One-step green method to prepare progressive burning gun propellant through gradient denitration strategy
2023-05-06ShiyingLiYuLiYajunDingHaoLiangZhongliangXiao
Shi-ying Li ,Yu Li ,Ya-jun Ding ,Hao Liang ,Zhong-liang Xiao ,*
a School of Chemical Engineering,Nanjing University of Science and Technology,Nanjing,210094,China
b Key Laboratory of Special Energy Materials (Nanjing University of Science and Technology),Ministry of Education,Nanjing,210094,China
Keywords:Gradiently denitrated gun propellant One-step green method Denitration Progressive burning Stability
ABSTRACT Gradiently denitrated gun propellant (GDGP) prepared by a “gradient denitration” strategy is obviously superior in progressive burning performance to the traditional deterred gun propellant.Currently,the preparation of GDGP employed a tedious two-step method involving organic solvents,which hinders the large-scale preparation of GDGP.In this paper,GDGP was successfully prepared via a novelty and environmentally friendly one-step method.The obtained samples were characterized by FT-IR,Raman,SEM and XPS.The results showed that the content of nitrate groups gradiently increased from the surface to the core in the surface layer of GDGP and the surface layer of GDGP exhibited a higher compaction than that of raw gun propellant,with a well-preserved nitrocellulose structure.The denitration process enabled the propellant surface with regressive energy density and good progressive burning performance,as confirmed by oxygen bomb and closed bomb test.At the same time,the effects of different solvents on the component loss of propellant were compared.The result showed that water caused the least component loss.Finally,the stability of GDGP was confirmed by methyl-violet test.This work not only provided environmentally friendly,simple and economic preparation of GDGP,but also confirmed the stability of GDGP prepared by this method.
1.Introduction
High muzzle velocity and long range are the goal of barrel weapons in modern war,and the preparation of gun propellant with progressive burning performance is a crucial approach to realize this goal [1—4].Gradiently denitrated gun propellant(GDGP),prepared by removing energetic functional groups(—O—NO2) in the surface layer of gun propellant,is a novel progressive burning gun propellant [5].Compared with traditional deterred gun propellant,GDGP exhibits remarkable advantages,such as avoiding usage of toxic phthalate deterrent agent,no migration of deterrent component,less muzzle smoke flame and residue [6—8].More recently,Li et al.[5,9]have successfully prepared GDGP by employing single-base gun propellant grains with a help of a “first swelling and then denitration” two-step method.The single-base gun propellant grains were pretreated with an appropriate organic swelling solvent in order to increase molecular chains space,thus facilitating the penetration of the denitration agent into the nitrocellulose chains and prepare GDGP under mild conditions.However,the organic swelling solvents would cause environmental pollution and waste of resources,which is against the conception of green chemistry and sustainable development[10,11].Meanwhile,the use of organic solvents may lead to the loss of stabilizers in the propellant (such as diphenylamine and centralite II) and the dissolution of surface nitrocellulose.Stabilizer is an important functional component of propellant,which is designed to absorb nitrogen oxides generated during propellant decomposition,producing various stable derivatives to reduce or terminate the autocatalytic decomposition of propellant [12,13].Therefore,the loss of stabilizer may reduce the storage life of propellant,and even cause spontaneous combustion,explosion and other security hazards [14,15].
The greening of chemical reaction solvents is an inevitable trend of green chemistry [16,17].Recently,chemists have been eagerly looking for non-toxic,environmentally friendly,recyclable green reaction solvents,such as water [18,19],ionic liquids [20],supercritical carbon dioxide [21],polyethylene glycol [22]and other green reaction media[23].Among them,water is an ideal reaction medium to replace traditional organic solvents because it is abundant,cheap,non-toxic and non-polluting.In the treatment of waste explosive,the waste nitrocellulose was normally denitrified in the water.Christodoulatos et al.[24]studied the relationship between the hydrolysis conditions and the denitration of nitrocellulose waste in water.Li et al.[25]studied the denitration process of military nitrocellulose waste in water,and the reaction yield model of nitrocellulose alkaline hydrolysis reaction was developed.Based on the mentioned above,in this study,we first report the use of hydrazine hydrate aqueous solution as a denitration reagent to prepare GDGP via a simple and green one-step route,in which the energetic functional groups in the surface of the propellant were removed directly.The gradient distribution of nitrate groups from the surface to the core along the radius of the prepared GDGP was confirmed by structural characterization.Meanwhile,a comparative study regarding the effects of preparation conditions on the energy,burning properties and components loss of propellant was performed.This method not only simplified the previously reported two-step process,but also made the preparation of GDGP better in line with the concept of green chemistry better.The use of water solvent,simple preparation process and low component loss rate of propellant pave the way for the large-scale preparation of GDGP.
