A chiral metal-organic framework {(HQA)(ZnCl2)(2.5H2O)}n for the enantioseparation of chiral amino acids and drugs
2023-05-29XingtiZhengQiZhngQinjieXinyuLiLingZhoXiodongSun
Xingti Zheng , Qi Zhng , Qinjie M , Xinyu Li , Ling Zho ,Xiodong Sun ,*
a Shanghai Engineering Research Center of Organ Repair, School of Medicine, Shanghai University, Shanghai, 200444, China
b School of Pharmacy, Jiangsu University, Zhenjiang, Jiangsu, 212013, China
c Department of Pharmacy, Shanghai Baoshan Luodian Hospital, Shanghai, 201908, China
d Luodian Clinical Drug Research Center, Institute for Translational Medicine Research, Shanghai University, Shanghai, 200444, China
Keywords:
Capillary electrochromatography
Metal-organic framework
Enantioseparation
Chiral stationary phase
Open-tubular column
Dansyl amino acids
ABSTRACT Chiral metal-organic frameworks (CMOFs) with enantiomeric subunits have been employed in chiral chemistry.In this study, a CMOF formed from 6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid(HQA) and ZnCl2, {(HQA)(ZnCl2)(2.5H2O)}n, was constructed as a chiral stationary phase (CSP) via an in situ fabrication approach and used for chiral amino acid and drug analyses for the first time.The{(HQA)(ZnCl2)(2.5H2O)}n nanocrystal and the corresponding chiral stationary phase were systematically characterised using a series of analytical techniques including scanning electron microscopy, X-ray diffraction, Fourier transform infrared spectroscopy, circular dichroism, X-ray photoelectron spectroscopy, thermogravimetric analysis, and Brunauer-Emmett-Teller surface area measurements.In opentubular capillary electrochromatography (CEC), the novel chiral column exhibited strong and broad enantioselectivity toward a variety of chiral analytes, including 19 racemic dansyl amino acids and several model chiral drugs (both acidic and basic).The chiral CEC conditions were optimised, and the enantioseparation mechanisms are discussed.This study not only introduces a new high-efficiency member of the MOF-type CSP family but also demonstrates the potential of improving the enantioselectivities of traditional chiral recognition reagents by fully using the inherent characteristics of porous organic frameworks.
1.Introduction
Enantiodiscrimination is a topic of interest in separation science because enantiomers of chiral compounds often differ in their biological activities[1,2].The increasing use of enantiopure drugs in pharmaceuticals has boosted research in the field of highperformance chiral separation [3,4].Amino acids (AAs) are essential compounds that contain both amino and carboxyl groups[5,6]and play important roles in several biochemical systems.They exhibit different biological behaviours owing to their chiral characteristics[7,8].Among these, L-AAs are widely used in food[9,10],medicine [11,12], and other fields.However, the potential of D-AAs in numerous biological processes has been recently demonstrated[13,14] through their connection to the treatment of metabolic disorders, including schizophrenia [15], Alzheimer's disease [16],and cancer [17].Hence, the development of methods for enantioseparating chiral AAs and drugs is crucial to many fields of science.
Metal-organic frameworks (MOFs) are composed of inorganic metal ions and organic ligands and exhibit many advantageous characteristics, such as ultra-high specific surface areas, tuneable uniform pore sizes, and ease of functional modification.MOFs are versatile porous materials for multiple applications including gas storage [18,19], catalysis [20,21], drug delivery [22,23], and molecular separation.Recent studies have successfully employed chiral MOFs with porous supramolecular architectures as the opentubular capillary electrochromatography (OT-CEC) stationary phases for chiral separation.For example,Wang et al.[24]developed a mesoporous Fe-based γ-cyclodextrin MOF as the chiral stationary phase in OT-CEC for the enantioseparation of 14 racemic drugs and one chiral alcohol.Sun et al.[25]established a chiral OT-CEC system based on the MOF L-His-MIL-53 via post-synthesis modification.The enantioresolution of several chiral drugs was significantly better than that of the bare capillary column or other modified capillary columns before post-synthesis modification.The major limitations of reported MOF-type capillary columns are their relatively poor enantioselectivities and complicated column preparation processes.The development of chiral MOFs as stationary phases in OT-CEC can solve these problems by enhancing the surface concentration of chiral selectors [26].Nevertheless, current reports on chiral MOF-based CEC methodologies are sporadic and high-performance MOF@capillaries remain scarce.
