation [74,75].Among patients undergoing chest radiation therapy,those with lung cancer are the most likely to develop lung injury[76].
Inflammatory lung injuries not attributed to radiation are characterized by a local imbalance between proteases and antiproteases [76,77].The imbalance of pro-inflammatory and antiinflammatory factors is correlated with tissue damage and systemic involvement [78].In radiation-induced lung injuries, homeostasis between pro- and anti-inflammatory regulators is necessary to mitigate the inflammatory process, in which NE is heavily involved.Under physiological conditions, neutrophils,macrophages,and ECs produce various NE inhibitors in the tissues at the injury site or the liver, which upregulate the response to inflammatory stimuli [79,80].The reduction of NE inhibitors may lead to excessive proteolytic damage and poor prognosis [81].
Lung irradiation can induce release of cytokines immediately,which in turn activates neutrophil migration and NE release.Researchers have speculated that blocking NE may lead to the suppression of further release of cytokines and subsequent lung injury.Sivelestat,as a specific inhibitor of NE,has been used to investigate the mitigation of radiation-induced lung injury in mice, and can effectively reduce radiation-induced lung injuries in mice by suppressing NE activity and excessive inflammatory reactions.All of the above results indicate that NE plays a part in radiation-induced lung injury [82] (Fig.4).
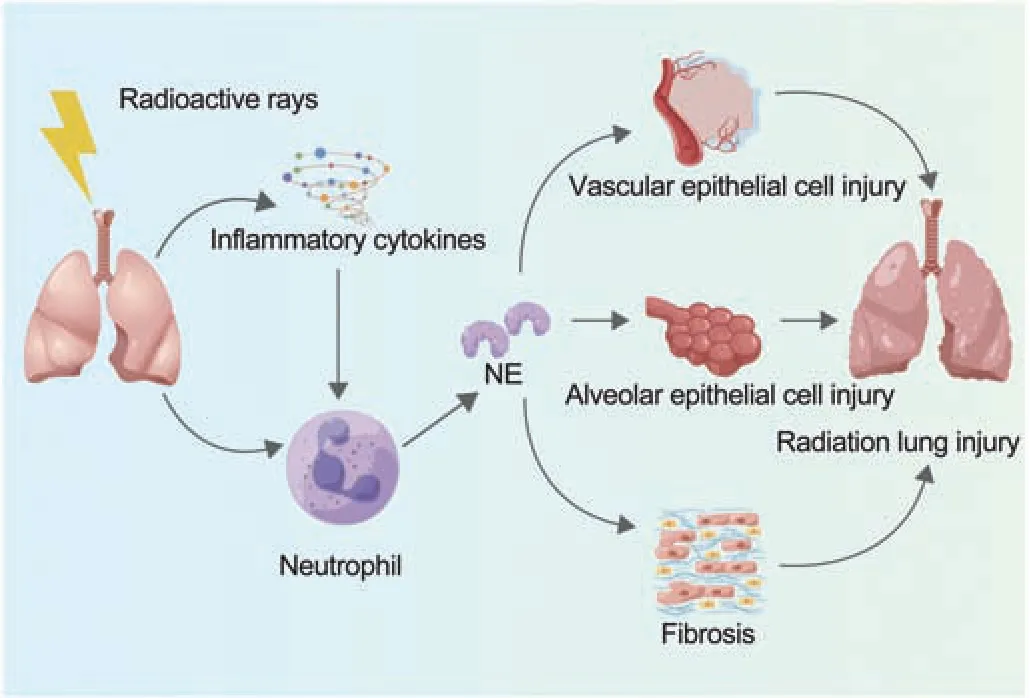
Fig.4 .NE mediates radiation-induced lung injuries.NE: neutrophil elastase.
2.9.Role in immune-associated pneumonia
Immunotherapy is an effective tumor treatment strategy that has benefitted many patients.Immune checkpoint inhibitors(ICIs)typically target proteins that negatively regulate the T cellmediated host immune response to tumor cells, including programmed death 1, programmed death-ligand 1, and cytotoxic Tlymphocyte-associated protein 4 (CTLA-4), to enhance immune activation and antitumor responses [83].ICIs have been approved by the US Food and Drug Administration for use against various solid and hematological malignancies [84].The use of ICIs is predicted to increase; however, with the increasing use of immunotherapy, the incidence of immune-related adverse events (irAEs)has also increased.The damage caused by irAEs is usually severe and permanent, including decreased quality of life and low treatment efficacy or death[85].Multiple organs and organ systems are affected by irAEs, such as the gastrointestinal tract, kidney, liver,skin, musculoarticular system, and hematologic system.Checkpoint inhibitor pneumonitis (CIP) is the commonest irAE [86].