2.Experiment
2.1.Materials
Extruded perforated single-base gun propellant grains (nitrocellulose content is 96%,nitrogen content of nitrocellulose is 13.15%,diphenylamine content is 0.85%,centralite II content is 1.46%,mean web thickness is 0.8 mm,mean perforation diameter is 0.2 mm,mean length is 3.0 mm)were provided by Luzhou North Chemical Industries Co.,Ltd.(Luzhou,China).Hydrazine hydrate(N2H4∙H2O,AR) was purchased from Chengdu Kelong Chemical Co.,Ltd.(Chengdu,China).Ethanol (AR) and ethyl acetate (AR) were purchased from Sinopharm Chemical Reagent Co.,Ltd.(Shanghai,China).Deionized water was produced in laboratory.All chemicals above were used directly without any further treatment.
2.2.One-step green preparation process of GDGP
One-step green preparation of GDGP is illustrated in Fig.1.Specifically,the dried single-base gun propellant grains(200 g)was added to a 1 L three-necked flask containing hydrazine hydrate aqueous solution (400 g).The mixture was stirred with a mechanical stirrer at 80°C in a constant temperature water bath.Different reaction times(30 min and 120 min)and concentration of hydrazine hydrate aqueous solution (10 wt% and 15 wt%) were implemented and investigated.After the reaction,the as-prepared samples were quickly moved to a 2 L beaker containing 1.5 L of deionized water in a constant temperature water bath of 80°C.The mixture was magnetically stirred vigorously for 2 h.Lastly,the GDGP samples were obtained by filtering and drying in a water bath oven at 60°C for 48 h.The labels and experimental parameters of raw single-base gun propellant and GDGP were listed in Table 1.
The denitration reaction mechanism of nitrocellulose and hydrazine hydrate can be summarized as follows [26]:
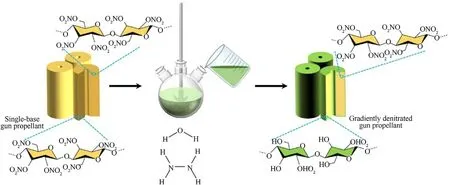
Fig.1.Illustration of one-step green preparation for GDGP.
In the first place the nitrate groups of the nitrocellulose on the surface of gun propellant reacts with the hydrazine molecule to generate the nitrohydrazine cation and the corresponding anion.The intermediate product further reacts,and part of original nitrate groups on the surface layer of gun propellant are converted into a hydroxyl group,and the hydrazine molecule is converted into a nitrohydrazine.Subsequently,nitrohydrazine reacts with excess hydrazine to produce tetrazene,nitrous acid and diazene.Then the tetrazene decomposes to form ammonia and nitrogen.Meanwhile,the diazene produces tetrazene,hydrazine and nitrogen.Hydrazoic acid and ammonia are then formed by the tetrazene decomposition.
2.3.The effects of different solvents on the loss of propellant components
In order to investigate the influence of organic solvents and deionized water on the loss of propellant components,samples were prepared according to the experimental parameters in Table 2.Specifically,dried single-base gun propellant grains(10 g±0.001 g)were mixed with 40 mL reaction solutions(solvents include ethanol-ethyl acetate,ethanol and deionized water),and performed magnetic stirring at different reaction temperature(40°C and 80°C) for 1 h.Finally,the samples were collected,washed and dried in a water bath oven at 60°C for 48 h.
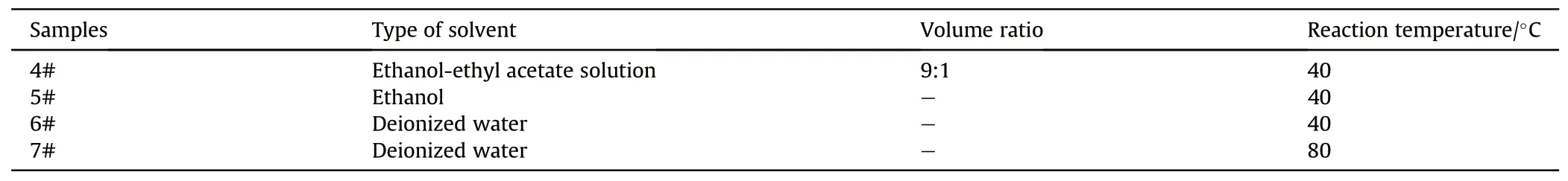
Table 2 Experimental parameters of propellant in different solvents.
2.4.Characterization
2.4.1.Structural characterization
The functional groups of the samples were characterized by Fourier transform infrared spectrometer (FT-IR,Thermo Fisher Nicolet iS10,America),the spectral resolution was 4 cm-1and the range of measurement was 4000—700 cm-1.Raman spectrometer(Renishaw in Via,UK)was used to obtain the information of nitrate groups of the sample at different depths.Specifically,a specific section of the sample was scanned in mapping mode by Live Track function with scanning area of 100 × 40 μm2,step length of 1 × 1 μm2,and exposure time of 1 s.All Raman spectra were obtained in the wavenumber range from 900 cm-1to 1500 cm-1using a laser at 532 nm.Scanning electron microscope (SEM,FEI Quanta 250 FEG,America)with 5 kV accelerating voltage was used to characterize the microstructure of the samples.Surface elemental analysis of the samples were performed by an X-ray photoelectron spectrometer (XPS,Thermo Scientific ESCALAB 250Xi,America) probed with a monochromated Al Ka radiation source (hν=1486.65 eV) in a vacuum chamber.The binding energies of the spectra were calibrated according to the C 1s peak at 284.6 eV.