In this study, a chiral MOF, {(6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid(HQA))(ZnCl2)(2.5H2O)}n,was synthesised and successfully immobilised on the inner wall of a capillary column using an in situ approach.Specifically,HQA was synthesised by the slow oxidation of quinine, a natural selector, followed by selfassembly of a 1D chain coordination framework.The chiral MOF and corresponding capillary column were characterized by scanning electron microscopy (SEM), X-ray diffractometry (XRD),energy-dispersive X-ray spectrometry (EDS), X-ray photoelectron spectroscopy (XPS), Fourier-transform infrared spectroscopy (FTIR), thermogravimetric analysis (TGA), circular dichroism (CD)spectroscopy, and Brunauer-Emmett-Teller surface area measurements (BET).A systematic OT-CEC experiment was conducted to demonstrate the excellent enantioseparation performance of this novel MOF-based capillary column.
2.Material and methods
2.1.Chemicals
L-proline(L-Pro), D-Pro, L-cysteine(L-Cys), D-Cys, L-alanine(L-Ala),D-Ala, L-arginine (L-Arg), D-Arg, L-glutamic acid (L-Glu), D-Glu, Lserine (L-Ser), D-Ser, L-tyrosine (L-Tyr), L-isoleucine (L-Ile), D-Ile, Lcystine(L-Cys), D-Cys,L-threonine(L-Thr), D-Thr, L-tryptophan(L-Trp),D-Trp, L-methionine (L-Met), D-Met, L-citrulline (L-Cit), D-Cit, D-histidine (D-His), and L-lysine (L-Lys) were purchased from Macklin Biochemical Co., Ltd.(Shanghai, China).Dansyl (DNS) chloride, Lleucine (L-Leu), D-Leu, L-valine (L-Val), D-Val, D-Tyr, L-asparagine (LAsn), D-Asn, L-phenylalanine (L-Phe), D-Phe, (S)-ibuprofen ((S)-IBU),(R)-IBU, and IBU were purchased from Shanghai Haohong Pharmaceutical Co.,Ltd.(Shanghai,China).D-Lys and L-His were purchased from Sigma-Aldrich(Shanghai,China).(S)-metoprolol((S)-MET)and(R)-MET were purchased from Beijing Bellwether Technology Co.,Ltd.(Beijing, China).(S)-chlorpheniramine maleate ((S)-CPM) and(R)-CPM were purchased from Shenzhen Zhenqiang Biotechnology Co.,Ltd.(Shenzhen,China).MET and CPM were provided by Jiangsu Institute for Food and Drug Control (Nanjing, China).3-aminopropyltriethoxysilane (APTES), glutaraldehyde (50% in H2O),and other chemicals were of analytical grade or higher and purchased from Macklin Biochemical Co., Ltd..Bare-fused silica capillaries (75 μm i.d.; 365 μm o.d.) were purchased from Yongnian Ruifeng Chromatography Devices Co.,Ltd.(Handan,China).
2.2.Apparatus
CEC experiments were performed using a CL1030 capillary electrophoresis system (Beijing Huayang Liming Instrumental Co.,Beijing, China) equipped with a ultraviolet (UV) detector(190-700 nm) and a 30 kV high-voltage power supply.The morphologies of the synthesised MOF {(HQA)(ZnCl2)(2.5H2O)}nand MOF-functionalized capillary ({(HQA)(ZnCl2)(2.5H2O)}n@capillary)were characterised using SEM (FEI Quanta 250 FEG, Waltham, MA,USA).XPS spectra were collected using a Shimadzu AXIS spectrometer(Kyoto,Japan).XRD data were recorded using a Bruker D8 ADVANCE X-ray powder diffractometer(Saarbrucken,Germany).FTIR spectra were recorded using a Nicolet IS50 spectrometer(Thermo Scientific Inc., Waltham, MA, USA) using KBr pellets.The thermostability of the MOF coatings was measured by TGA using a NETZSCH STA 449F3 instrument(Selb,Germany)in the temperature range of 25-800°C.The optical activities were tested by CD spectroscopy using a JASCO J-815 spectrometer(Easton,MD,USA)spectrometer in a trifluoroethanol:H2O(1:1,V/V)solution.The BET surface area and total pore volume of the MOF material and MOF capillary coating were determined using a JW-BK200B instrument(JWGB Sci.&Tech.Ltd., Beijing, China) instrument and the N2adsorption-desorption isotherm method.