The pathogenesis of CIP has not been clarified; the proposed mechanisms include four aspects:1)continuously activated T cells simultaneously attacking the tumor and other autoantigens;2)the activation of preexisting autoantibodies; 3) excessive secretion of inflammatory factors; 4) complement-mediated inflammatory responses caused by direct binding of the CTLA-4 antibody to CTLA-4 expressed in healthy tissues.In addition,the genetic characteristics of individuals and the composition of microorganisms may play roles in the pathogenesis of CIP.CIP may also be related to the inflammatory state of the lungs and the tumor inflammatory microenvironment [87].NE is one of the most important factors mediating pulmonary inflammatory states [1] and tumor inflammatory microenvironments [59].
2.10.Selective killing of cancer cells and attenuation of tumorigenesis
Cancer is a mutational disease with high spatial and temporal genetic heterogeneity.The goal of cancer treatment is to overcome this heterogeneity and eradicate tumor cells while preserving healthy tissues.Therefore, it is challenging to develop drugs that have broad efficacy against multiple cancer types while maintaining specificity with minimal damage to surrounding healthy tissues.The innate immune system protects against bacteria, fungi,and other pathogenic microorganisms,which have far more genetic variability than cancer.This may provide new insights and possibilities for the study of highly specific methods to kill tumor cells[88].As a critical strategy of the innate immune system,neutrophils can kill pathogens with a wide range of genetic diversity;the killing may also be effective against cancer cells, and the therapeutic potential of neutrophils has been explored in clinical trials [89,90].
According to existing reports, neutrophils can promote tumorigenesis and may have the potential to kill tumors.These contrasting functions may be attributed to differences between species, tissue source, or activation status [91-93].Meanwhile,studies on mice have revealed that tumor cells hijack neutrophils to release molecules that promote metastasis and diffusion [94].Increased tumor-associated neutrophil accumulation is a marker of poor prognosis in several types of cancer.
However, some papers have reported that neutrophils mediate the specific killing of tumor cells via the release of NE[2].In human cells,neutrophils release catalytically active NE to kill various types of tumor cells, and the killing effect is highly specific and weak against non-cancer tissues, which may be associated with the mutual effect of NE with histone H1 isoforms.After entering cancer cells,NE can hydrolyze and release CD95 death domain,which will ultimately damage DNA, release ROS, and induce cell apoptosis.Additionally,the mutual effect of NE with histone H1 isoforms may produce CD8+ T cell-mediated effects in vitro to prevent distant metastasis.NE can kill various types of cancer cells, while its toxicity to non-cancer cells is minimal,suggesting its potential as a broad anticancer therapy [2,56,95] (Fig.5).