2.4.2.Energy performance evaluation
The energy performance could be reflected by the explosion heat(Qv)[27,28],measured by adiabatic calorimetric oxygen bomb under vacuum condition with samples weighing 6 g±0.0002 g.The explosion heat was then calculated based on the Hess's law,according to Eq.(6).TheQvof each sample was measured three times with the average results reported.
whereQv: explosion heat of the sample (J/g);Δt: temperature variation(K);C:heat capacity value of the calorimeter system(J/K);E: ignition energy (J);m: mass of the sample (g).
2.4.3.Burning performance evaluation
The constant volume combustion and progressive burning performance of gun propellant were evaluated by a 49.5 cm3closed bomb test at a loading density of 0.2 g/cm3[29].Nitrocellulose(0.486 g,nitrogen percent is 12.0 wt%)was used as ignition powder and the initial pressure of the ignition powder was 10 MPa.The dynamic vivacity versus relative pressure (L-B) curve was calculated in terms of Eq.(7) and Eq.(8) [5].
whereLis dynamic vivacity(MPa-1∙s-1);pmis maximum pressure of gas (MPa);andBis relative pressure.
2.4.4.Propellant components content test
Reversed-phase high performance liquid chromatograph (RPHPLC,WUFENG LC-100,China) was used to test the content of diphenylamine and centralite II in the propellant.The test method refers to the Chinese Military Standard GJB770B-2005.The samples(1.0000 g ± 0.0005 g),the standard solution of internal standard and 20 mL acetone were added into a dry conical flask with astopper,followed by stirring in a magnetic stirrer until sample completely dissolved,adding 15 mL methanol.The nitrocellulose in the solution was flocculated by adding 15 mL of deionized water under constant shaking.The compound was poured into the centrifuge tube evenly and centrifuged for 20 min.5 μL centrifuged sample solution was injected into the chromatograph for three times to acquire the average height value of each chromatogram peak.The mass fraction of each componenti(Wi)in the sample was calculated by Eq.(9):
wheremsiis the mass value of the internal standard substance in the sample (g);hiis the peak height value of componention the chromatogram (mV);fsiis the relative calibration factor of componentiand internal standard substance;mis the mass value of the sample (g);hsis the peak height value of the internal standard substance on the chromatogram (mV).
Nitrocellulose content was studied by nitrocellulose subtraction method according to Chinese Military Standard GJB770B-2005.
2.4.5.Stability evaluation
The stability of gun propellant was evaluated by a methyl violet constant temperature tester (JJZ-A,China).The test method refers to the Chinese Military Standard GJB770B-2005.Sample was heated in a test-tube at 134.5°C with a sample mass of 2.5 g±0.1 g in the presence of a methyl violet paper.Subsequently,time was measured in minutes until the color of the paper changes from purple to orange.The discoloration time of methyl violet constant temperature tester more than 70 min can prove that the stability of the sample was qualified.
3.Results and discussions
3.1.Structural characterization of GDGP
FT-IR was applied to investigate the structure and functional group composition of the raw single-base gun propellant and GDGP prepared by one-step green method.Fig.2(a) displays the FT-IR spectra of 0# and 3#.The characteristic peaks at 1654 cm-1,1272 cm-1and 830 cm-1are attributed to the —NO2group of nitrocellulose[30—32].The peak at 1160 cm-1refers to C—O group asymmetric stretching vibration of nitrocellulose [33,34].These characteristic peaks were still observed as main peaks without significant intensity change of 3#,indicating the well-preserved nitrocellulose structure after denitration reaction.The broad peak near 3400 cm-1belongs to —OH group [35,36].It was also remarkable from Fig.2(a)that 3#outer displayed a relative increase in the peak intensities of hydroxyl group with respect to 0# outer,which indicated the denitration reaction converted part of the nitrate ester groups in the surface layer of GDGP into hydroxyl groups[37,38].Meanwhile,the reduction of nitrate groups could be characterized byIx/I1160,wherexis the wave number corresponding to the nitrate groups characteristic peak position.TheIx/I1160values of 0#outer,3#inner and 3#outer were shown in Fig.2(b).TheIx/I1160values of the 0#outer and 3#inner were almost equal and higher than those of 3# outer,which indicated the denitration reaction only occurred in the outer layer of gun propellant.
To further understand the distribution of nitrate groups in the GDGP surface layer,we need to obtain more experiment results to support it.Taking the 3# as a representative sample,the sectional sample of 3# was measured at 2 μm,5 μm,20 μm,40 μm,60 μm and 80 μm from the surface respectively by Raman,shown in Fig.3(a).The characteristic peak near 1270 cm-1belongs to NO2group and the characteristic peak near 1100 cm-1is attributed to C—O—C [39].The intensity of nitrate groups increased along with the deepened distance to the surface of propellant.