2.3.Synthesis of HQA
HQA was synthesized according to the literature [27].Briefly,100 mL of a KMnO4solution(32.4 mmol)was mixed with 14.6 mL of a quinine solution in 10% H2SO4(12.35 mmol)at 0°C.Next,200 mL of ethanol (EtOH) was added.The reaction mixture was stirred overnight at room temperature and refluxed at 70°C for 30 min.The reaction mixture was purified by filtration to remove MnO2byproduct, and the residual solvent was evaporated under reduced pressure at 100°C.HQA was obtained as a light-brown solid(27.6% yield) after recrystallization from H2O and EtOH.The reaction scheme for HQA synthesis and its characterisation data are shown in Figs.S1-S4.
2.4.Synthesis of {(HQA)(ZnCl2)(2.5H2O)}n
The chiral MOF {(HQA)(ZnCl2)(2.5H2O)}nwas synthesised by heat treatment [28].Briefly, 30 mL of a methanol (MeOH):H2O(1:1, V/V) solution containing HQA (1 mmol) and ZnCl2(1 mmol)was heated at 70°C for 4 days.HQA was obtained as a colourless product in 78.9% yield (Fig.S5).
2.5.Preparation of the {(HQA)(ZnCl2)(2.5H2O)}n coated OT capillary column
A 50-cm untreated fused-silica capillary was continuously rinsed with 1 M NaOH (1 h), H2O (15 min),1 M HCl (30 min),H2O(15 min),and MeOH(30 min).After removing residual MeOH with N2gas,the capillary column was dried at 100°C in a vacuum oven for 1 h.Next,a 50% APTES solution in MeOH was pumped into the capillary for 15 min.The capillary was sealed with rubber septa and allowed to react in a water bath maintained at 55°C for 12 h to fully bind the amino groups.A 2% glutaraldehyde (V/V, pH 11) solution was then pumped into the APTES-modified column for 2 h, followed by injecting a 0.1 M KMnO4solution into the capillary for 1 h using a syringe pump.
{(HQA)(ZnCl2)(2.5H2O)}n@capillary was fabricated using a simple in situ approach.The carboxyl-functionalized capillary column was rinsed with a 3 mL of MeOH:H2O(1:1,V/V)solution containing HQA (1 mmol) and ZnCl2(1 mmol) for 3 h.The capillary was then sealed with rubber septa and allowed to react in a water bath maintained at 70°C for three days to obtain the final functionalized capillary.
2.6.Preparation of standard solutions
All DNS AAs were prepared according to the literature [29].Briefly,100 μL of an AA solution(1 mg/mL in 0.1 M HCl containing 30% MeOH),20 μL of 2 M NaOH,and 30 μL of concentrated NaHCO3were mixed with 500 μL of a DNS chloride solution (10 mg/mL in acetone).The mixed solution was allowed to react at 4°C for 45 min,and the reaction terminated by adding 10 μL of 25% NH4OH.Racemic drugs (0.5 mg/mL) were dissolved directly in 50% MeOH:H2O (1:1, V/V) solution [30].
2.7.CEC procedures
A 50 cm {(HQA)(ZnCl2)(2.5H2O)}n@capillary was used for CEC,with an effective length of 41.5 cm.An applied voltage of 15 kV was used (unless stated otherwise) and the temperature was maintained at 25°C.The detection wavelength was set at 254 nm.A 20 mM phosphate solution containing 10% MeOH was used as the running buffer, and its pH was accurately adjusted by adding a small volume of HCl(10%)or NaOH(5 M)with a micro syringe.All solutions were filtered through a 0.45-μm membrane filter prior to use.Thiourea was used as a neutral marker to indicate the electroosmotic flow (EOF).The capillary column was conditioned with running buffer between CEC analyses until a stable baseline was obtained.
