Iron/aluminum nanocomposites prepared by one-step reduction method and their effects on thermal decomposition of AP and AN
2023-05-06YongKouYiWngJunZhngKigeGuoXiolnSong
Yong Kou ,Yi Wng ,Jun Zhng ,Ki-ge Guo ,Xio-ln Song
a School of Environment and Safety Engineering,North University of China,Taiyuan,Shanxi,030051,China
b School of Materials Science and Engineering,North University of China,Taiyuan,Shanxi,030051,China
c School of Ordance Engineering,Naval University of Engineering,Wuhan,Hubei,430033,China
d School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing,Jiangsu,210094,China
Keywords:Aluminum Fe/Al composite fuel High reactivity Thermal decomposition AP AN
ABSTRACT Aluminum (Al)powder is widely used in solid propellants.In particular,nano-Al has attracted extensive scholarly attention in the field of energetic materials due to its higher reactivity than micro-Al.However,the existence of aluminum oxide film on its surface reduces the heat release performance of the aluminum powder,which greatly limits its application.Hence,this paper used iron,a component of solid propellant,to coat micron-Al and nano-Al to improve the heat release efficiency and reactivity of Al powder.SEM,TEM,EDS,XRD,XPS,and BET were used to investigate the morphological structure and properties of pure Al and Fe/Al composite fuels of different sizes.The results show that Fe was uniformly coated on the surface of Al powder.There was no reaction between Fe and Al,and Fe/Al composite fuels had a larger specific surface area than pure Al,which could better improve the reactivity of pure Al.Besides,the catalytic effects of pure Al and Fe/Al composite fuels of different sizes on ammonium perchlorate and ammonium nitrate were explored.The results show that the catalysis of pure Al powder could be greatly improved by coating Fe on the surface of Al powder.Especially,the micron-Fe/Al composite fuel had a higher catalytic effect than the pure nano-Al powder.Hence,Fe/Al composite fuels are expected to be widely used in solid propellants.
1.Introduction
Aluminum (Al) powder enjoys wide application in the field of energetic materials due to its high density,low oxygen consumption,high combustion heat and volumetric heat [1—5].The heat released by the redox reaction can improve the damage capability and range of the ammunition.Nanometerization of Al powder can be added to pyrotechnic powder to improve its stability and durability [6].It can also significantly improve the energy performance of solid propellants,such as their specific impulse [4—6].However,the high melting point of the Al2O3oxide layer on the surface of aluminum powder makes it difficult to ignite.The incomplete oxidation-reduction reaction of Al powder will lead to its poor exothermic performance and combustion characteristics,such as a high melting point (boiling point) and easy sintering of the combustion products.In addition,the practical application of Al is also limited by its low melting point (660°C) [7—9].
Considering the unique comprehensive properties of Al powder,many researchers have been trying to improve its thermal properties.As some studies have found,reducing particle size and using organic fluoride (polytetrafluoroethylene) [10],carbon materials(carbon,graphene oxide) [11,12],transition metal oxide (Co3O4,Fe2O3,ZnO,etc.) [13—16],nanomaterials (Ni,Cu,Co,and NiO)[7,8,17,18],and other materials for particle surface coating can effectively improve the thermal properties of Al powder.However,some components that are not contained in energetic materials occupy a certain proportion;they reduce the heat release efficiency of the system due to their low heat release,but this problem can be solved via using nano metal powder (Co,Cu,Fe,etc.) to coat Al powder.In this study,Fe powder was used to coat Al powder and its thermal properties were explored.Coating Al powder with metallic Fe has unique advantages and characteristics.First,the nano-Fe particles on the surface of Al powder can prevent the bridging of molten Al due to its relatively high melting point (1535°C)compared to that of Al powder.Second,more energy can be released by the thermite reaction and alloying reaction with Al at high temperatures.Third,coating Fe on the surface of Al powder can improve the dispersibility of nano-Fe particles.It is well known that the specific surface area of Al powder increases as the particle size decreases,which can enhance its reaction rate and combustion rate.However,the increase in the specific surface area also leads to the increase in the Al2O3oxide layer on the surface of the Al powder,which hinders the ignition and makes the redox reaction incomplete.Yet,the content and area of Al2O3can be effectively reduced by coating Fe on the surface of the nano-Al powder,thereby increasing the heat release rate of Al [7,8].
Ammonium perchlorate (AP) and ammonium nitrate (AN) are oxidants widely used in composite solid propellants.The effective oxygen content of AP is almost the highest among oxidants,and the oxygen balance reaches +34.04%,which is beneficial to the oxidation and combustion of other components in the solid propellant.It also has high energy conversion efficiency due to the high hydrogen content,meaning that more work can be done by releasing the same heat energy [19—21].AN is a halogen-free oxidant that can replace AP.It is more environmentally friendly because the combustion products do not contain hydrogen chloride and other compounds.AN also has low combustion characteristics;that is,almost no white smoke or white mist is generated during combustion [22,23].The thermal decomposition characteristics of AP and AN have always been the focus of research,because they directly affect the combustion performance of propellants [24].In this paper,Al powders of two sizes were coated by iron to prepare Fe/Al core-shell structure,and their effects on the thermal decomposition of ammonium perchlorate and ammonium nitrate were explored.
2.Experimental approach
2.1.Materials
Nanometer Al powder and micrometer Al were purchased.FeCl3∙6H2O (AR) was purchased from Tianjin Fengchuan Chemical Reagent Technology Co.,Ltd.NH4F(AR)was bought from Shanghai Macklin Biochemical Co.,Ltd.Ammonium perchlorate and ammonium nitrate were bought from Tianjin Guangfu Chemical Co.,Ltd.Distilled water was used in all experiments.
2.2.Preparation of Fe/Al composite fuels
First,0.2 g of micron-Al powder was added to 25 ml of aqueous solution and ultrasonically vibrated to form a uniform Al suspension.Second,0.253 g of FeCl3∙6H2O was dissolved in 25 ml of aqueous solution,which was stirred with a glass rod to accelerate dissolution.Third,0.7363 g of NH4F was dissolved in 3 ml of water to form NH4F solution.Next,the FeCl3∙6H2O solution and the NH4F solution were successively poured into the continuously stirred Al suspension,and the replacement reaction between Al and Fe3+took place immediately when the Al2O3oxide layer on the aluminum surface was dissolved by F-.The brownish yellow in the solution disappeared when Fe3+was reduced to Fe.Therefore,the product was magnetically separated and washed twice with distilled water,absolute ethanol,and acetone after the yellow color of the solution faded.Finally,the micron-Fe/Al composite fuel was obtained by freeze drying(-40°C).The same method was used to prepare the nano-Fe/Al composite fuel.The mass of Al powder,FeCl3∙6H2O,and NH4F was changed to 0.2 g,0.652 g,and 1.015 g,respectively,and 1.015 g of NH4F was dissolved in 4 ml of water to form the solution.Next,the FeCl3∙6H2O solution and the NH4F solution were poured into the continuously stirred Al suspension.The replacement reaction between Al and Fe3+proceeded when the Al2O3oxide layer on the surface of the aluminum powder was dissolved under the action of F-.Then,the nano-Fe/Al composite fuel was obtained by repeating the above steps.Noteworthily,the whole procedures mentioned above were carried out at room temperature,and the preparation process is shown in Fig.1.
2.3.Characterization
The ZEISS MERLIN Compact scanning electron microscope and the FEI Tecnai G2 F30 transmission electron microscope were used to investigate the microscopic morphology of the samples.The energy spectra of the samples were obtained using Oxford x-max.XRD(Bruker Advance D8)was used to determine the crystal phase of the samples.XPS was performed on Thermo Scientific K-Alpha to probe the surface elements of the samples.The Brunauer-Emmett-Teller (BET) measurements were performed utilizing the nitrogen adsorption with the Quadrasorb SI instrument.Thermal decomposition process was investigated with DSC(TA Model Q600)at the heating rates of 5,10,15,and 20°C/min.