The distribution of nitrate groups in samples was clarified by performing cross sectional Raman image mapping of sample 0#and 3#.The scanning area was indicated by the squared region,as shown in Fig.3(b).Different nitrate group content showed different Raman activity and signal intensity,which could be characterized by the ratio value ofI1270/I1160.The Raman image mappings of sample 0# and 3# were shown in Fig.3(c) and (d).Fig.3(c) displayed that the uniform spatial distribution of nitrate groups in the surface layer of 0#.The Raman image mapping of sample 3#clearly demonstrated the content of nitrate groups increases gradually from the surface to the inner 50 μm and the content of the nitrate groups tended to be a constant value at a depth of 50 μm—100 μm,which correlated with the results of FT-IR (Fig.2) quite well.This result further confirmed that the nitrate groups gradiently increased from the surface to the core in the surface layer of GDGP.
The effect of the denitration reaction on the microstructure of the gun propellant was studied by SEM and the results were shown in Fig.4.The surface layer and internal structures of the 0#sample were shown in Fig.4(a) and (c),respectively.The surface and internal structure of the 3# sample were shown in Fig.4(b) and (d).As seen from Fig.3,the results of Raman indicated that the denitration depth of 3#sample was about 50 μm,from Fig.4(a)and(b),the 0#and 3#samples contained a lot of fibrous substances in the range of 50 μm from the surface,consistent with the results reported in reference[40].In addition,the surface layer of the denitrified sample was more compact than the raw gun propellant.The nitrocellulose could from hydrogen bonds with the —OH groups through the oxygen atoms of the—OH groups,the oxygen atoms in the main chain,the oxygen and nitrogen atoms of the—NO2group,and the oxygen atoms attached to the —NO2group.Based on the results of FT-IR (Fig.2),the denitration reaction increased the number of —OH groups,resulting in more hydrogen bonds and stronger polymer chain forces[41,42].The internal structure of the 0#sample and the 3#sample were the same,which indicated the inner layer was not involved in the denitration reaction.This result was in agreement with the result of FT-IR and Raman spectroscopy.
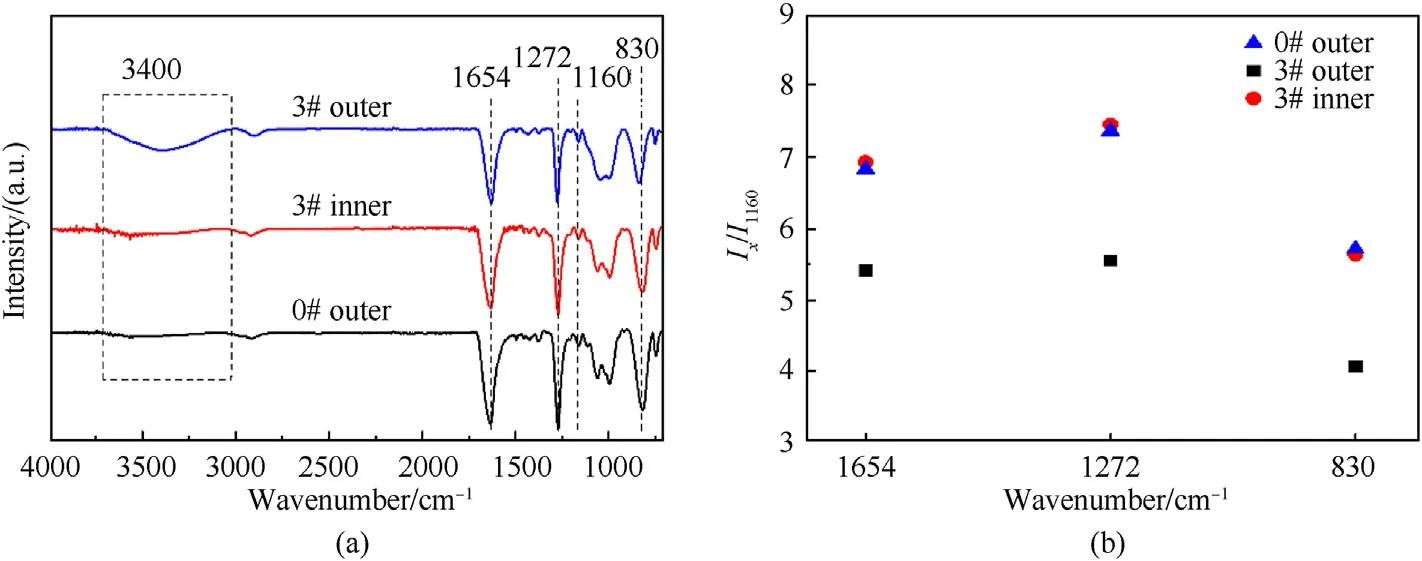
Fig.2.FT-IR spectra of samples:(a)curve 0#outer:the surface of raw signal-base propellant;curve 3#inner:the inner of 3#;curve 3#outer:the surface of 3#;(b)the values of the Ix/I1160 (x=1654 cm-1,1272 cm-1 and 830 cm-1) of 0# outer,3# outer and 3# inner.