3.Results and discussion
3.1.Characterization of {(HQA)(ZnCl2)(2.5H2O)}n
The SEM analysis showed that {(HQA)(ZnCl2)(2.5H2O)}nhas a triclinic morphology, which matches the theoretical architecture(Fig.1A).To confirm the successful synthesis and purity of{(HQA)(ZnCl2)(2.5H2O)}n,powder XRD (PXRD) was performed at room temperature using a powdered MOF sample (Fig.S6).PXRD showed that this MOF crystallises in a triclinic morphology, as observed in the SEM image, with two crystallographically independent Zn centers.The experimental PXRD pattern showed peaks at 9.8°, 12.9°, 13.3°, 14.8°, 20.0°, and 25.2°, which are consistent with the simulated pattern.The FT-IR spectra of HQA and MOF product (Fig.1B) showed a characteristic band in the region of 1450-1360 cm-1corresponding to the asymmetric stretching vibrations of the carboxylate functional groups and benzene rings[31].In addition, an intense peak corresponding to the stretching vibration of the carbonyl group was observed at 1710 cm-1, indicating the presence of freely available carboxyl groups in{(HQA)(ZnCl2)(2.5H2O)}n.The bands at 1620 cm-1and 1598 cm-1corresponded to symmetric and asymmetric stretching of carboxyl groups bonded to Zn[32,33].Coordination between Zn ions and O atoms of the HQA ligand causes the electron cloud densities of carbon-nitrogen bonds to be between that of C=O and C-O bonds,shifting its stretching vibration to a low frequency [34].A peak at 1685 cm-1indicated the presence of a zwitterionic moiety.Notably,two peaks at 3433 cm-1and 3300 cm-1indicated the presence of uncoordinated H2O molecules, further confirming the successful synthesis of {(HQA)(ZnCl2)(2.5H2O)}n.
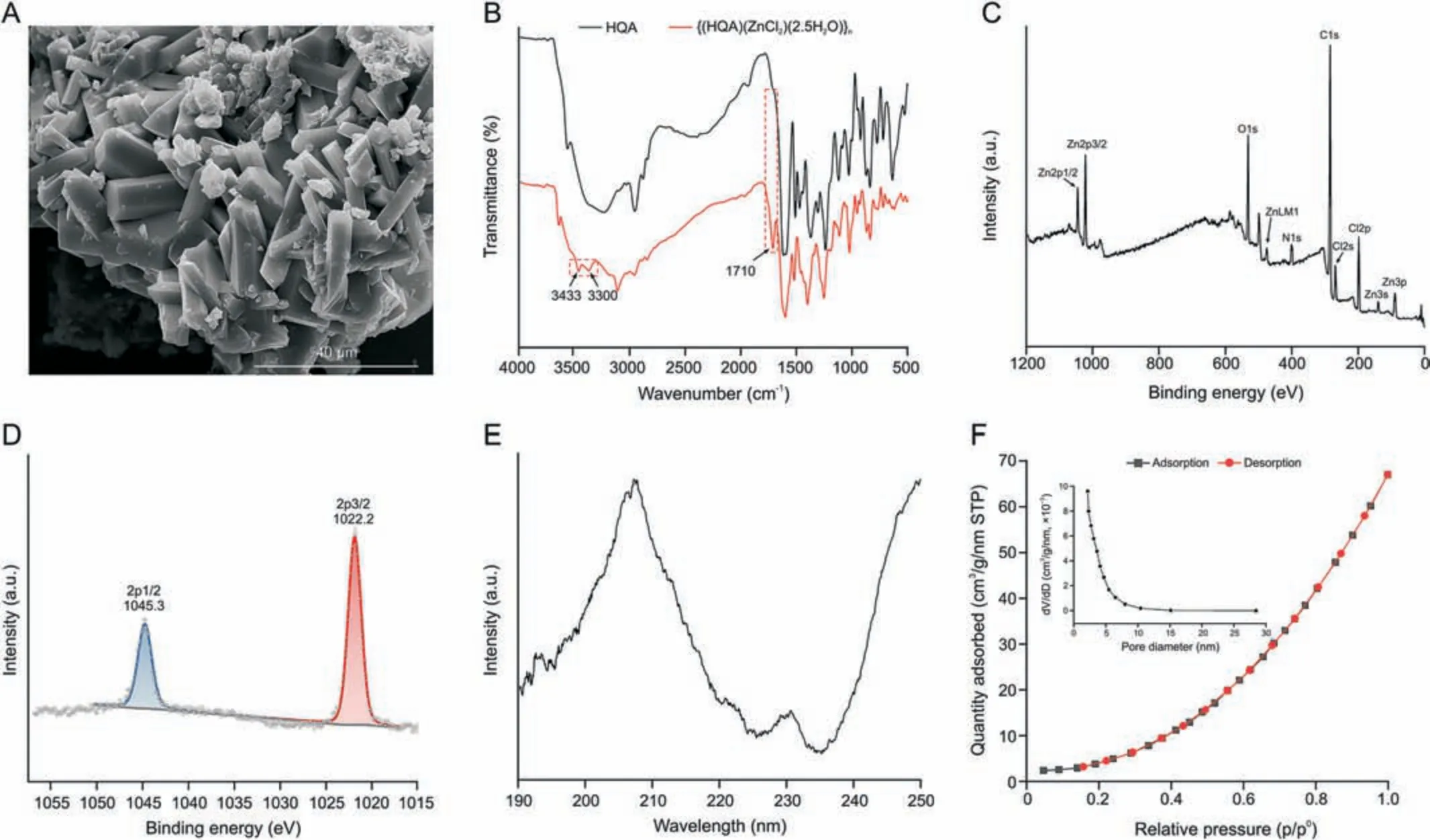
Fig.1 .Characterization of{(HQA)(ZnCl2)(2.5H2O)}n.(A)Scanning electron microscopy(SEM)image.(B)Fourier transform infrared(FT-IR)spectra.X-ray photoelectron spectroscopy(XPS) spectra of (C) survey spectrum and (D) Zn2p spectrum.(E) Circular dichroism (CD) spectra.(F) N2 adsorption-desorption isotherms (inset: pore size distribution).HQA: 6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid; STP: standard temeprature and pressure.