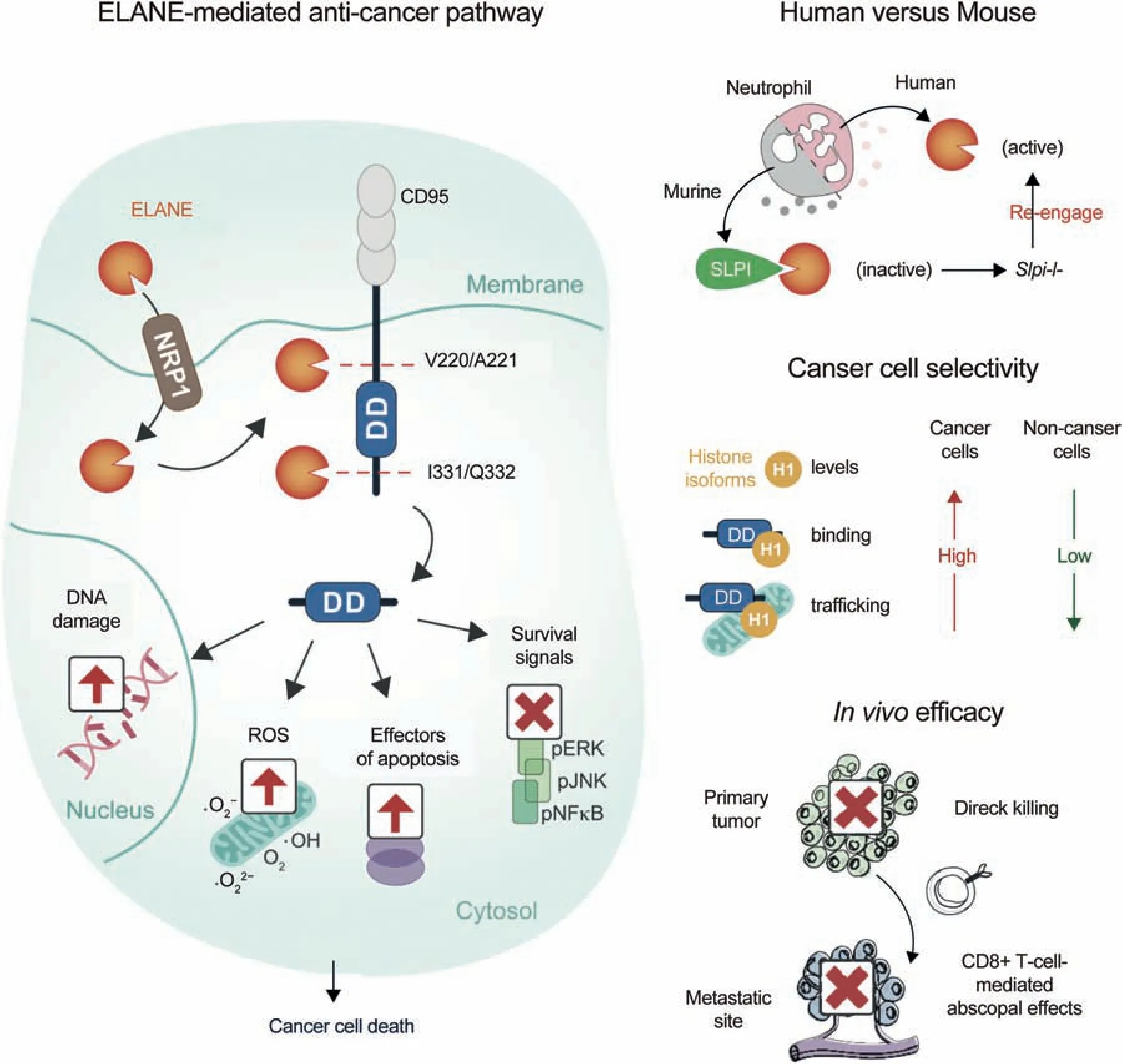
Fig.5 .NE kills cancer cells and attenuates tumorigenesis.NE: neutrophil elastase; ELANE: neutrophil elastase; ROS: reactive oxygen species; pJNK: phosphorylated c-Jun N-terminal kinase;pERK:phosphorylated extracellular regulated protein kinases;pNFκB:phosphorylated nuclear factor-kappa-B;DD:death domain;NRP1:neuropilin 1;SLPI:secretory leukocyte protease inhibitor.Reprinted from Ref.[2] with permission.
NE has a positive effect on the human body under physiological conditions.This indicates that the suppression of NE to treat uncontrolled NE damage must be conducted carefully.
3.The potential clinical applications of sivelestat
3.1.Sivelestat for the treatment of COVID-19
Most COVID-19 patients present with symptoms of fever, dry cough, myalgia, tiredness, and diarrhea.However, symptoms vary based on patient age and comorbidities.Due to patient immobility,conditions of inflammation, hypoxia, and disseminated intravascular coagulation (DIC), the risk of thromboembolism in patients with COVID-19 is high.During COVID-19, fibrin degradation products significantly increase, and significant reductions in platelet levels have been observed in severe cases and deaths [96].