3.Results and discussion
The dense Al2O3oxide film on the surface of the aluminum powder has stable chemical properties,which would prevent the oxidation-reduction between Al and Fe3+.It is thus necessary to add F-to dissolve the Al2O3film on the surface of the Al powder during the reaction [7,8,25].The Fe powder generated by the oxidation-reduction reaction will adhere to the surface of the Al particles after the Al powder is exposed.In this paper,the SEM test was carried out on the raw Al powder and Fe/Al composite particles of different sizes to observe the coating effect of Fe.The results are shown in Fig.2.Fig.2(a)and(b)demonstrate the SEM images of the micron-Al powder.We could see that the micron-Al powder had good dispersibility,and all of the particles were spherical with smooth surfaces.The diameter distribution of micron-Al powder was obtained by measuring the diameters of about 100 particles.Then,the volume distribution could be achieved based on the frequency distribution curve,and the results are displayed in Fig.2(c)and(d).It can be concluded from Fig.2(c)that the average diameter of micron-Al powder was 11.86 μm,being consistent with the median diameter of 11.9 μm in Fig.2(d).Fig.2(e)and(f)display the Fe/Al core-shell structure.The surfaces of the particles were uneven,which was caused by the destruction of the Al2O3oxide layer by F-and the adsorption of the reduced Fe particles on the surface of the Al powder.The reduced Fe particles were coated on the surface of Al powder to form a core-shell structure,which was consistent with the expected structure in Fig.1.Fig.2(g) and (h)show the morphology of nano-Al powder.We could see that the nano-Al particles were all tiny and spherical with smooth surfaces,but agglomeration phenomenon appeared.The particle size was also analyzed,as shown in Fig.2(i) and (j).Fig.2(i) shows that the average diameter of nano-Al powder was 118.06 nm,which was basically consistent with the median diameter of 115.8 nm shown in Fig.2(j),and it is within the error tolerance range.The morphology of Fe coated on the surface of nano-Al is shown in Fig.2(k) and (l).Clearly,Fe particles with smaller diameters were uniformly attached to the surface of nano-Al powder[11,17,26,27].
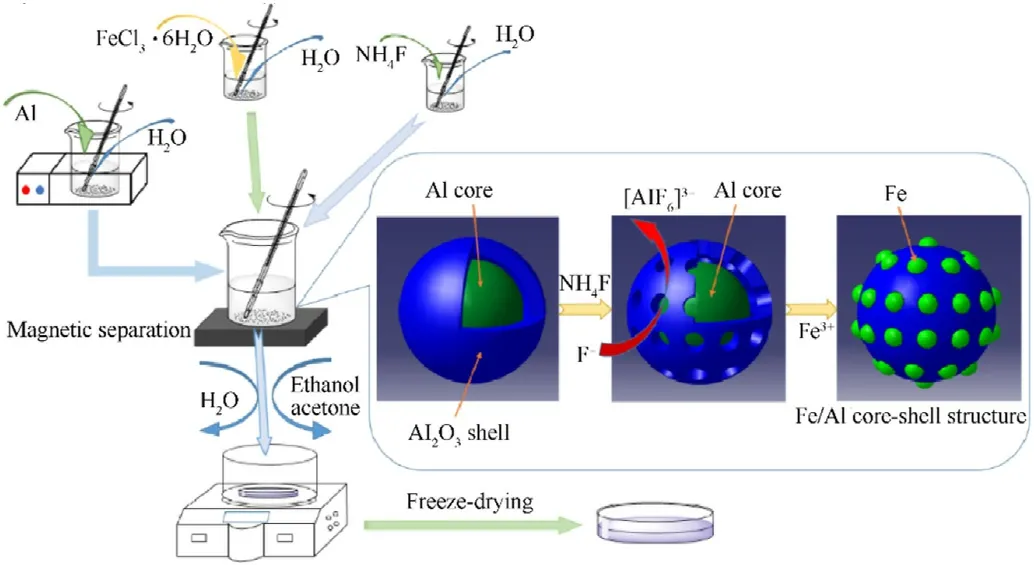
Fig.1.Preparation process of the Fe/Al core-shell structure.
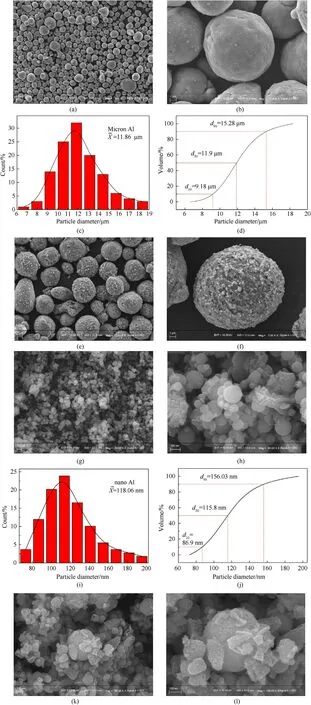
Fig.2.SEM images and diameter distribution of the samples: (a),(b),(e),(f),(g),(h),(k),(l) SEM images;(c),(i) Frequency curves;(d),(j) Volume curves.
TEM measurement was conducted to characterize the more detailed morphological features of nano-Al powder and nano-Fe/Al composite fuel.The results are displayed in Fig.3 [7,8].Micron-Al and micron-Fe/Al composite fuel were not tested by TEM because their particles were larger than 5 μm.Fig.3(a),(c),and (e) are the electron diffraction patterns of nano-Al powder and TEM images with different resolutions,and Fig.3(b),(d),and (f) are those of nano-Fe/Al composite fuel.Fig.3(a) and (b) display the crystal planes of raw aluminum powder and composite fuel,respectively.Fig.3(c) shows that the surface of pure Al powder was smooth.Comparing Fig.3(d) and (c),it can be concluded that the iron particles were evenly coated on the surface of the nano-Al powder.Fig.3(e)and(f)are high-resolution TEM(HRTEM)images of pure Al powder and Fe/Al composite particles.We could observe a wellresolved lattice fringe with an interplanar spacing of 0.23 nm,which matched well with the distance of the (111) planes of Al powder.The areas enclosed by the red boxes in Fig.3(e) and (f)were the aluminum oxide layer,which was coated on the surface of Al powder.The area marked by the blue circle in Fig.3(f) was Fe particles,which were attached to the surface of Al powder[28—31].The above conclusions revealed that Fe particles were successfully coated on the surface of nano-Al powder,which was consistent with the results of SEM.
