Divalent nitrogen-rich cationic salts with great gas production capacities
2023-05-06HaoGuChengchuangLiChanghaoDaiDongxuChenHongweiYangGuangbinCheng
Hao Gu,Cheng-chuang Li,Chang-hao Dai,Dong-xu Chen,Hong-wei Yang,Guang-bin Cheng
School of Chemistry and Chemical Engineering,Nanjing University of Science and Technology,Xiaolingwei 200,Nanjing,210094,PR China
Keywords:Fused cyclic compound Triazole Divalent cation Gas production Energetic materials
ABSTRACT Monocyclic nitrogen-rich 3-(aminomethyl)-4,5-diamine-1,2,4-triazole (1) and fused cyclic 3,7-diamine-6-(aminomethyl)-[1,2,4]triazolo [4,3-b][1,2,4]triazole (9) were synthesized through the convenient cyclization reaction from the readily available reactant.Their energetic salts with high nitrogen content were proved to be rare examples of divalent monocyclic/fused cyclic cationic salts according to the single crystal analyses.The structure of intermediate B was also identified and verified by its trivalent cation crystal 17.5H2O indirectly.Energetic compounds 2—8 and 10—17 were fully characterized by NMR spectroscopy,infrared spectroscopy,differential scanning calorimetry,elemental analysis.These energetic salts exhibit good thermal stability with decomposition temperatures ranged from 182 °C to 245 °C.The sensitivity of compounds 2, 6,10 and 14 is similar or superior to that of RDX while the others were much more insensitive to mechanical stimulate.Furthermore,detonation velocity of 10 (8843 m/s)surpass that of RDX(D=8795 m/s).Considering the high gas production volume(≥808 L/kg) of 2,4,10 and 12,constant-volume combustion experiments were conduct to evaluate their gas production capacities specifically.These compounds possess much higher maximum gas-production pressures (Pmax:7.88—10.08 MPa) than the commonly used reagent guanidine nitrate (GN: Pmax=4.20 MPa),which indicate their strong gas production capacity.
1.Introduction
As an important role in the research of energetic materials,gas generant has very extensive applications in the military field as well as the civil [1—5].The main component of gas generant is usually high nitrogen energetic compounds with great gas production capacities.However,the traditional gas generant represented by sodium azide suffers from some remarkable drawbacks,especially the toxicity of azides [6—8].Therefore,the increased requirements,such as cost reduction,less harsh synthesis conditions,moderate detonation performance,good stability and low toxicity,must be taken into consideration for developing the new candidates for gas generant.
The synthesis of nitrogen-rich energetic salts is a feasible method to seek qualified component for gas generant [9—13].According to the reported literature,the nitrogen-rich heterocyclic energetic salts have been proved to possess several advantages over conventional neutral molecules,such as thermal stability and lower vapor pressure as well as good stabilities towards mechanical stimulation [14—16].Generally,the heterocyclic rings including pyrazole,triazole and tetrazole are essential structure for high nitrogen energetic salts.Among them,1H-1,2,4-triazole was favored by scientists for its high nitrogen content(60.84%),positive heat of formation (182 kJ/mol) and flexible modifiability [17—20].Some classic high nitrogen cations were based on 1,2,4-triazole ((a)-(c)).Besides,the fused cyclic heterocyclic structure based on 1,2,4-triazole has attracted great attention in the past few years.Because the delocalization of electron distribution in fused ring backbone,the fused heterocyclic system exhibits much more aromatic and better planarity than the single triazole framework.The resulting strong π-π conjugation effect contributes to better stability [21].The massive C—N and N—N bonds in the fused ring structure give the fused heterocycle huge energy reserves,which contribute markedly to the high heat of formation[22—24].Owing to the electron-donating effect of the amino group,polyamine fused hetercycles tend to form energetic cationic salts when react with energetic acids.In recent years,some newly reported nitrogen-rich fused cyclic cations,such as 3,7-diamino-[1,2,4]triazolo [4,3-b][1,2,4]triazolium (d),3,6,7-triamino-[1,2,4]triazolo[4,3-b][1,2,4]triazolium(e)and 3,6-diamino-[1,2,4]triazolo[4,3-b][1,2,4]triazolium (f),were reported as monovalent cations (Fig.1)[25—27].In contrast,divalent fused cyclic cations were rarely reported due to the limitation of cationic site.Compared with the monovalent energetic salts,divalent energetic salts would have better detonation properties because they can aggregate more high-energy components at the same time [28,29].Furthermore,the large amounts of amino groups in the cation make it advantageous to develop intra-and intermolecular hydrogen bond interactions with an anion containing strong polar groups (e.g.nitro or nitramine groups),which could stabilize the compound structure and alleviate its sensitivity to mechanical stimulation [30,31].