Patients with COVID-19 can exhibit severe symptoms within a few days,including acute lung injury(ALI)/acute respiratory distress syndrome(ARDS), respiratory failure,sepsis, and heart failure [97].Animal models have revealed that significant levels of inflammatory and immune responses may lead to cytokine storms and apoptosis of ECs[98].Vascular permeability and leakage increase,as well as T cells and macrophages responding abnormally,results in ALI/ARDS,which may ultimately lead to death.Viral infections may induce the production of oxidized products or oxidative stress, thereby exacerbating inflammation-mediated COVID-19 pathology via neutrophils and other inflammatory cells[99].
SARS-CoV-2 involves structural proteins and non-structural proteins, among which the structural proteins play a key role in the pathogenesis of SARS-CoV-2.They mainly include membrane,envelope, nucleocapsid, and spike proteins, which are involved in the process of virus entry into host cells for replication [96,100].Studies have shown that NE activates the spike protein of coronavirus,which changes the state of the virus[101].Spike protein can be activated by angiotensin-converting enzyme 2(ACE2)in human cells, which is the main way for the SARS-CoV-2 to infect human cells.Because ACE2 is found in multiple organs throughout the body (such as the esophagus, lungs, liver, and small intestine),SARS-CoV-2 can cause infection in multiple organs throughout the body [102].The formation of interstitial fibrosis and chronic inflammation has been found in the lung histological examinations of patients who died from COVID-19,suggesting that the enhanced NE activity led to the abnormal remodeling and degeneration of the lungs[103].The application of sivelestat in the treatment of COVID-19 has two main purposes.First, sivelestat can specifically inhibit the effect of NE to reduce lung injury in COVID-19 patients;second,sivelestat can inhibit the action of spike protein to limit the transmission ability of the virus [104].
Sivelestat, as an inhibitor of NE, may reverse neutrophilmediated vascular permeability by ameliorating alveolar epithelial and vascular endothelial damage.Sivelestat can inhibit the over-activation of signaling pathways and neutrophil chemotaxis to improve the survival rate of ALI/ARDS patients[4].The function of NE in promoting DIC in ARDS and sepsis patients has been highlighted, and COVID-19 patients are more prone to thromboembolic events.In a clinical study, sivelestat was administered to patients upon admission to the Intensive Care Unit (ICU) and continued for 5 days in patients with ALI/ARDS and DIC.Compared to the control group, patients who received sivelestat had significantly improved lung injury scores and survival rates, a shortened length of stay in the ICU, and a reduced incidence of thrombosis [105].These findings suggest sivelestat as a promising agent for the management of ALI/ARDS or coagulopathy in patients with COVID-19 [4].
Sivelestat is the only drug approved for the treatment of ALI/ARDS.It provides physicians with a pharmaceutical treatment option for critically ill patients with respiratory diseases [106].
Sivelestat was the first investigational drug approved for COVID-19.However, Sivelestat is not currently included in treatment guidelines.The adverse effects of sivelestat include dyspnea,leukopenia, thrombocytopenia, liver damage, and jaundice.In clinical practice,the toxicity and side effects of sivelestat should be comprehensively considered.Patients should stop using sivelestat if serious adverse reactions occur.
3.2.Sivelestat for the treatment of pulmonary fibrosis
Pulmonary fibrosis is caused by various injuries to the lungs,involving acute lung injuries, irradiation, and drugs.As current therapies for pulmonary fibrosis are ineffective or only marginally effective, the major determinant of clinical outcomes in patients with fibrotic pulmonary diseases is the degree of fibrosis[107].
车间在需求申请赋码阶段计量单位基于车间的实际定额需求确定,比如油漆m,计量单位可以是升,也可以是kg;比如绑扎带,计量单位可以是个,也可以是m;而后期采购环节对于计量单位的要求,往往不完全与定额管理计量单位一致。
Interstitial lung diseases (ILDs) are characterized by alveolar inflammation of lung parenchyma and interstitial fibrosis pathological lesions, which result in active breathing difficulties, X-ray chest radiograph diffuse infiltrating shadows,restrictive ventilatory dysfunction, dispersion function decreases, and hypoxemia with different types of clinical manifestations of diseases tied to the core clinical and pathological entity.It is estimated that ILDs account for nearly one fifth of all lung diseases.Idiopathic pulmonary fibrosis(IPF) is characterized by a thickening of the alveolar wall with collagen deposition, an increased extracellular matrix, and focal monocyte infiltration [108,109].Since several forms of pulmonary fibrosis are associated with the initial inflammatory response,immune-mediated inflammation in the lungs is considered as a vital factor in the pathogenesis of IPF and other forms of fibrotic ILDs.NE may play a critical part in damaging the blood vessels in the lungs and alveolar walls.