EDS test was performed on four samples to identify their elemental composition.Fig.4(a) displays the energy spectrum of micron-Al powder.It only contained Al and O elements,thus being in line with the element composition of Al.The small amount of O element was related to the Al2O3layer on the surface of Al.Mapping scan was performed on the particles enclosed in the red box in Fig.4(b),and the results are shown in Fig.4(c)and(d).The element distribution of Al and O was consistent with the shape of Al powder particles in Fig.4(b)[26,27].The sum of the mass percentages of O and Al in Fig.4(e)and the atomic percentages of the two elements in Fig.4(f) were both 100% [32,33].Fig.4(g) shows the energy spectrum of the micron-Fe/Al core-shell structure.It can be concluded that the Fe particles were successfully coated on the surface of the Al powder due to the presence of Fe,Al,and O.The mapping diagrams of the composite particles in Fig.4(h)are shown in Fig.4(i),(j),and (k).It can be seen that the distribution of different elements was consistent with the Fe/Al core-shell structure in Fig.4(h)[26,27].Fig.4(l)and(m)show that the sum of the mass percentages and atomic percentages of Fe,O,and Al were 100%,indicating that only Fe was introduced during the preparation of the composite particles,and no additional impurities were introduced [32,33].Fig.5(a) is the energy spectrum of nano-Al powder,which contained only Al and O elements.The mapping diagrams of Al particles in the red frame in Fig.5(b) are shown in Fig.5(c) and (d).The mass percentage and atomic percentage of different elements are shown in Fig.5(e)and(f).It can be observed that the nano-Al powder contained only Al and O elements,and the result was consistent with the micron-Al powder.However,the content of O in nano-Al powder was significantly higher than that in micron-Al powder,indicating that the proportion of Al2O3oxide layer in nano-particles was larger than that in micron-Al powder.The higher content of Al2O3oxide layer will affect the thermal performance of Al powder,which highlights the necessity of using Fe instead of Al2O3oxide layer to coat the surface of Al powder[8].Fig.5(g)is the energy spectrum of nano-Fe/Al composite fuel.Three elements,namely Fe,O,and Al,were obtained,indicating that Fe particles had successfully coated on the surface of Al powder.The mapping diagrams of the particles in the red box in Fig.5(h) are shown in Fig.5(i),(j),and (k).Fig.5(l) and (m) demonstrate the mass percentages and atomic percentages of the three elements,the set of which were all 100%,indicating that no other impurities were introduced during the process of Fe coating Al particles.The content of O element in nano-Fe/Al composite fuel was significantly lower than that in pure nano-Al powder,revealing that the proportion of Al2O3was reduced,which is beneficial to increasing the heat release rate of Al powder [26,27,32,33].
XRD was performed to characterize the phase composition of pure Al and Fe/Al composite fuels of different sizes.The results are shown in Fig.6.As displayed in Fig.6(a),the pure Al powder had strong peaks at 38.6°,44.8°,65.2°,and 78.3°,which were consistent with the standard card of Al (JCPDS card 04—0787) and corresponded to(111),(200),(220),and(311)planes,respectively[7,8].In Fe/Al composite fuels,except for the characteristic peaks of Al powder,the diffraction peaks of Fe powder also appeared at 44.85°and 65.2°,which were related to the crystal planes of Fe at (110)and (200) [25,34,35].Compared with pure micron-Al powder,the diffraction peak intensity of micron-Fe/Al composite particles was reduced,and the peak width did not change significantly.Fig.6(b)shows the diffraction peaks of raw nano-Al and nano-Fe/Al composite particles.The characteristic diffraction peaks of nano-Al powder and nano-Fe/Al composite particles were consistent with those of pure micron-Al powder and micron-Fe/Al composite particles,respectively.But several tiny peaks appeared in Fig.6(b)due to the smaller size of nano-Al and nano-Fe/Al composite particles[7,8,25,34,35].The samples stored for six months were characterized by XRD to verify the presence of Fe in the composite fuels and also to determine whether the reduced Fe was oxidized.Fig.6(a)and (b) show that the characteristic peaks of Al coincided with those of Fe in the XRD test,resulting in insufficient evidence of the presence of Fe in the composite fuels.Concentrated hydrochloric acid could dissolve aluminum powder and has almost no effect on Fe particles.Therefore,the composite fuels were dissolved in an appropriate amount of concentrated hydrochloric acid,which could corrode the active aluminum powder inside.Then,the remaining nanoparticles were collected for XRD testing.The results are displayed in Fig.6(c) and (d),which show that the nanoparticles collected from composite fuels had strong diffraction peaks at 44.72°and 65.08°,corresponding to the crystal planes of Fe at(110)and (200),respectively.The strong diffraction peaks of collected nanoparticles at 44.72°and 65.08°were the same as the diffraction peaks of standard XRD pattern of Fe (JCPDS card 06—0696),indicating that the collected nanoparticles were Fe which had successfully coated on the surface of Al powder.It can also be concluded that the Fe nanoparticles obtained by the replacement reaction were not oxidized since the diffraction peaks of the collected Fe shell were the same as the peaks of standard XRD pattern of Fe,without any redundant diffraction peaks.
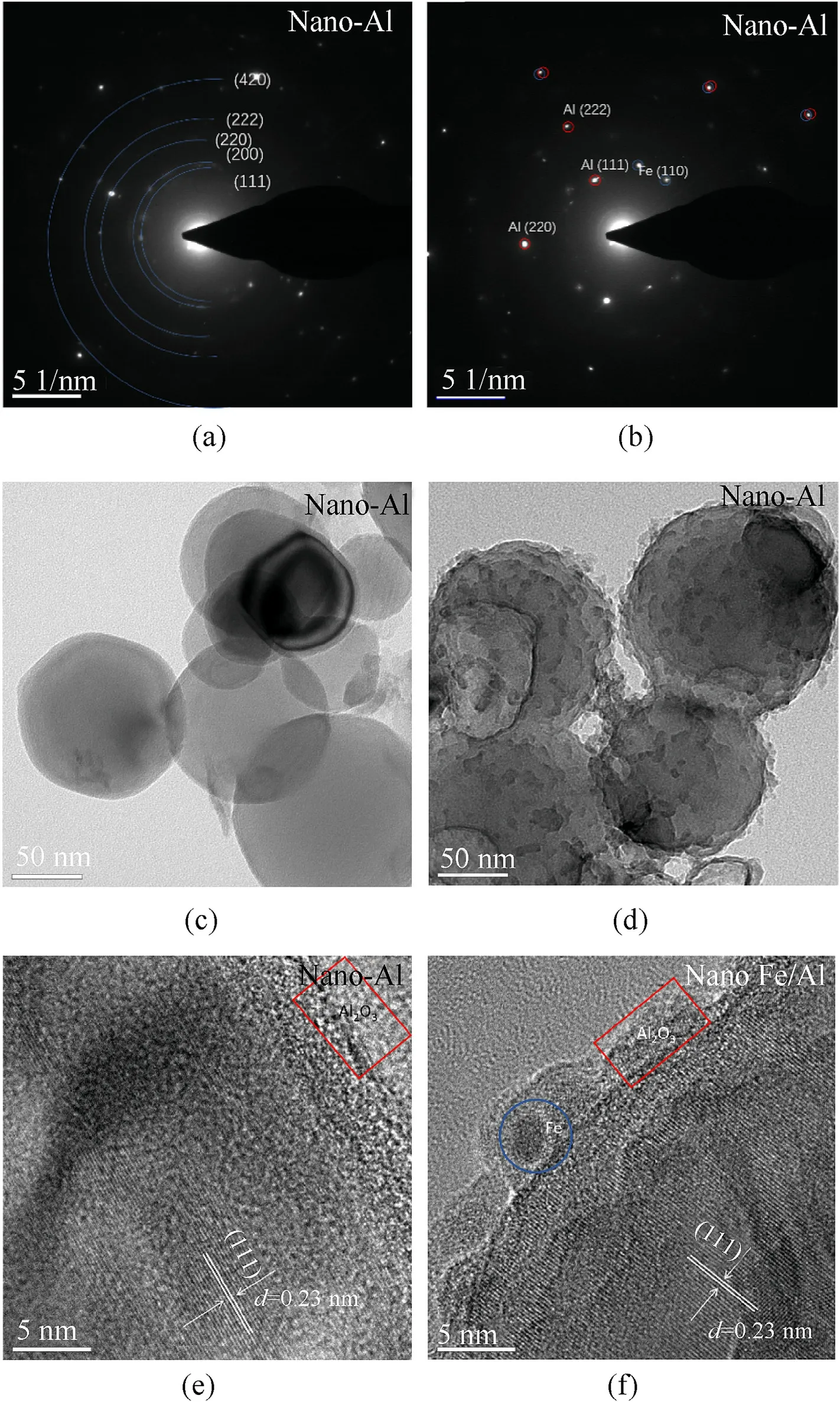
Fig.3.Images of nano-Al powder and nano-Fe/Al composite fuel: (a),(b) Electron diffraction patterns;(c),(d) TEM images;(e),(f) HRTEM images.