XPS experiments were also performed to determine the elemental composition of {(HQA)(ZnCl2)(2.5H2O)}n.The full-scan XPS profiles of the MOF samples are shown in Figs.1C and D.As expected,the characteristic peaks of C1s(284.5 eV),N1s(401.5 eV),O1s(532.5 eV),Zn2p(1022.2 eV and 1045.3 eV),and Cl2p(198.5 eV)were observed.The two characteristic peaks in the Zn2p XPS spectrum, situated at 1045.3 eV and 1022.2 eV, corresponded to Zn2p1/2 and Zn2p3/2,respectively.This indicated that Zn(II)exists in the MOF, matching the SEM-EDS results.CD spectra were recorded in the far-UV region (190-250 nm) to determine the optical activity of the MOF (Fig.1E).The thermal stability of the MOF product was tested using TGA (Fig.S7).The weight change profile remained constant up to a temperature of 80°C, at which point the weight reduction increased with the temperature up to 120°C.This weight loss corresponded to the release of coordinated water molecules.A second significant weight loss occurs at 250°C,the onset of instability temperature of {(HQA)(ZnCl2)(2.5H2O)}n.The TGA weight curve indicated a steep reduction in weight under extreme thermal stress, corresponding to the collapse of the MOF structure.To determine the porosity of the prepared MOF product,N2adsorption-desorption isotherms were measured using the BET technique.The N2adsorption-desorption isotherms were identified as type III isotherms (Fig.1F), and their pore size distribution indicated the presence of pores smaller than 30 nm(inset of Fig.1F).Using the Barrett-Joyner-Halenda (BJH) model and the desorption branch of the N2isotherm,the average pore size was calculated to be 5.739 nm.Multipoint BET method and BJH analysis showed that the surface area was 139.5 m2/g and pore volume was 0.048 cm3/g.
3.2.Characterization of {(HQA)(ZnCl2)(2.5H2O)}n@capillary
The SEM images clearly showed the presence of a homogeneous coating on the inner surface of the {(HQA)(ZnCl2)(2.5-H2O)}n@capillary and the smooth inner wall of the bare capillary(Figs.2A and B).The surface elemental compositions of bothcapillaries were analysed using SEM-EDS (Figs.2A and B).The differences in the elemental compositions of the two capillaries further demonstrated that the {(HQA)(ZnCl2)(2.5H2O)}nMOF was successfully immobilised onto the capillary inner surface(Table 1).The multipoint BET method showed that the surface area of the{(HQA)(ZnCl2)(2.5H2O)}n@capillary (78.801 m2/g) was significantly larger than that of the bare capillary (33.889 m2/g).BJH analysis and the desorption branch of the N2isotherm showed that the (MOF-coated and bare capillaries had pore sizes of 4.897 and 4.671 nm and pore volumes of 0.085 and 0.057 cm3/g,respectively.These results indicated the successful synthesis and high porosity of the MOF coating(Figs.2C and D).The effect of pH on the EOF is shown in Fig.S8.The {(HQA)(ZnCl2)(2.5H2O)}n@capillary exhibited different EOF behaviours than the bare capillary column but with similar trends; its decreased EOF confirms the successful synthesis and coating of {(HQA)(ZnCl2)(2.5H2O)}non the inner surface of the capillary.

Table 1 The differences in the elemental compositions of the two capillaries.