In a bleomycin-induced pulmonary fibrosis mouse model, the intraperitoneal injection of sivelestat significantly reduced collagen content, pulmonary fibrosis scores, and the number of inflammatory cells in bronchoalveolar lavage fluid,all of which are indicators of the severity of pulmonary fibrosis[110].In this experiment,mice treated with high-dose sivelestat showed greater improvement in lung function than those treated with low-dose sivelestat.Therefore, sivelestat may be useful for the treatment of pulmonary fibrosis.However, further clinical trials are required.
3.3.Sivelestat for the treatment of COPD
When the lungs are exposed to harmful particles or gases, immune inflammatory cells (including neutrophils) in the airways respond, thereby inducing airway inflammation, which may eventually lead to COPD.Neutrophils are often recruited directly from the peripheral blood to increase their number.At the same time,neutrophils can produce elastolysis enzymes,including NE,to promote lung parenchymal destruction [25].
Neutrophils release excess NE into the extracellular medium during the COPD inflammatory process, which in turn degrades structural proteins, participates in pathogen killing, and plays a critical part in the modulation of inflammatory responses.Endogenous inhibitors of NE, such as elastase-specific inhibitor, α1-proteinase inhibitor, and SLPI, can protect against the damaging effects of extracellular NE.However, the imbalance between proteases and antiproteases may lead to tissue damage under certain pathological conditions.This imbalance may cause excessive NE to hydrolyze elastin,a structural protein that provides elasticity to the lungs.Excess extracellular NE may also lead to the cleavage of matrix proteins and inflammatory mediators [111].
In addition, NE can activate more proteases and induce the expression of neutrophil-associated chemokines and cytokines.An ultimate result of this is polymorphonuclear neutrophil-dominated airway inflammation.
Therefore, NE is a promising target, and sivelestat protects the lungs from NE-mediated tissue damage and controls excessive inflammatory responses.While there are various clinical treatments for COPD, the most effective option is to correct the imbalance between proteases and antiproteases and inhibit excess NE.Sivelestat is used clinically in China and Japan with satisfactory therapeutic results [112].
3.4.Sivelestat for the treatment of chronic liver disease and cirrhosis
PHTN is the major cause of liver transplantation and even death in patients with cirrhosis.The treatment of PHTN remains challenging; an understanding of the pathogenesis of PHTN will provide deeper insights into developing new therapeutic strategies[29].Current treatments for PHTN include drug therapies, endoscopic esophageal variceal ligation, endoscopic injection sclerotherapy,and transjugular intrahepatic portosystemic shunts(TIPS).These methods have significant curative effects and low invasiveness; however, endoscopic esophageal variceal ligation has a high rebleeding rate and poor long-term efficacy and may result in esophageal ulcers or fatal esophageal fistulas.Despite the presence of membrane-coated stents, TIPS treatment cannot completely prevent thrombosis and postoperative shunt stenosis.Hence, it is necessary to develop new, valid treatment strategies for hepatic encephalopathy and other complications of chronic liver disease.
In a mouse model of the partial ligation of the superior and inferior hepatic vena cava, mice treated with NE inhibitors had significantly reduced portal vein pressure and expressions of some markers of liver fibrosis compared with control mice.In addition,mice receiving sivelestat had significantly lower PHTN, less liver fibrin production, and reduced hepatic sinusoidal MPO precipitation than mice receiving a placebo,indicating that the formation of cirrhosis was slowed by sivelestat [26].
NETs participate in vascular thrombosis, and NE is a key component of the formation of NETs.Inhibiting NE through genetic or pharmacological means can reduce portal vein pressure in several models of chronic liver injury, indicating that inhibiting neutrophil recruitment and NE may be an effective therapeutic strategy against PHTN.
3.5.Sivelestat use during the perioperative period in patients with esophageal cancer
Despite the increasing use of multimodal therapy, surgery remains the main treatment for esophageal cancer.But in fact, the resection of esophageal cancer is highly invasive and prone to perioperative complications.The way to reduce the incidence of perioperative complications of esophageal cancer is to administer protease inhibitors or corticosteroids before the operation.Videoassisted thoracoscopic surgery (VATS) for esophagectomy was introduced in 2003 to reduce surgical injuries.