XPS analysis was performed to detect the surface elements of the samples.The results of pure Al powder and Fe/Al composite particles are displayed in Fig.7 and Fig.8,respectively.Fig.7 clearly shows that both micron-Al powder and nano-Al powder contained Al and O elements.The peak positions of micron-Al powder and nano-Al powder were calibrated using C1s (in Fig.7(c) and (d)) of contaminated carbon.In Fig.7,the positions of the diffraction peaks of Al with different particle sizes were all at 73 eV,and the positions of the O1s peaks were also at 531.18 eV.This demonstrated that the elements contained in the Al powders of the two sizes were the same [7,8,26,27].Then,the elements of Fe/Al composite fuels of different sizes were characterized,and the results are shown in Fig.8.We could see that in addition to the Al and O elements,the Fe/Al composite fuels also contained Fe.The peak positions of Al and O elements in the composite fuels were consistent with those of the pure Al powder.The diffraction peaks of Fe appeared at 710.9 eV(Fe2p3/2)and 724.58(Fe2p1/2)eV in both micro-composite fuel and nano-composite fuel.The above conclusions indicated that no new groups or chemical bonds were generated during the preparation of Fe/Al composite fuels [26,27,32,33].
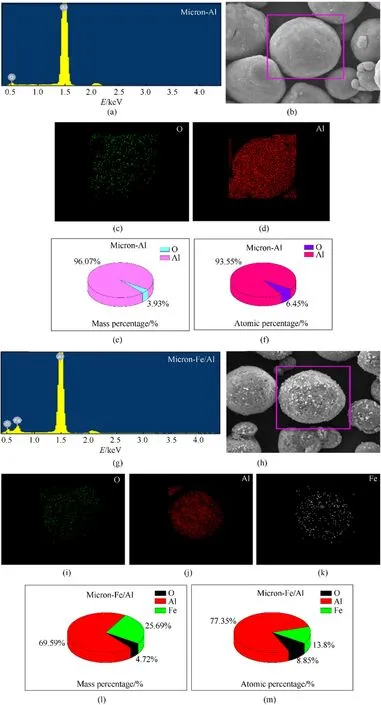
Fig.4.EDS spectra of micron-Al powder and micron-Fe/Al composite particles:(a)The energy spectrum of micron-Al powder;(b)The SEM picture of micron-Al;(c)(d)The mapping diagrams of micron-Al;(e)The mass percentage of O and Al;(f)The atomic percentage of O and Al;(g)The energy spectrum of micron-Fe/Al;(h)The SEM image of micron-Fe/Al;(i)(j)(k) The mapping diagrams of the micron-Fe/Al;(l)The mass percentages of O,Al,Fe;(m)The atomic percentages of O,Al,Fe.
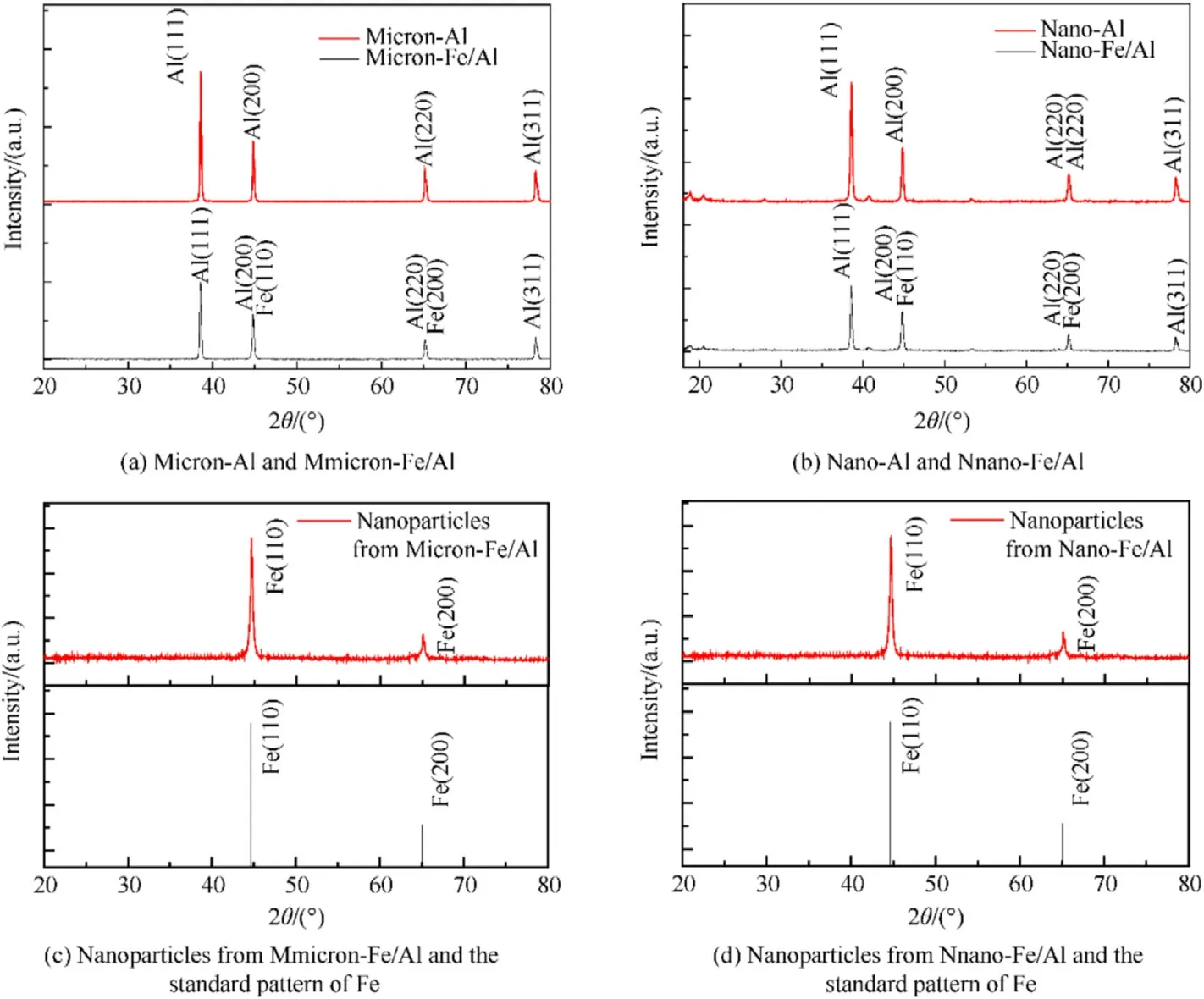
Fig.6.XRD patterns of the samples: (a) Micron-Al and micron-Fe/Al;(b) Nano-Al and nano-Fe/Al;(c) Nanoparticles from micron-Fe/Al and the standard pattern of Fe;(d)Nanoparticles from nano-Fe/Al and the standard pattern of Fe.