3.3.Enantiomer separation
The enantioseparation performance of the {(HQA)(ZnCl2)(2.5-H2O)}n@capillary was evaluated using various chiral compounds as model analytes.Under the selected CEC conditions,almost all chiral analytes were successfully resolved, as summarized in Table 2.Representative electropherograms are shown in Fig.3.Note that free AAs cannot be enantioseparated in this MOF-coated capillary because they do not have suitable functional groups to interact with selectors[35].However,derivatizing agents can be applied to both the amino and carboxylic groups to allow intermolecular interactions with the selectors.The chiral MOF-type stationary phase achieved excellent enantioselectivities toward DNS-AAs, with an enantioresolution of 13.56 for DNS-Phe.Notably, no enantioresolution was achieved using the bare capillary (Table 2).These results demonstrate the essential role of the chiral MOF coating during CEC separation.This MOF-coated capillary is also capable of resolving acidic or basic drug enantiomers, which is a significant advantage over most reported chiral capillary columns that typically exhibit relatively low enantioselectivities.

Fig.3 .Performance of open-tubular capillary electrochromatography-based enantioseparation of (A) nineteen dansyl amino acids (DNS-AAs) and (B) three model racemic drugs conducted using{(HQA)(ZnCl2)(2.5H2O)}n@capillary.Conditions:20 mM phosphate buffer containing 10% methanol(MeOH)at pH 6.75;applied voltage,15 kV.HQA:6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid; Ala: alanine; Arg: arginine; Asn: asparagine; Cit: citrulline; Cys: cysteine; Cys-Cys: cystine; Glu: glutamic acid; His: histidine; Ile:isoleucine; Leu: leucine; Lys: lysine; Met: methionine; Phe: phenylalanine; Pro: proline; Ser: serine; Thr: threonine; Trp: tryptophan; Tyr: tyrosine; Val: valine; CPM: chlorpheniramine maleate; IBU: ibuprofen; MET: metoprolol.
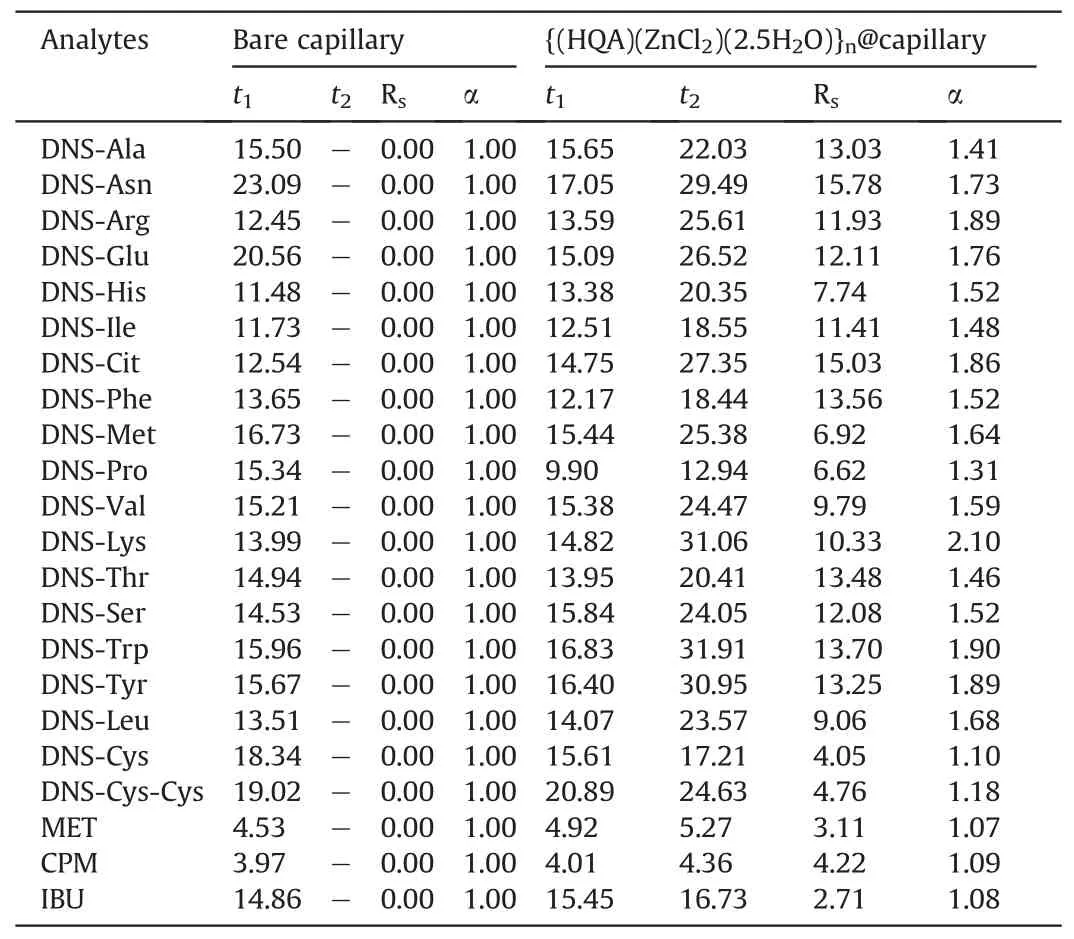
Table 2 Enantioseparation of dansyl (DNS)-amino acids and racemic drugs in different capillary column.