VATS has several advantages,such as reducing the production of NE and related cytokines, minimizing lung tissue damage, and producing therapeutic outcomes not inferior to those of traditional esophageal cancer resection.Although these treatment strategies have improved the postoperative courses of patients with esophageal cancer, postoperative pulmonary complications, such as ALI,remain common; therefore, new therapeutic strategies are warranted[113].
Sivelestat improves the respiratory status of patients with stressinduced ARDS/ALI, sepsis, and ventilator-induced lung injuries.Postoperative administration of sivelestat is effective in patients undergoing conventional transthoracic esophagectomy.In patients with thoracic esophageal cancer treated with VATS, postoperative administration of sivelestat improved postoperative hypoxia and reduced the number of days on mechanical ventilation.Hamster models of ALI suggest that pretreatment with sivelestat can suppress endothelial injuries caused by NE more effectively than treatment administered later in the disease course[114].
The prophylactic, perioperative administration of sivelestat in patients undergoing VATS esophagectomy is effective in relieving postoperative hypoxia, especially when administered during the initial stage of surgery.In addition,sivelestat partially shortens the duration of systemic inflammatory response syndrome to prevent ALI [115].Therefore, perioperative sivelestat may be effective against esophageal cancer.
3.6.Sivelestat used for alleviating early injuries after hepatectomy
The treatment strategies for patients with liver neoplasms depend on their physical condition; when possible, liver resection is performed.However, a large proportion of patients with liver tumors have cirrhosis.Partial hepatectomy reduces the volume of the functional liver, and patients with cirrhosis are more likely to have liver function deterioration.This makes appropriate and effective perioperative management extremely important.It has been reported that ischemic injuries initially activate inflammatory signals through the release of high mobility group box 1(HMGB1).HMGB1, a highly conserved nuclear protein widely distributed in mammalian cells, secreted by activated monocytes or macrophages, binds to DNA, participates in transcriptional regulation,induces the inflammatory response, participates in reproductive differentiation and the migration of tumor cells, and has other biological functions [26].
Sivelestat competitively inhibits NE activity with high specificity.There have been many studies on the function of sivelestat in reducing lung injury induced by endotoxin, and lung injury after ischemia-reperfusion.During simulated extracorporeal circulation,sivelestat preserves neutrophil deformability and reduces inflammatory mediators, while pretreatment with sivelestat prevents microcirculation disturbances, alveolar damage, and interstitial edema [116].
In a prospective clinical study of patients undergoing hepatic resections,patients who were administered sivelestat had reduced release of HMGB1 and more rapidly reduced levels of IL-6 than those who were administered a placebo.Thus, sivelestat may alleviate early injuries after hepatectomy [117].
3.7.Co-treatment with trastuzumab and sivelestat in anti-human epithelial growth factor receptor 2 (HER2) therapy
Approximately 25%-30% of patients with breast cancer have a positive expression of HER2, which is associated with a high potential for malignancy and poor prognosis.Trastuzumab causes HER2 down-regulation, thereby improving the efficacy of other treatments; however, drug intolerance must be considered when administering trastuzumab.TGF-α is a growth factor that can increase the invasion of tumor cells and interfere with HER2 downregulation through anticancer drugs.TGF-α inhibits the disintegration of HER2 through trastuzumab activation of endocytosis or lysosomes and promotes the recruitment of HER2 expression on the cell surface.The inhibition of these TGF-α effects may block tumor growth and drug resistance [118,119].
NE may split TGF-α and activate signal transduction in an autocrine manner, and increased NE is associated with worsened patient prognosis [58].Controlling inflammatory alternation may be a crucial step in the regulation of cancer progression.
To explore the effect of NE on the proliferation of breast cancer cells and the potential role of sivelestat in the treatment of HER2-positive breast cancer, each of their proliferative and inhibitory effects on breast cancer cell lines has been evaluated[120].NE can trigger the progression of breast cancer cells by activating TGF-α and is inhibited by sivelestat, while trastuzumab can downregulate HER2 levels, though TGF-α impairs this process.TGF-α promotes cell proliferation and enhances trastuzumab resistance by preventing HER2 downregulation.The combination of sivelestat and trastuzumab may suppress cancer cell proliferation more than either can alone, which may provide new insights into the treatment of HER2-positive breast cancer [120].