The N2adsorption-desorption isotherms of pure Al powder and Fe/Al composite particles are shown in Fig.9.The curves were all typical tetravalent adsorption isotherms,which are considered as class IV (H3-type hysteresis loop),indicating that all the four samples were mesoporous materials [27,36].At a lowp/p0,the isotherms increased slowly.However,with the increase inp/p0,the isotherms increased faster due to the onset of multilayer adsorption.Capillary condensation would cause the adsorption capacity to rise sharply at largep/p0.Generally,capillary condensation and capillary evaporation do not occur under the same pressure,thereby leading to the occurrence of hysteresis loops.The BET surface area,pore volume,and pore size of the samples are shown in Table 1.It can be observed that the pore size of the Fe/Al composite particles was smaller than that of the pure Al powder,but their BET surface area and pore volume were larger than those of pure Al powder [26,27,36].These findings indicated that the Fe/Al composite particles had higher reactivity and catalysis.
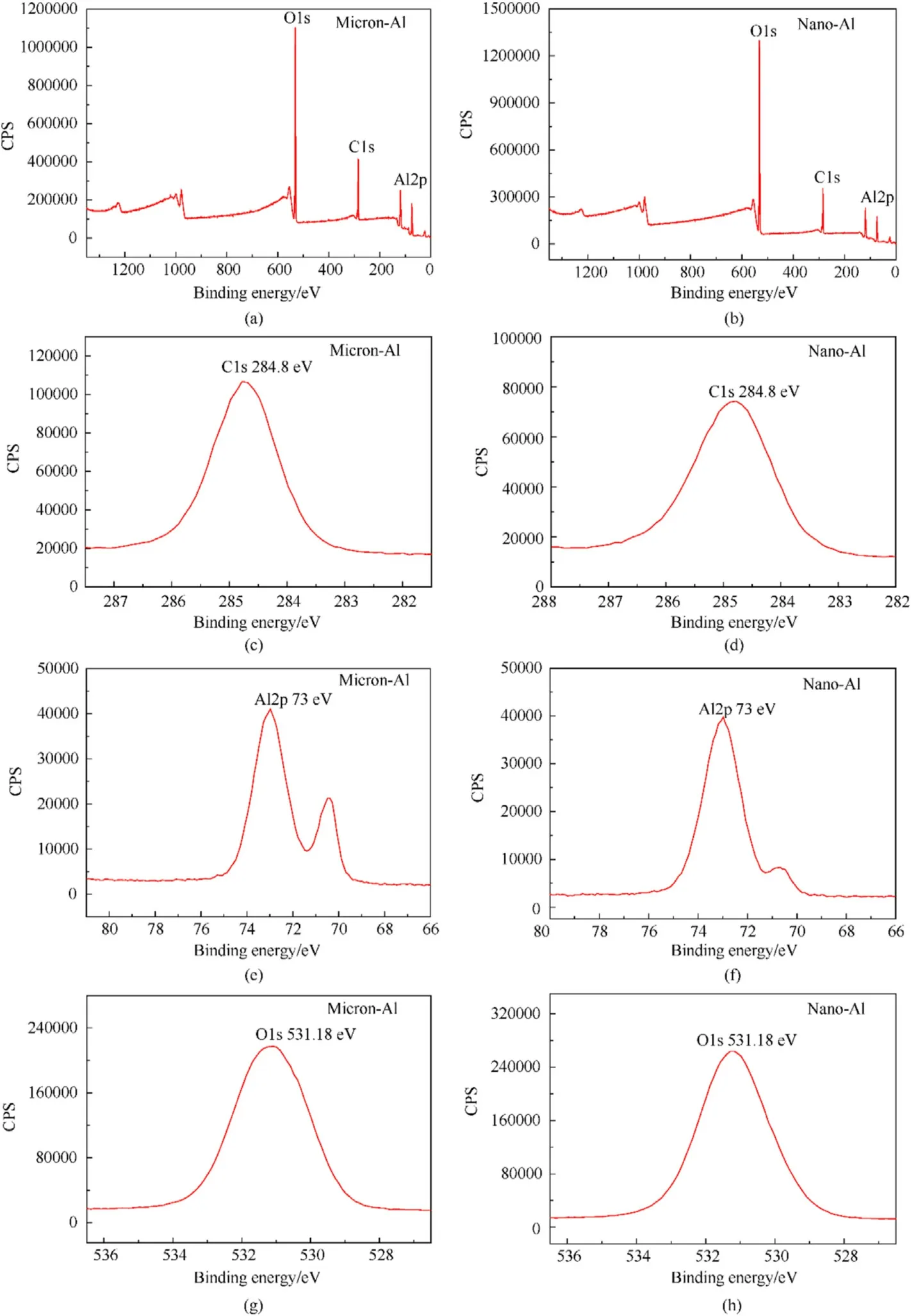
Fig.7.XPS spectra of pure Al powder: (a),(c),(e),(g) Micron-Al;(b),(d),(f),(h) Nano-Al.
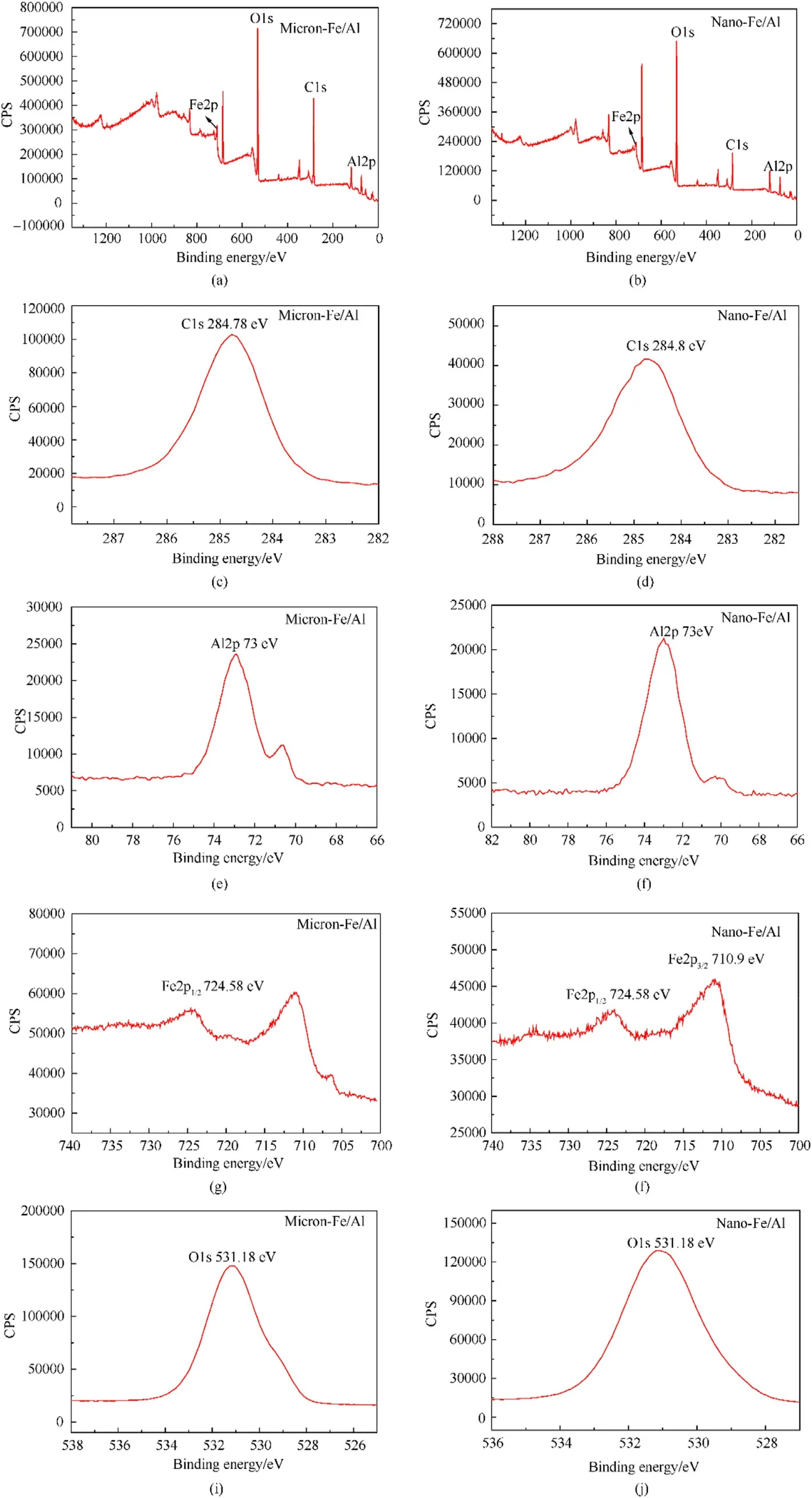
Fig.8.XPS spectra of the samples: (a),(c),(e),(g),(i) Micron-Fe/Al;(b),(d),(f),(h),(j) Nano-Fe/Al.