In this work,3-(aminomethyl)-4,5-diamine-1,2,4-triazole (1)and the fused cyclic compound 3,7-diamine-6-(aminomethyl)-[1,2,4]triazolo [4,3-b][1,2,4]triazole (9) were synthesized from common chemical raw material glycine via simple and short steps.Their divalent cationic salts were characterized by single crystal Xray diffraction,IR,NMR spectroscopy,elemental analysis and DSC.Besides,the synthetic intermediateBwas determined and clarified the process of the cyclization reaction.Furthermore,compounds2,4,10and12with good gas production capacities underwent the measurement of constant-volume combustion experiments to evaluate their potential as component of gas generant.
2.Experimental
2.1.Safety precautions
Cation! All the new compounds mentioned in this manuscript are potential energetic compounds with the possibility of explosion.Mechanical stimulation and static electricity should be avoided during synthesis and storage.Protective equipment,such as face shields,safety glasses,protective clothing,gloves,are strongly recommended for laboratory staffs.
2.2.Synthesis
The general synthesis route and the design thought of fused cycle were shown in Fig.2.The energetic anions donors used in the manuscript were synthesized according to the literatures[32—37].
2.2.1.3-(Aminomethyl)-4,5-diamine-1,2,4-triazole (1)
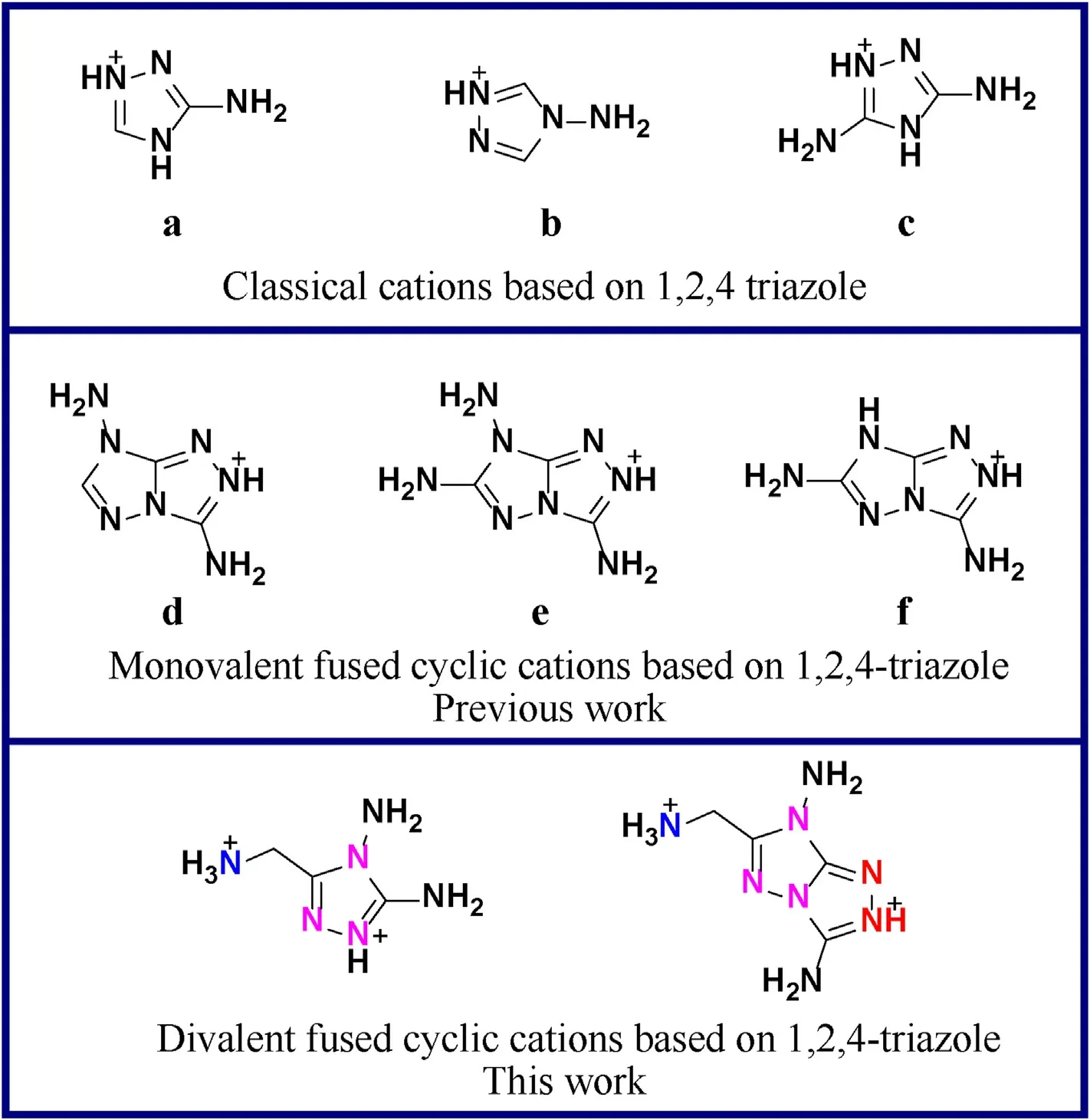
Fig.1. Scheme of monovalent and divalent heterocyclic cations based on 1,2,4-triazole.