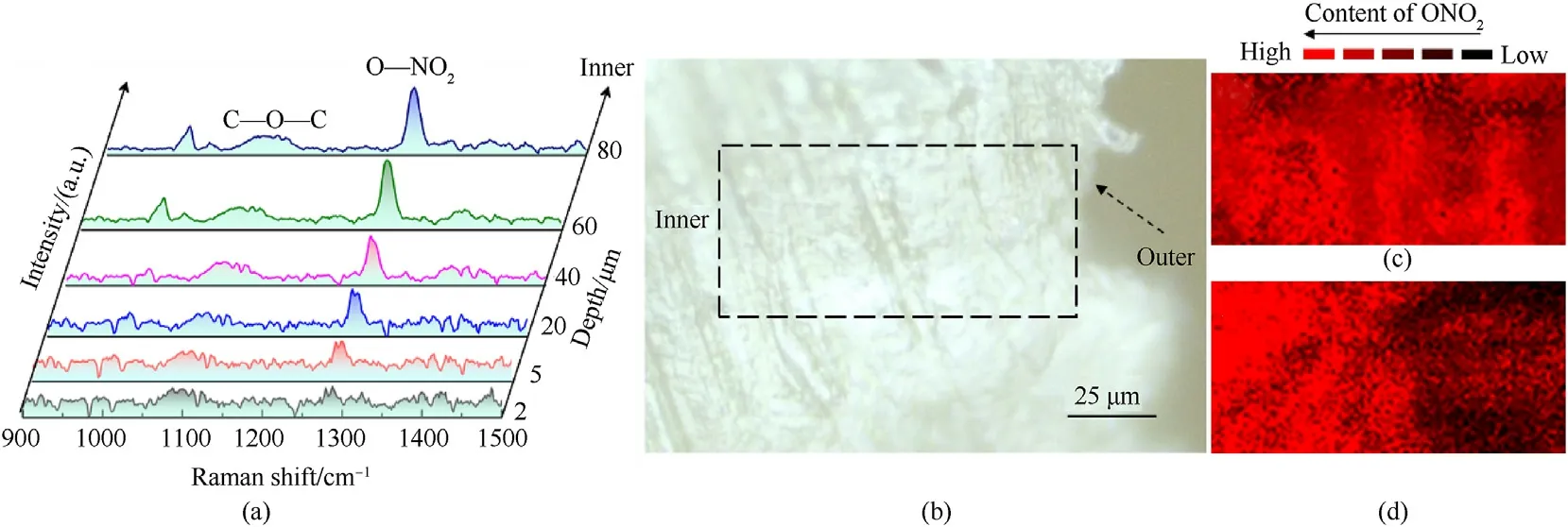
Fig.3.Raman spectra of samples:(a)3#sample Raman spectra of 2 μm,5 μm,20 μm,40 μm,60 μm and 80 μm from the outside;(b)microscope image of propellant sample profile and black rectangle defines the mapped area;(c) the ONO2 mapping of raw gun propellant;(d) the ONO2 mapping of 3#.

Fig.4.SEM images of profile of samples:(a),(b)the microstructure of the surface layer of 0# and 3#;(c),(d) the microstructure of the inner layer of the 0# and 3#.
We have also investigated the chemical property and chemical bonding of the propellant samples by carrying out XPS study.The full surface survey and individual spectra of sample 0#and 3#were shown in Fig.5.As shown in Fig.5(a),the total intensity for each sample has been normalized to unity and the survey spectrums clearly indicated the existence of C,N and O elements.The characteristic peak positions of the sample 0# and 3# did not change significantly before and after the denitration reaction experiment,suggesting that the denitration reaction method preserved the skeleton structure of nitrocellulose well,which was consistent with the result of SEM in Fig.4.Furthermore,compared with 3# inner and 0# outer surface,the characteristic peak of N element in 3#outer surface spectrum was obviously weakened,which confirmed that the denitration reaction occurred on the outer surface of propellant.
The curve fitting of the C 1s and O 1s spectra revealed the functional composition of the carbon and oxygen species for each sample,as shown in Fig.5(b)and(c).From Fig.5(b),the C 1s spectra of 0#outer,3#inner and 3#outer can be deconvoluted into three Gaussian peaks (C-1,C-2 and C-3).The C-1 corresponds to the bonds of C—C/C—H;the C-2 corresponds to the C—OH bonds;the C-3 is indexed to C—O—NO2[43—45].Compared with 3#inner and 0#outer,it can be found that the Gaussian peaks of 3#outer at C-2 and C-3 shift to lower bonding energy.The characteristic peak of C-2 is closer to the position of C—OH in cellulose [43,46].This phenomenon also indicated that the denitration reaction converted the C—O—NO2bound form to C—OH form in nitrocellulose.The O-1 peak is attributed to O—C bond;the O-2 peak represents the single bond of O—NO2[47,48].Comparing the spectra of different samples,it can be found that the position of the O 1s peak on the outer surface of 3# shifts to the direction of smaller binding energy.It may be caused by the increase of the amount of hydroxyl groups that increases the interaction between carbon and oxygen atoms.This indirectly proved that GDGP had been prepared by one-step green method successfully.