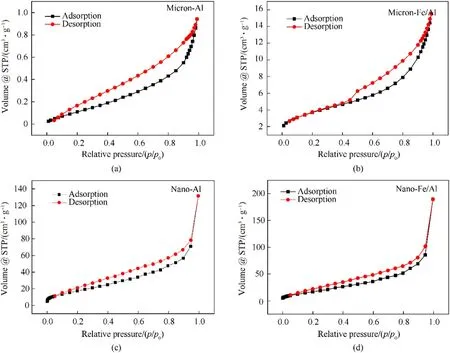
Fig.9.The BET date of the samples:(a)Micro-Al;(b)Micro-Fe/Al;(c)Nano-Al;(d)Nano-Fe/Al.
The pure Al (10% by mass) powder and Fe/Al composite fuels(10% by mass) of two sizes were added to AP to explore their catalytic effect on AP.The results are illustrated in Fig.10.Fig.10(a)is the thermal decomposition spectrum of pure AP.All the four curves showed an endothermic peak (240.7°C) and an exothermic peak(421.9—451.4°C),indicating the phase change and thermal decomposition of AP,respectively.Normally,there are two exothermic peaks in the thermal decomposition curve of AP,one related to the low-temperature decomposition,and the other corresponding to the high-temperature decomposition.However,only one decomposition peak appeared in Fig.10(a),which was caused by the failure to press the sample before the test.The test conditions of all samples were the same,so the experimental results were more reliable [20].Fig.10(b) is the thermal decomposition spectrum of AP catalyzed by pure micron-Al powder.Each of the four curves with different heating rates had an endothermic peak(240.1°C) and an exothermic peak (415—450.7°C),which were related to the phase change and thermal decomposition of AP.Fig.10(c)shows the thermal decomposition curves of AP catalyzed by micron-Fe/Al composite fuel.Each curve exhibited an endothermic peak(240.1°C)and an exothermic peak(365.2—399.8°C),representing the phase transition temperature and thermal decomposition temperature of AP,respectively.By comparing Fig.10(a)—(c),it was found that the phase transition peaks of AP did not change under the action of different catalysts.The micron-Al also had little effect on the thermal decomposition of AP.However,the decomposition peaks of AP under the catalysis of micron-Fe/Al composite fuel were advanced by about 55°C compared to that under the catalysis of pure Al powder.Fig.10(d) presents the DSC curves of AP catalyzed by nano-Al powder.It can be concluded from Fig.10(a)—(d) that the phase transition temperature of AP had not changed under the action of different catalysts,but the decomposition peaks had changed.As reflected by the decomposition peaks of AP,the catalytic effect of nano-Al powder was better than that of micron-Al powder,but worse than that of micron-Fe/Al composite fuel.Fig.10(e) is the DSC spectrum of AP catalyzed by nano-Fe/Al composite fuel.Likewise,the phase transition temperature of AP had not shifted.However,the decomposition peaks of AP in Fig.10(e) were the lowest under the action of different catalysts,indicating that nano-Fe/Al composite fuel had the best catalytic effect on AP.
The thermodynamic parameters of the samples were calculated by Formulas(1)-(5)to study the catalytic effect of Fe/Al composite fuels and pure Al powder on ammonium perchlorate.The results are shown in Table 2.Among them,the apparent activation energy(EK),pre-exponential factor (AK),and rate constant (k) were calculated by Kissinger equation (Eq.(1)) and Arrhenius equation (Eq.(2)).Activation enthalpy (ΔH≠),activation free energy (ΔG≠),and activation entropy(ΔS≠)were calculated by Eqs.(3)—(5)[27,33,36].The value of ΔH≠represents the energy required for molecular activation.As shown in Table 2,the ΔH≠values of AP catalyzed by composite fuels (Fe/Al) were less than those catalyzed by pure Al powder and pure AP,indicating that the reactions were easier to proceed when composite fuels were used to catalyze AP.ΔG≠means the chemical potential of the samples in the activation reaction.The ΔG≠values of the samples were all positive,indicating that the thermal decomposition reaction of the samples was not spontaneous,and energy absorption was needed.The value of ΔS≠is related to the disorder or randomness of the system.Its positive value means that the randomness of the system is increased after the reaction,while a negative value indicates that the disorder of the system is reduced when the reaction is completed.TheEKvalues of AP catalyzed by composite fuels were lower than those of raw AP and the AP catalyzed by pure Al powder,indicating that composite fuels had a better catalytic effect on AP [27,32,33,36].
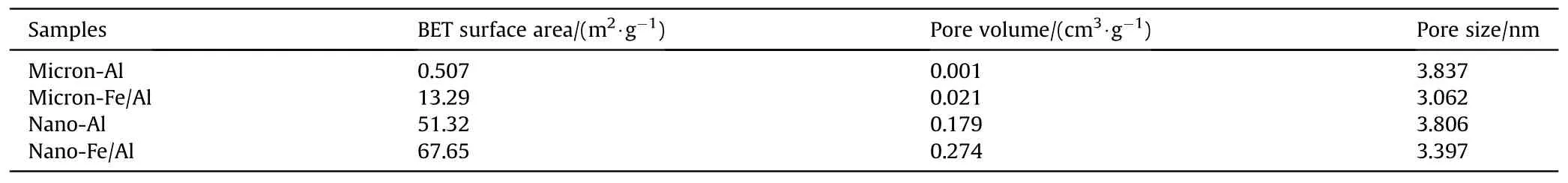
Table 1 BET surface area and the pore structure parameters of the nanoparticles.
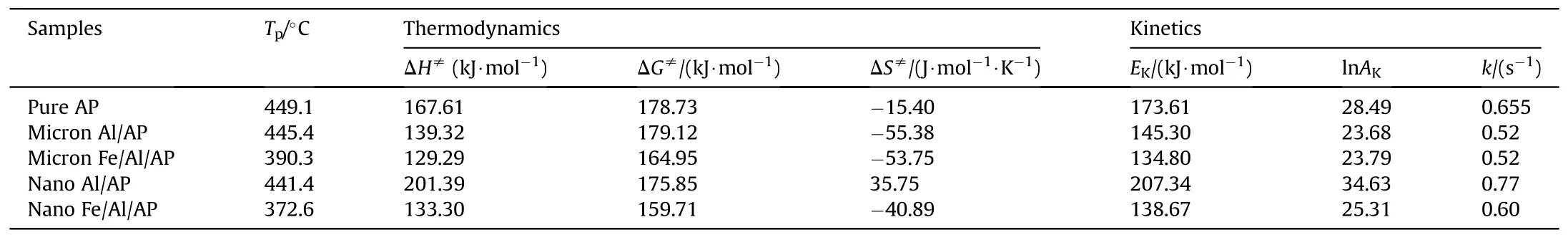
Table 2 Thermodynamics and kinetics derived from DSC traces.
whereTpis the temperature of the thermal decomposition peak in the DSC spectrum when the heating rate is 15°C/min,K;KBandhare Boltzmann (KB=1.381 × 10-23J/K) and Planck constants(h=6.626×10-34J/s),respectively;β is the heating rate;EKandAKare the activation energy and pre-exponential factor calculated by Kissinger equation;Ris the gas constant;kis the rate constant,s-1;ΔH≠is the activation enthalpy,J/mol;ΔG≠is the activation free energy,J/mol;and ΔS≠is the activation entropy,J∙mol-1∙K-1.