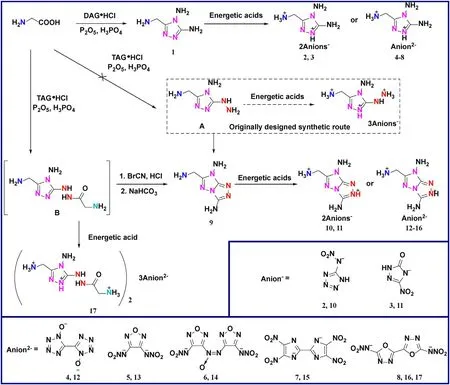
Fig.2.Scheme of synthesis of neutral compounds 1,9,divalent energetic salts 2—8,10—16 and trivalent energetic salt 17.
Phosphorus pentoxide (20.0 g) was dissolved in orthophosphoric acid(85 wt.%,70.0 g)in a three-necked flack under stirring at 40°C.Glycine (7.5 g,100.0 mmol) and diaminoguanidine hydrochloride (16.31 g,130.0 mmol) was added to the clear solution slowly to avoid agglomeration.The mixture was then heated gradually to 120°C and was gradually clarified during 12 h of reaction.After cooling naturally to 80°C,the reaction solution was poured to 200 g crushed ice.The resulting solution was carefully neutralized with saturated NaOH solution (50.0 g NaOH in 100.0 mL H2O)to pH=7 and stirred for 1 h.A large amount of white solid precipitates and was collected by filtration.It should be noted that the excessive addition of lye would make the precipitates dissolved again and the product could not precipitate again after reacidification.The crude product was purified by hot water and dried in the air to yield compound1as white solid.Yield: 9.58 g,73.1%.Compound 1 has poor solubility in most of the solvents and is hardly soluble in dimethylformamide (DMF) and dimethylsulfoxide (DMSO).IR (KBr pellet): 2862,2598,1695,1633,1557,1094,1044,978,914,873,788,738,703,655,584,556 cm-1.Elemental analysis calcd (%) for C3H8N6(128.13): C 28.12,H 6.29,N 65.59;found: C 28.16,H 6.33,N 65.64.
2.3.General procedure for energetic salts 2-8
The energetic acid(2.0 mmol for2and3,1.0 mmol for4—8)was dissolved in 5.0 mL deionized water,respectively.Subsequently,3-(Aminomethyl)-4,5-diamine-1,2,4-triazole (0.128 g,1.0 mmol) was added to the above solution.The reaction mixture was stirred at room temperature for 1 h.The precipitate was collected by filtration,washed with cold water (5.0 mL) and dried in the air to give the products2—8.
2.3.1.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium bis(5-nitraminotetrazolate) (2)
White solid.Yield:0.285 g,73.4%.1H NMR(500 MHz,DMSO‑d6):8.50 (s,3H),8.36 (s,2H),5.95 (s,2H),4.21 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6): 157.72,151.67,146.32,32.54 ppm.IR (KBr pellet): 3470,3092,2995,1688,1558,1519,1418,1306,1228,1171,1093,1055,1035,974,859,775,743,714,696,608 cm-1.Elemental analysis calcd(%)for C5H12N18O4(388.27):C 15.47,H 3.12,N 64.94;found: C 15.42,H 3.15,N 64.97.