3.2.Energy and burning performances of GDGP
The oxygen bomb method was used to measure the explosion heat (Qv) of samples prepared under different conditions.The energy reduction of gun propellant caused by the denitration process can be evaluated,which can inflect the denitration degree.The results and preparation conditions were listed in Table 3.From Table 3,the denitration degree of gun propellant was improved by prolonging the denitration time and increasing the concentration of denitration reagent.
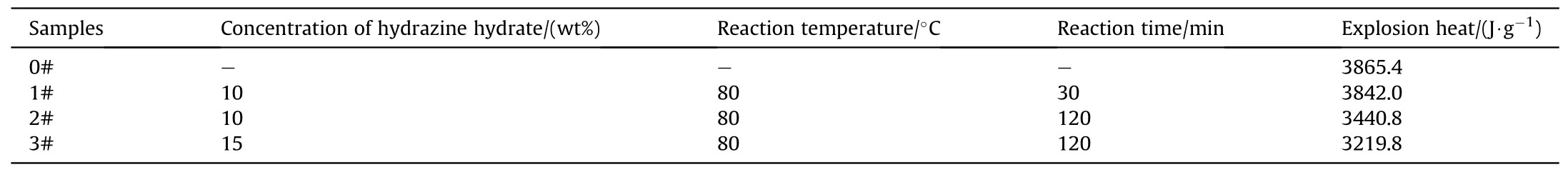
Table 3 The effect of denitration process on explosion heat.
The burning performance is one of the most important properties for gun propellant,thep-tandL-Bcurves of GDGP were shown in Fig.6.The combustion characteristic parameters were listed in Table 4.As shown in Fig.6(a) and Table 4,the maximum burning pressurepmof raw single-base gun propellant was 269.8 MPa and the time corresponding to the maximum burning pressure(tm)was 4.9 ms.After denitration treatment of single-base gun propellants,thepmfor the GDGP reduced andtmincreased.This can be attributed to the reduction of energy during the denitration proved by the explosion heat test.The excellent progressive burning performance for gun propellant is usually accompanied by increase of ΔLandBm[9].For tube weapons,it is necessary to make propellant burning faster for eliminating the influence of increasing volume behind the bullet forwarding.In other words,the gas generated in the decline section ofL-Bcurve is not enough to propel the bullet faster.Thus,the progressive burning performance refers to the rising section ofL-Bcurve.It can be seen that the raw singlebase gun propellant almost did not exhibit progressive burning performance,while samples 1#,2# and 3# exhibited excellent progressive burning performance.Meanwhile,in the experiment range,prolonging the denitrification treatment time or increasing the concentration of hydrazine hydrate aqueous solution could promote the progressive burning performance.
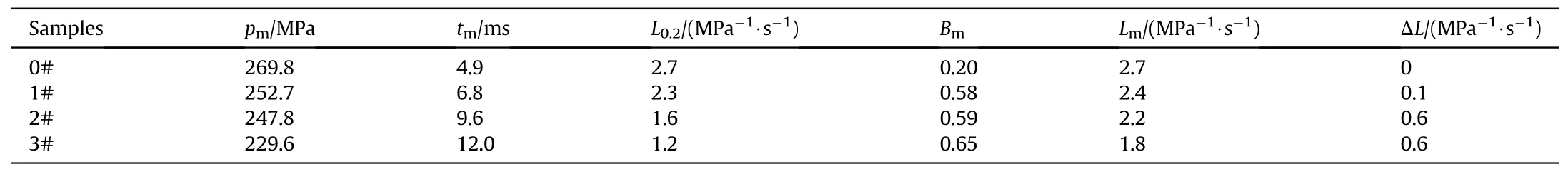
Table 4 Combustion characteristic parameters of samples.
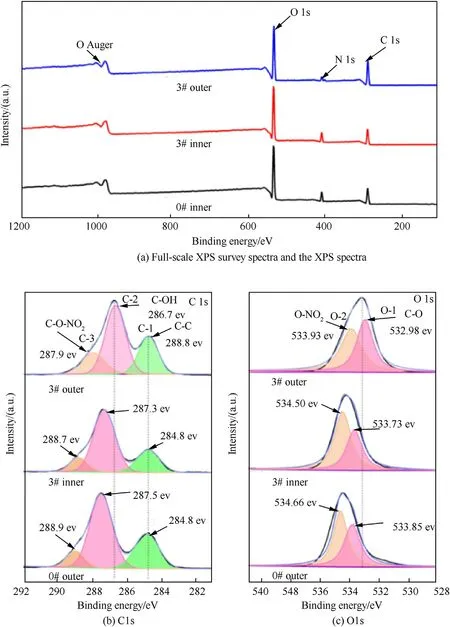
Fig.5.XPS spectra of samples: (a) Full-scale XPS survey spectra and the XPS spectra of (b) C 1s and (c) O 1s.