3.4.Separation mechanisms
Quinine possesses multiple chiral recognition sites that allow for excellent enantioselectivity; hence, it is commonly used as a chiral acid selector.Furthermore,the planar quinoline ring and the carbamate group are potential chiral binding sites.A series of experiments has revealed that the quinine-modified stationary phase can enantioseparate chiral aromatic carboxylic acids, aromatic oxocarboxylic acids, various N-derived AAs, and other structural AAs[36-38].The H-bonding,dipole-dipole,and π-π interactions with the carbamate groups and the large steric effect of quinine itself toward analytes promotes chiral recognition[39].However,it is not possible to achieve both versatility and enantioselectivity using a single chiral stationary phase-modified capillary column,possibly because of the negative interactions between recognition domains and the relatively low surface chiral selector concentrations in a limited support surface area [35].To overcome this limitation, we can enhance the surface concentration of chiral selectors in the stationary phase.Compared with previous research on quinine-modified stationary phases[36,37],this study showed significantly increased enantioseparation performance(Rs=13.56).This improvement may be attributed to the high MOF surface area, offering intense surface concentration and high porosity,resulting in abundant chiral recognition sites for selectorselectant interactions.These factors may facilitate the access of guest enantiomers to the host framework, leading to efficient enantioseparation in CEC(Fig.4).To verify this hypothesis,quinine was coated onto an NH2-modified capillary column to prepare a similar stationary phase with low surface area (Fig.S9).The enantioseparation performance of this stationary phase(Rs=1.51)was inferior than that of {(HQA)(ZnCl2)(2.5H2O)}n@capillary, confirming our hypothesis.Regarding analyte enantioseparation, the tertiary amine of the quinine group is fully protonated at the working pH of the mobile phases, enabling an ion pairing mechanism and strong Coulomb attractions with deprotonated analytes.Therefore, the analytes form transient ion pairs with the chiral selectors through the molecular electrostatic field surrounding the chiral association sites.Moreover,molecular interactions between analytes and the chiral stationary phase such as H-bonding,dipole-dipole forces, π-π interactions, and steric attraction or repulsion, cause enantiodiscrimination and enantioresolution of chiral analytes.Notably,the enantiomer migration orders were not always consistent with the results of the enantiomeric separation,including those of DNS-Cys and DNS-Cys-Cys.Here, enantioresolution was not equal to that of the other DNS-AAs, with a deficiency in the single enantiomer peak area induced by the coelution of faster migrating enantiomers.

Fig.4 .Schematic illustration of {(HQA)(ZnCl2)(2.5H2O)}n.HQA: 6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid.
3.5.Optimization of CEC conditions
To improve CEC performance, we optimised the different types(MeOH,EtOH,and acetonitrile)and proportions(0-20%)of organic modifiers,buffer pH(6.00-7.00),and applied voltages(12-20 kV)(Fig.5 and Tables S1-S4).Our results showed that the chiral column is compatible with various organic additives.Considering both the enantioresolution and retention time of the enantiomers,20 mM phosphate buffer(pH=6.75)containing 10% MeOH was the optimal buffer, with an applied voltage of 15 kV.Under optimised CEC conditions,all model analytes were baseline-separated within 20 min.The theoretical plate numbers of the enantiomer peaks were usually higher than 17,000, suggesting excellent separation efficiency.
Fig.5 .Effect of different conditions on the efficiency of the enantioseparation of dansyl(DNS)-phenylalanine(Phe)using{(HQA)(ZnCl2)(2.5H2O)}n@capillary.(A)Different types of organic solvents.(B)Different concentrations of organic solvent.(C)Different applied voltages.(D)Different values of pH of phosphate buffer.HQA:6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid; Cl: chloride; EtOH: ethanol; Rs: resolutions; α: relative retention value; ACN: acetonitrile; MeOH: methanol.