2.3.2.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium bis(3-nitro-5-one-1,2,4-triazolate) (3)
White solid.Yield:0.315 g,81.1%.1H NMR(500 MHz,DMSO‑d6):8.02 (brs,5H),6.11 (s,2H),4.17 (s,2H) ppm.13C NMR (125 MHz,D2O-d2):159.1,152.2,151.4,145.9,32.86 ppm.IR(KBr pellet):3370,3220,3121,2971,1695,1540,1340,1045,1016,980,915,874,786,756,725,702,656,547,541 cm-1.Elemental analysis calcd (%) for C7H12N14O6(388.26):C 21.65,H 3.12,N 50.51;found:C 21.61,H 3.13,N 50.55.
2.3.3.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium 1,1′-diolate-5,5′-bistetrazolate (4)
White solid.Yield:0.254 g,85.1%.1H NMR(500 MHz,DMSO‑d6):8.57 (s,3H),8.38 (s,2H),5.97 (s,2H),4.20 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6):155.3,144.5,135.0,32.9 ppm.IR(KBr pellet):3343,3314,3153,3052,1690,1615,1522,1410,1348,1282,1238,1164,1062,1042,1016,998,990,920,861,726,668,607,594,576,544 cm-1.Elemental analysis calcd (%) for C5H10N14O2(298.23): C 20.14,H 3.38,N 65.75;found: C 20.17,H 3.42,N 65.78.
2.3.4.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium 3,4-di(nitramino)furazanate (5)
White solid.Yield:0.251 g,78.8%.1H NMR(500 MHz,DMSO‑d6):7.92 (brs,5H),6.00 (s,2H),4.31 (s,2H) ppm.13C NMR (125 MHz,DMSO‑d6): 152.8,152.3,146.2,32.7 ppm.IR (KBr pellet): 3298,3060,1677,1627,1544,1508,1482,1419,1289,1121,1064,1020,997,897,882,836,769,742,725,687,610,591 cm-1.Elemental analysis calcd(%)for C5H10N12O5(318.21):C 18.87,H 3.17,N 52.82;found:C 18.91,H 3.14,N 52.81.
2.3.5.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium 4,4′-bis(nitramino)azoxyfurazanate (6)
Yellow solid.Yield: 0.385 g,89.4%.1H NMR (500 MHz,DMSO‑d6): 8.50 (s,3H),8.39(s,2H),5.94(s,2H),4.21 (s,2H) ppm.13C NMR (125 MHz,DMSO‑d6): 154.8,153.8,151.5,151.1,146.3,32.5 ppm.IR(KBr pellet):3322,3158,1691,1562,1527,1490,1438,1388,1312,1049,1011,945,820,770,754,708,674,619,607,586 cm-1.Elemental analysis calcd (%) for C7H10N16O7(430.26): C 19.54,H 2.34,N 52.09;found: C 19.51,H 2.36,N 52.06.
2.3.6.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium 4,4′,5,5′-tetranitro-bis-imidazolate (7)
Red solid.Yield: 0.333 g,75.2%.1H NMR (500 MHz,DMSO‑d6):8.82 (s,3H),8.34 (s,2H),6.04 (s,2H),4.37 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6): 151.7,146.4,143.6,140.1,32.6 ppm.IR (KBr pellet): 3597,3553,3225,3145,1689,1522,1494,1485,1463,1397,1366,1308,1227,1207,855,812,794,754,718,699,576,559 cm-1.Elemental analysis calcd (%) for C9H10N14O8(442.26): C 24.44,H 2.28,N 44.34;found: C 24.42,H 2.25,N 44.31.
2.3.7.3-(Ammoniomethyl)-4,5-diamino-1,2,4-triazol-1-ium 5,5’-dinitramino-2,2’-bi(1,3,4-oxadiazolate) (8)
White solid.Yield:0.281 g,72.4%.1H NMR(500 MHz,DMSO‑d6):8.54 (s,3H),8.32 (s,2H),5.94 (s,2H),4.20 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6): 165.9,151.6,148.1,146.3,32.5 ppm.IR (KBr pellet): 3269,3154,2993,1716,1632,1607,1514,1491,1435,1423,1392,1303,1273,1155,1068,1039,1017,964,891,851,771,752,720,661,610,586 cm-1.Elemental analysis calcd (%) for C7H10N14O6(386.24):C 21.77,H 2.61,N 50.77;found: C 21.74,H 2.59,N 50.75.