The decomposition mechanism of ammonium perchlorate catalyzed by different samples was analyzed,and the results are shown in Fig.11.During the process of low-temperature decomposition,the thermal decomposition products of AP wereand.Then,the electrons were transferred fromto NH4+to generate NH3and HClO4.The generated NH3and HClO4were adsorbed on the surface of AP to prevent its further decomposition.In the process of high-temperature decomposition,HClO4was decomposed into O2,ClO3,ClO,and H2O,where O2would formafter getting the electrons.then reacted with NH3to produce some products such as NO,N2O,and NO2.Numerous studies have shown that the transfer of electrons fromtois a control step in the low-temperature decomposition process,and the transfer of electrons to O2to formis a key step in the hightemperature decomposition process.Therefore,enhancing the transfer of electrons and the adsorption and desorption of NH3and HClO4during the thermal decomposition of AP are conducive to accelerating the thermal decomposition of AP[13,30,37].For the Fe/Al composite fuels,three reasons may well explain their positive effect on AP thermal decomposition.First,Fe instead of A2O3adhered to the surface of Al powder,which greatly improved the heat release efficiency and catalytic activity of Al powder.Second,there were partially filled 3d orbitals in Fe atoms,which established a bridge for electron transfer based on the electron transfer theory.Third,the Fe/Al composite fuels prepared in this study had a larger specific surface area than pure Al,which was considered to be another important factor in promoting the decomposition of AP[7,8,30].
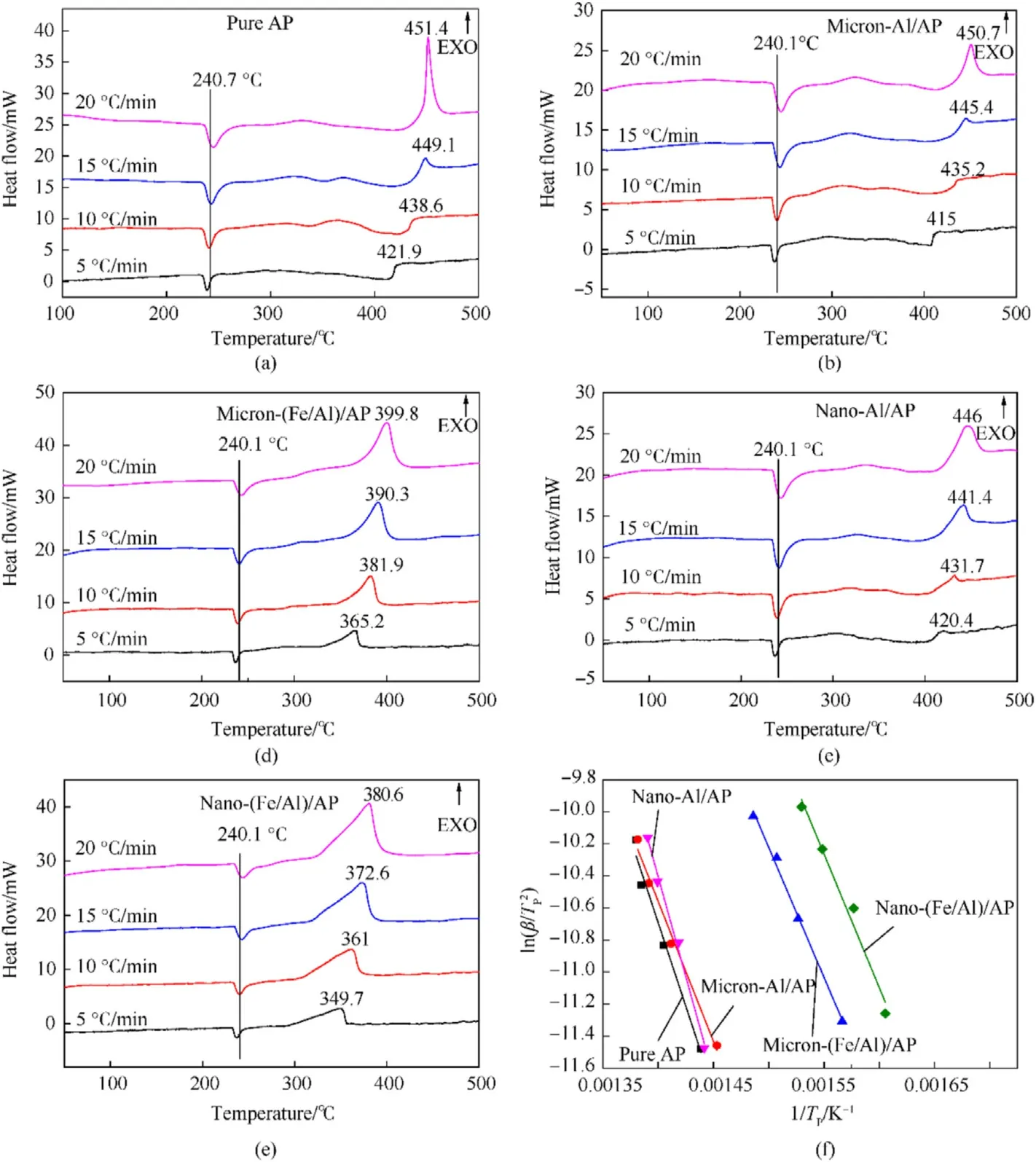
Fig.10.Thermal analysis curves of the samples: (a),(b),(c),(d),(e) DSC spectra;(f) Dynamic line diagram.
The catalytic effects of pure Al powder and Fe/Al composite fuels(10%by mass)of different sizes on AN were also explored through the thermal decomposition curves.The results are displayed in Fig.12.We could see that each curve contained three small endothermic peaks and one large endothermic peak.The peaks at 56.6°C and 128.6°C represent the phase transition of AN.The endothermic peak at 166.7°C stands for the melting point of AN.A large endothermic peak appeared as the temperature continued to rise,which was related to the thermal decomposition of AN.Comparing the DSC spectra of the five samples,it was found that the peaks at 56.6,128.6,and 166.7°C basically did not shift when different catalysts were used to catalyze AN,indicating that the pure Al powder and composite fuels did not change the phase transition temperature and melting point of AN.However,the decomposition peaks shifted greatly when different catalysts were used [20].And the decomposition temperature of AN was significantly reduced under the action of Fe/Al composite fuels.The thermodynamic parameters of the five samples were also calculated using Eqs.(1)—(5)to further explore the catalytic effects of Fe/Al composite fuels and pure Al powder on AN.The results are shown in Table 3.The ΔH≠of AN catalyzed by Fe/Al composite fuels was higher than that of raw AN and the AN catalyzed by pure Al powder,indicating that the decomposition of (Fe/Al)/AN needed more energy.The values of ΔG≠were positive,meaning that the thermal decomposition reaction of the five samples did not proceed spontaneously,but it needed to absorb heat.The ΔS≠values of pure AN,micron-Al/AN,micron-(Fe/Al)/AN,and nano Al/AN were negative,showing that the disorder or randomness of the system was reduced.Meanwhile,the positive value of ΔS≠with nano-(Fe/Al)/AN was related to an increase in the randomness of the system when the reaction was completed.TheEKvalues of(Fe/Al)/AN and Al/AN of different sizes were all higher than those of raw AN,indicating that the thermal decomposition reaction of Fe/Al/AN and Al/AN needed to absorb more energy,which was consistent with the result of ΔH≠[27,32,33,36].However,the results contradicted the conclusions drawn from Fig.12,which was caused by the increase in the exponential factor [20].It is undeniable that the thermal decomposition peak of AN was significantly advanced under the action of Fe/Al and Al with different diameters.In particular,the Fe/Al composite fuels had better catalytic effect on AN.