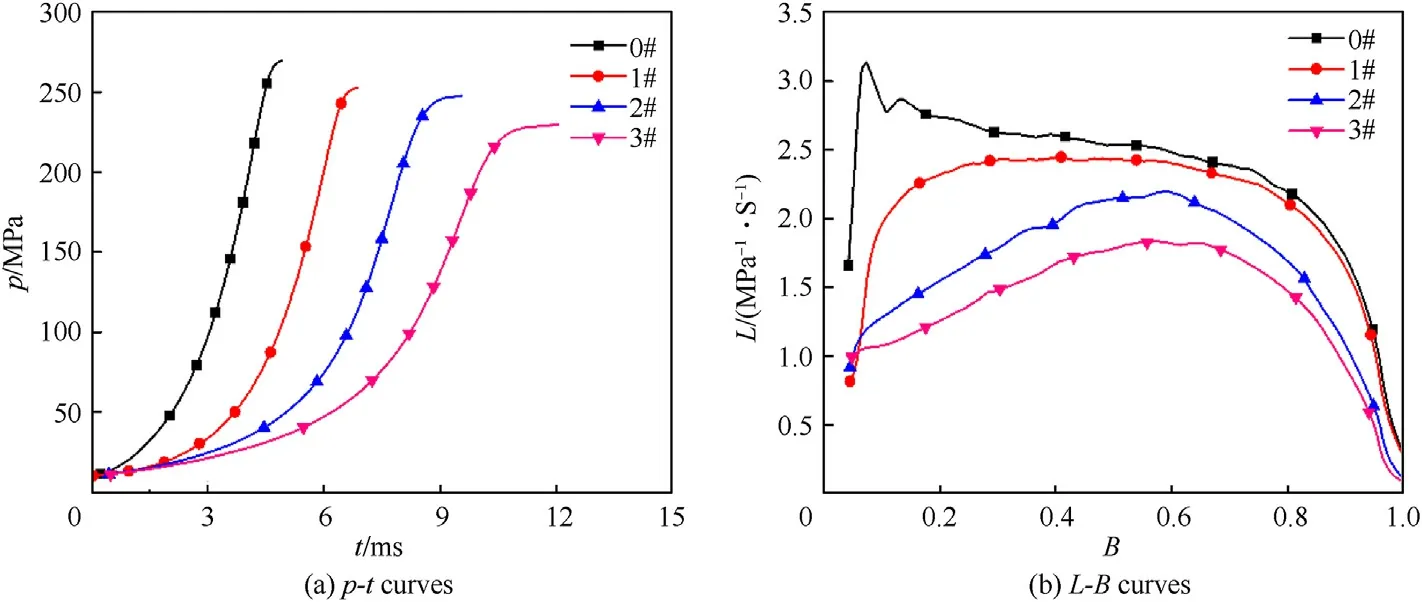
Fig.6.Burning performance of GDGP: (a) p-t curves;(b) L-B curves.
3.3.Loss of propellant components
In order to investigate the influence of organic solvents on the loss of propellant components,samples were prepared according to the experimental parameters in Table 2,based on RP-HPLC and subtraction method.The energy component of single-base propellant used in all samples was nitrocellulose (NC).The functional additives were diphenylamine (DPA) and centralite II (C2),which could enhance the stability of propellant and prolong the storage life [49,50].Similar to the organic solvents reported in previous work[5,9],the loss of propellant components in water was studied.DPA Loss Rate(DPALR),C2 Loss Rate(C2LR)and NC Loss Rate(NCLR)were calculated in Eq.(10),Eq.(11) and Eq.(12) respectively:
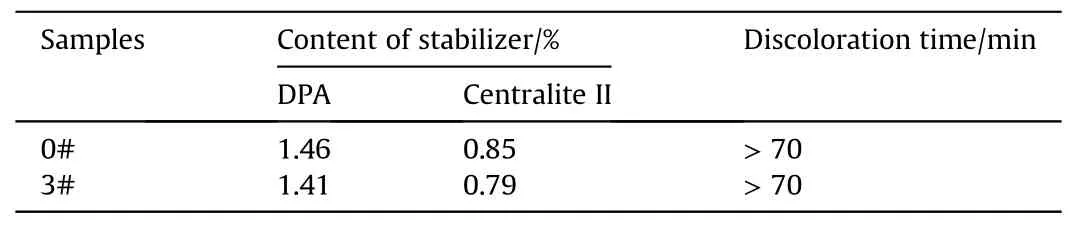
Table 5 Propellant stabilizer content and methyl violet discoloration time.
wDPA0andwDPA1are the mass percentages of DPA before and after preparation process,wt%;wC20andwC21are the mass percentages of C2 before and after preparation process,wt%;m0andm1are the mass of the samples before and after preparation process,g.