2.3.8.3,7-Diamine-6-(aminomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazole (9)
The preparation of intermediateAis slightly different from that of compound1.Glycine(7.5 g,100.0 mmol)and triaminoguanidine hydrochloride (21.07 g,150.0 mmol) was slowly added to the prepared solution of phosphorus pentoxide (25.0 g) and orthophosphoric acid(85 wt.%,80.0 g)in a three-necked flack under stirring at 40°C.After reacting for 24 h at 120°C,the reaction mixture turned into a pale yellow clear solution.The resulting solution was subsequently poured into 250 g crushed ice.Then it was carefully neutralized with saturated NaOH solution (50.0 g NaOH in 100.0 mL H2O) to pH=6 and stirred for 48 h.The precipitate was collected and washed with ice water.The precipitate was dried in the air to afford 5.73 g of a white solid.The product was used directly in the next step without further purification.The intermediateB(3.0 g) was dissolved in 50.0 mL diluted hydrochloric acid(2.0 M).The cyanogen bromide(6.36 g,60.0 mmol)was added instantly.The resulting solution was stirred at 60°C for 3 h.Then it was heat to reflux for additional 10 h.After cooling to room temperature,the solvent was removed and 15.0 mL deionized water was added to re-dissolve the residue.The solution was neutralized carefully by sodium bicarbonate and stirred for 30 min.The precipitate was filtrated,washed by cold water and dried in the air to give 3,7-diamine-6-(aminomethyl)-[1,2,4]triazolo [4,3-b][1,2,4]triazole as white solid.Yield: 1.54 g,61.4%.Compound9has poor solubility in most of the solvents and is hardly soluble in dimethylformamide (DMF) and dimethylsulfoxide (DMSO).IR (KBr pellet):3127,2966,1698,1679,1638,1546,1398,1344,1281,1179,1042,980,862,847,730,659,626,603,576 cm-1.Elemental analysis calcd (%) for C4H8N8(168.16): C 28.57,H 4.80,N 66.63;found: C 28.52,H 4.76,N 66.59.
2.3.9.General procedure for energetic salts 10-16
The energetic acid(2.0 mmol for10and11,1.0 mmol for12—16)was dissolved in 5.0 mL deionized water,respectively.Subsequently,3,7-diamine-6-(aminomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazole (0.168 g,1.0 mmol) was added to the above solution.The reaction mixture was stirred at 50°C for 1 h.The precipitate was collected by filtration,washed with cold water(5.0 mL)and dried in the air to give the products10—16.
2.3.10.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium bis(5-nitraminotetrazolate) (10)
White solid.Yield:0.296 g,69.1%.1H NMR(500 MHz,DMSO‑d6):8.50 (s,3H),8.43 (s,2H),5.96 (s,2H),4.22 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6): 157.7,155.3,148.3,142.1,33.3 ppm.IR (KBr pellet): 3306,3133,2967,2730,1681,1601,1533,1502,1441,1419,1338,1304,1223,1145,1043,990,854,767,729,626,592,573 cm-1.Elemental analysis calcd (%) for C6H12N20O4(428.29): C 16.83,H 2.82,N 65.41;found: C 16.80,H 2.85,N 65.40.
2.3.11.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium bis(3-nitro-5-one-1,2,4-triazolate) (11)
White solid.Yield:0.303 g,70.7%.1H NMR(500 MHz,DMSO‑d6):8.49 (s,3H),8.41 (s,2H),5.94 (s,2H),4.20 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6): 157.6,154.7,151.5,149.8,143.4,33.1 ppm.IR(KBr pellet): 3191,2968,1682,1545,1344,1281,1179,1043,1017,983,848,828,786,754,723,629,601,573,553 cm-1.Elemental analysis calcd(%)for C8H12N16O6(428.29):C 22.44,H 2.82,N 52.33;found: C 22.41,H 2.86,N 52.36.
2.3.12.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium 1,1′-diolate-5,5′-bistetrazolate (12)
White solid.Yield:0.203 g,60.0%.1H NMR(500 MHz,DMSO‑d6):9.13 (s,3H),8.80 (s,2H),5.93 (s,2H),4.37 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6): 157.8,148.2,142.1,135.6,32.6 ppm.IR (KBr pellet): 3136,3122,3025,1678,1498,1411,1351,1242,1230,1166,1081,1003,992,906,887,847,726,625,611,590,573,565 cm-1.Elemental analysis calcd (%) for C6H10N16O2(338.25): C 21.31,H 2.98,N 66.26;found: C 21.28,H 2.95,N 66.22.