3.6.Repeatability
Repeatability of the new CEC system was evaluated by calculating the relative standard deviations(RSDs)of the retention times of the first eluted peak(t1).As shown in Table 3,the run-to-run(n=5)and day-to-day (n = 5) repeatability was less than 1.74% and 1.66%,respectively.The column-to-column repeatability was also tested using {(HQA)(ZnCl2)(2.5H2O)}n@capillaries synthesised from different batches,with the RSD(n=3)lower than 3.40%.Therefore,{(HQA)(ZnCl2)(2.5H2O)}n@capillary exhibited good repeatability.

Table 3 Repeatability data for {(HQA)(ZnCl2)(2.5H2O)}n@capillary.
3.7.Stabil ity
The stability of the {(HQA)(ZnCl2)(2.5H2O)}n@capillaries was verified by repeatability tests,as shown in Fig.6.Using DNS-Phe as the analyte, there were no significant changes in resolution after 100 separation runs,thus confirming the stability and durability of the {(HQA)(ZnCl2)(2.5H2O)}n@capillaries.

Fig.6 .Durability of {(HQA)(ZnCl2)(2.5H2O)}n@capillary.Conditions: analytes, dansyl(DNS)-phenylalanine (Phe); 20 mM phosphate buffer containing 10% methanol(MeOH) at pH 6.75; applied voltage,15 kV.HQA: 6-methoxyl-(8S,9R)-cinchonan-9-ol-3-carboxylic acid; Cl: chloride.
3.8.A comparison of the present OT-CEC system with previous studies
A comparison of the advantages of different chiral column preparation methods, chiral coating materials, analytes, and previous representative CEC studies is summarized in Table S5[40-43].Generally, MOFs have large specific surface areas and porosities, and tuneable micropores/channels that can accommodate various chiral compounds.Researchers can ‘‘tailor” the properties of MOF materials by changing the ligand or performing chemicalmodifications.Themajoradvantageof{(HQA)(ZnCl2)(2.5H2O)}n@capillary over other CEC studies is its broad and high enantioresolution as well as simple column preparation.A similar conclusion can be drawn when comparing it with commercial chiral stationary phases (Table S6).
4.Conclusions
A chiral MOF, {(HQA)(ZnCl2)(2.5H2O)}n, was successfully synthesised and employed to prepare a novel chiral capillary column.The MOF nanocrystal and the corresponding chiral OT column were characterised using several analytical techniques, including SEM,XRD, FT-IR, CD, XPS, TGA, and BET.This is the first detailed characterisation of this type of MOF.The new OT-CEC system showed broad enantioselectivity towards a variety of chiral analytes,including 19 racemic DNS-AAs and several chiral drugs(both acidic and basic).The enantioselectivity of the quinoline precursor (e.g.,hydrophobic effect,π-π interactions,and H-bond interaction)was greatly improved by the high porosity of the chiral MOF coating,enhancing the enantiorecognition capability of the chiral stationary phase.This study introduced a new high-efficiency MOF-type chiral column and demonstrated traditional chiral selectors can be improved by exploiting the inherent characteristics of porous organic frameworks.
CRediT author statement
Xiangtai Zheng:Writing-Original draft preparation,Reviewing and Editing, Supervision;Qi Zhang: Writing - Original draft preparation,Reviewing and Editing;Qianjie MaandXinyu Li:Writing-Reviewing and Editing;Liang Zhao: Supervision, Methodology,Funding acquisition;Xiaodong Sun: Methodology, Writing -Reviewing and Editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (Grant No.: 82003705) and the Shanghai Science and Technology Innovation Foundation (Grant Nos.: 23010500200 and 23ZR1422700).
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jpha.2023.03.003.
杂志排行
Journal of Pharmaceutical Analysis的其它文章
- Recent progress in aptamer-based microfluidics for the detection of circulating tumor cells and extracellular vesicles
- Breath-by-breath measurement of exhaled ammonia by acetonemodifier positive photoionization ion mobility spectrometry via online dilution and purging sampling
- Solriamfetol impurities: Synthesis, characterization, and analytical method (UPLC-UV) validation
- Quantitative characterization of cell physiological state based on dynamical cell mechanics for drug efficacy indication
- Recent applications and chiral separation development based on stationary phases in open tubular capillary electrochromatography(2019-2022)
- High-throughput transcriptional profiling of perturbations by Panax ginseng saponins and Panax notoginseng saponins using TCM-seq