In addition, related studies show that sivelestat inhibits the growth of gastric cancer cells.Its action is inhibiting the release of TGF-α stimulated by NE,which usually occurs after surgical stresses[62].
At present, sivelestat has been clinically approved for the treatment of ALI/ARDS, and it has an inhibitory effect on DIC promoted by NE [105].Both ALI/ARDS and DIC are commonly observed in patients with severe COVID-19; thus, sivelestat is likely to be an effective strategy for the treatment of COVID-19[4].In addition, sivelestat can specifically inhibit the effect of NE to reduce lung damage in COVID-19 patients and inhibit the effect of spike protein to limit the transmission of the virus [104].Although clinical trials describing the clinical outcome of sivelestat for the treatment of COVID-19 are currently lacking, the potential of sivelestat for the treatment of COVID-19 and associated lung injuries is significant from a mechanistic and pharmacological perspective, as well as regarding the rapid approval of sivelestat for the treatment of COVID-19.Surrounding the treatment of COVID-19, papers report that the lymphocyte antigen 6 complex, locus E (LY6E), can regulate the immune function of T cells.Moreover, the encoding gene of LY6E can inhibit membrane fusion mediated by the spike protein of SARS-CoV-2 to limit the infectivity of SARS-CoV-2 [121,122].This raises the possibility that LY6E gene activators can contribute to the treatment of COVID-19 [123].Separately, some natural phytomedicines have been found to protect the lungs and may also improve treatments of COVID-19 [124].Research on drugs to treat COVID-19, including but not limited to sivelestat, requires more clinical trials to further determine the efficacy of treatments.
4.Conclusion
NE lowers inflammation levels,mediates pulmonary ventilation dysfunction, accelerates the progression of chronic liver disease and cirrhosis, activates dormant cancer cells, promotes tumor cell proliferation and distant metastasis, and changes the tumor microenvironment.It also aggravates radiation-induced lung injuries and immune-associated pneumonia, selectively kills cancer cells, and attenuates tumorigenesis.
Sivelestat may have several clinical applications, including the treatment of COVID-19, pulmonary fibrosis, and chronic liver disease and cirrhosis; it can also be administered during the perioperative period for patients with esophageal cancer and to prevent early injuries following hepatectomy.Sivelestat may also be combined with trastuzumab to treat HER2-positive breast cancer.The quick approval of sivelestat for the treatment of COVID-19 emphasizes the urgent need to further study the pathophysiological processes mediated by NE and the clinical applications of sivelestat;hence, studies focusing on the clinical applications of sivelestat,including phase III clinical trials, remain warranted.
CRediT author statement
Weilin Zeng:Investigation,Writing-Original draft preparation,Reviewing and Editing;Yingqiu Song:Writing - Reviewing and Editing;Runze Wang:Visualization;Rong He:Writing-Reviewing and Editing, Project administration;Tianlu Wang:Project administration, Conceptualization, Resources, Supervision.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This work has been supported by the Liaoning Province Natural Science Foundation (Grant Nos.: 2020-ZLLH-47, 2020-MS-065,2021-YGJC-02, and 2017225054).Figures in the paper were drawn using Figdraw, and we sincerely thank the free drawing support provided by the Figdraw platform (www.fgdraw.com).We also would like to thank Editage(www.editage.cn)for English language editing.
猜你喜欢
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Recent progress in aptamer-based microfluidics for the detection of circulating tumor cells and extracellular vesicles
- Breath-by-breath measurement of exhaled ammonia by acetonemodifier positive photoionization ion mobility spectrometry via online dilution and purging sampling
- Solriamfetol impurities: Synthesis, characterization, and analytical method (UPLC-UV) validation
- Quantitative characterization of cell physiological state based on dynamical cell mechanics for drug efficacy indication
- Recent applications and chiral separation development based on stationary phases in open tubular capillary electrochromatography(2019-2022)
- A chiral metal-organic framework {(HQA)(ZnCl2)(2.5H2O)}n for the enantioseparation of chiral amino acids and drugs