The result of loss rates of DPA,C2 and NC were shown in Fig.7.As illustrated in Fig.7,sample 4#and 5#exhibited a greater loss of components,while the components loss for sample 6#and 7#was weaker.This result can be explained by the following reasons.First,for NC,ethyl acetate is a good solvent so that the dissolution of part of NC in the propellant during swelling process is difficult to be avoided.Second,for stabilizers,the swelling solvent (ethyl-ethyl alcohol mixed solution) weakened the intermolecular force of the NC and accelerated the migration of the stabilizers.In addition,the reaction solvent (ethanol) is a good solvent for the stabilizer and can also cause loss of stabilizer.Hence,the use of organic solvents during preparation of GDGP would result in a large loss of components of gun propellant,as well as other problems such as waste of resources,environmental pollution,increased costs.Contrary to organic solvents,using water as a solvent,the component loss of the propellant was greatly reduced because water is a poor solvent for all components in the propellant.
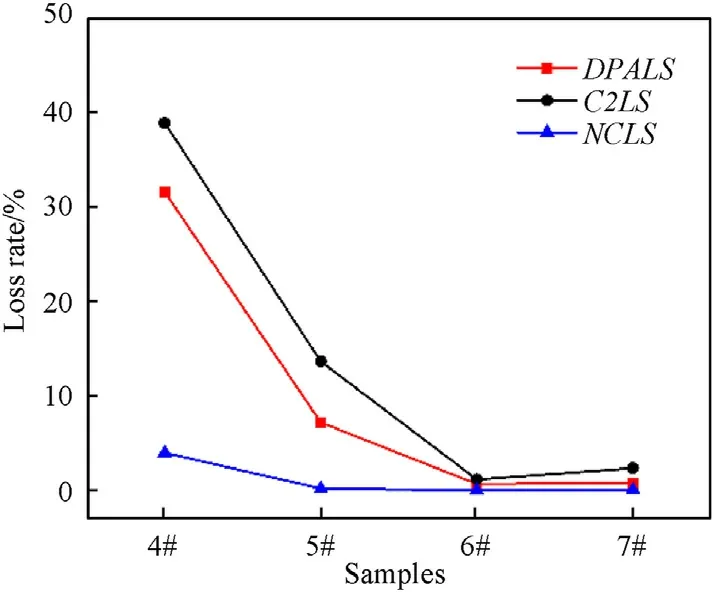
Fig.7.The influence of solvents on the loss rate of components in propellant.
3.4.Stability of GDGP
Nitrocellulose is chemically unstable and easy to undergo autocatalytic decomposition during storage due to the low bond energy of nitrate groups.Autocatalytic decomposition occurs in the presence of water and oxygen,producing alkyl radicals and acidic materials[51,52].In order to study the stability of GDGP,the 0#and 3# samples with the highest denitration degree were analyzed by RP-HPLC and methyl violet test.The results were shown in Table 5.
As shown in Table 5,the denitration process of gun propellant exerted little effect on the loss of stabilizer.The stability kept unchanged from the results of methyl violet test.The stabilizer would absorb the nitrogen oxides produced by the decomposition of the gun propellant,generating a variety of stable derivatives,and reduce or terminate the autocatalytic decomposition of the propellant [53].At the same time,the GDGP preparation process reduced the content of nitrate groups in the propellant and thus reduced the autocatalytic substances,which was conducive to improving the storage stability of the propellant.
4.Conclusions
In this paper,a novelty,environmentally friendly,resourcesaving and simple one-step method was applied to prepare GDGP in a water system successfully.The obtained GDGP were studied from aspects of structures,energy,burning properties,components loss of propellant and chemical stability.The results showed as follows:
(1) The GDGP was successfully prepared by one-step method in water system for the first time.Characterized by FT-IR,Raman,SEM and XPS,the nitrate groups gradiently increased from the surface to the core in the surface layer of GDGP,and the surface layer exhibited a higher compaction than raw gun propellant,with a well-preserved nitrocellulose structure.
(2) The one-step green denitration enabled the propellant surface with regressive energy density and good progressive burning performance,as confirmed by oxygen bomb and closed bomb test.In the experiment range,prolonging the denitrification treatment time or increasing the concentration of hydrazine hydrate aqueous solution could promote the progressive burning performance.
(3) The GDGP prepared by one-step method in water system showed stable and safe long-term storage performance.Based on RP-HPLC,subtraction method and methyl-violet test,a comparative analysis of the effects of different solvents(water,ethanol and ethanol-ethyl acetate mixed solution) on the loss of gun propellant components during the preparation of GDGP and the stability of the prepared GDGP was employed.The results demonstrated that the GDGP prepared by the one-step method of the water system had a lower component loss rate and a better long-term storage performance.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
杂志排行
Defence Technology的其它文章
- Dual Attribute Adversarial Camouflage toward camouflaged object detection
- Electrophoretic deposition of hybrid organic-inorganic PTFE/Al/CuO energetic film
- A bi-population immune algorithm for weapon transportation support scheduling problem with pickup and delivery on aircraft carrier deck
- Benchmark calculations and error cancelations for bond dissociation enthalpies of X—NO2
- GO/HTPB composite liner for anti-migration of small molecules
- Resilient tightly coupled INS/UWB integration method for indoor UAV navigation under challenging scenarios