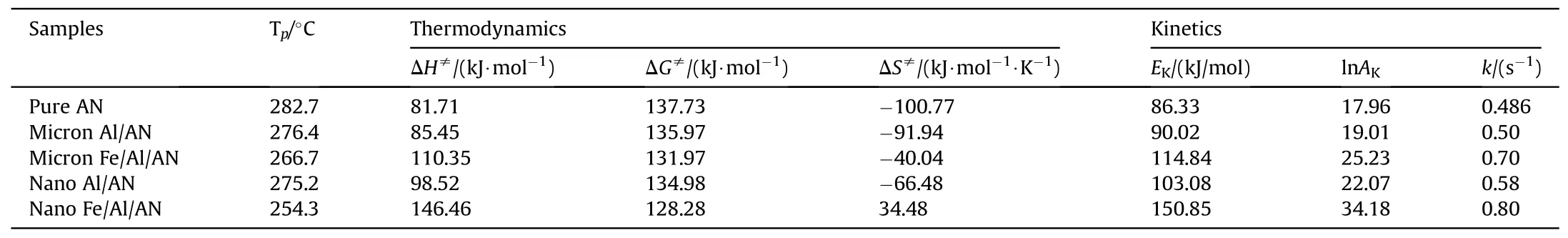
Table 3 Thermodynamics and kinetics derived from DSC traces.
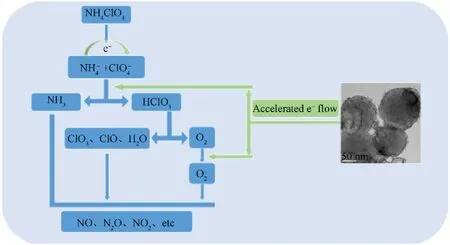
Fig.11.Thermal decomposition mechanism diagram of AP decomposition catalyzed by different samples.
4.Conclusions
In this study,micro/nano Fe/Al composite fuels have been prepared by a one-step reduction method.The results of the microscopic morphology,surface elements,and XRD analysis have confirmed that Fe was uniformly coated on the surface of Al powder,and there was no reaction between Fe and Al to generate new groups or bonds,indicating that the two have good compatibility.The BET results have shown that the prepared Fe/Al core-shell structure had a larger specific surface area,which significantly improved the heat release efficiency of Al.The excellent performance of Fe/Al core-shell structure has been fully reflected in the process of catalyzing the thermal decomposition of AP and AN,and the decomposition peaks of AP and AN have been significantly reduced.Therefore,Fe/Al composite fuels have broad application prospects in solid propellants.
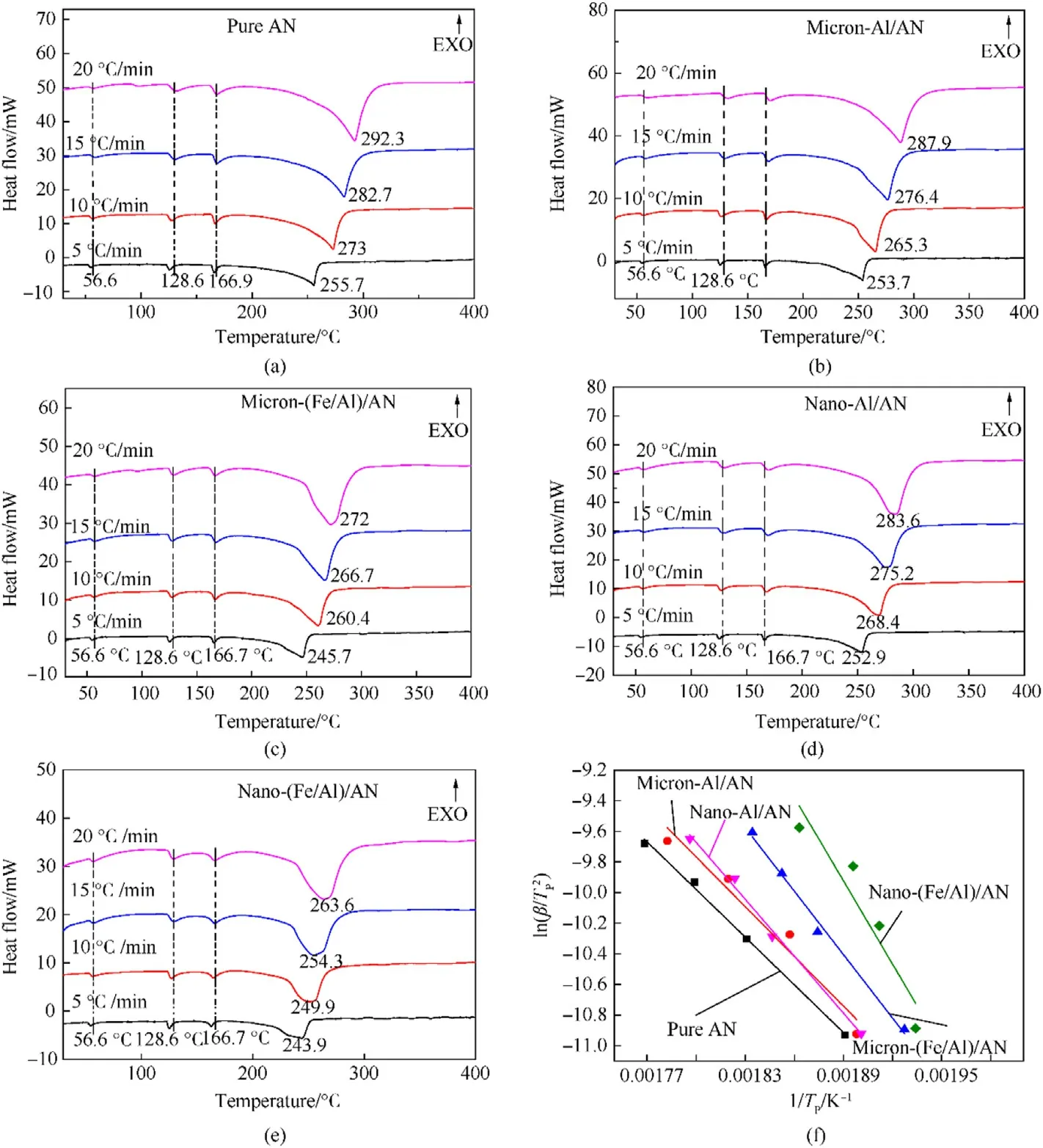
Fig.12.Thermal analysis curves of the samples: (a),(b),(c),(d),(e) DSC spectra;(f) Dynamic line diagram.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
杂志排行
Defence Technology的其它文章
- Dual Attribute Adversarial Camouflage toward camouflaged object detection
- A bi-population immune algorithm for weapon transportation support scheduling problem with pickup and delivery on aircraft carrier deck
- One-step green method to prepare progressive burning gun propellant through gradient denitration strategy
- Benchmark calculations and error cancelations for bond dissociation enthalpies of X—NO2
- GO/HTPB composite liner for anti-migration of small molecules
- Resilient tightly coupled INS/UWB integration method for indoor UAV navigation under challenging scenarios