2.3.13.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium 3,4-di(nitramino)furazanate (13)
White solid.Yield:0.256 g,71.4%.1H NMR(500 MHz,DMSO‑d6):8.17 (brs,5H),6.02 (s,2H),4.31 (s,2H) ppm.13C NMR (125 MHz,DMSO‑d6):156.4,152.0,148.9,142.6,33.3 ppm.IR(KBr pellet):3308,31,236,3024,1715,1660,1605,1523,1480,1425,1366,1282,1232,1155,1112,1068,1037,1004,986,906,890,880,832,798,776,728,776,690,612,573 cm-1.Elemental analysis calcd (%) for C6H10N14O5(358.23): C 20.12,H 2.81,N 54.74;found: C 20.15,H 2.85,N 54.71.
2.3.14.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium 4,4′-bis(nitramino)azoxyfurazanate (14)
Yellow solid.Yield: 0.326 g,69.3%.1H NMR (500 MHz,DMSO‑d6): 8.69 (s,3H),8.62 (s,2H),6.18 (s,2H),4.38 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6):157.5,154.7,153.8,151.1,148.1,142.0,33.1 ppm.IR (KBr pellet): 3319,3121,3073,2996,1683,1607,1551,1528,1466,1444,1419,1385,1304,1266,1041,947,903,849,820,768,716,651,638,589,572 cm-1.Elemental analysis calcd (%) for C8H10N18O7(470.28): C 20.43,H 2.14,N 53.61;found: C 20.40,H 2.10,N 53.65.
2.3.15.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium 4,4′,5,5′-tetranitro-bis-imidazolate(15)
Orange solid.Yield: 0.398 g,82.5%.1H NMR (500 MHz,DMSO‑d6):8.89(s,3H),8.66 (s,2H),6.24(s,2H),4.53(s,2H) ppm.13C NMR (125 MHz,DMSO‑d6): 157.6,148.2,142.6,142.0,139.7,33.2 ppm.IR(KBr pellet):3598,3401,3322,3134,1700,1684,1607,1529,1482,1457,1399,1368,1335,1312,1271,1112,1020,946,881,853,811,752,698,572,551 cm-1.Elemental analysis calcd (%) for C10H10N16O8(482.29): C 24.90,H 2.09,N 46.47;found: C 24.86,H 2.05,N 46.45.
2.3.16.3,7-Diamino-6-(ammoniomethyl)-[1,2,4]triazolo[4,3-b][1,2,4]triazol-2-ium 5,5′-dinitroamino-2,2′-bi(1,3,4-oxadiazolate)(16)
White solid.Yield:0.355 g,83.2%.1H NMR(500 MHz,DMSO‑d6):8.53 (s,3H),8.41 (s,2H),5.95 (s,2H),4.21 (s,2H) ppm.13C NMR(125 MHz,DMSO‑d6):164.6,157.5,148.2,146.7,142.0,33.2 ppm.IR(KBr pellet): 3486,3285,3141,3051,1682,1540,1514,1487,1427,1325,1288,1154,1079,993,953,876,847,770,753,709,653,603,587,573 cm-1.Elemental analysis calcd (%) for C8H10N16O6(426.27):C 22.54,H 2.36,N 52.57;found:C 22.51,H 2.34,N 52.59.
2.3.17.4-Amino-5-(2-(2-ammonioacetyl)hydrazineyl)-3-(ammoniomethyl)-1,2,4-triazol-1-ium 5,5′-dinitramino-2,2′-bi(1,3,4-oxadiazolate) (17)
The intermediateB(2.0 mmol) and 5,5′-dinitramino-2,2′-bi(1,3,4-oxadiazolate) (3.0 mmol) were mixed in 25.0 mL deionized water.The mixture was stirred at 60°C for 2 h.The precipitate was collected by filtration,washed with cold water(10.0 mL)and dried in the air to give compound17.The crude product was recrystallized from hot mixed solution(H2O:N,N-dimethylformamide=2:1).
White solid.Yield:0.855 g,72.8%.1H NMR(500 MHz,DMSO‑d6):10.94(s,1H),8.59(s,3H),8.22(s,3H),6.16(s,2H),4.26(s,2H),3.73(s,2H) ppm.13C NMR (125 MHz,DMSO‑d6): 166.5,165.5,152.6,147.9,147.7,42.0,32.4 ppm.IR (KBr pellet):3271,2919,1719,1589,1516,1492,1424,1285,1156,1072,1017,953,940,773,660,690,580 cm-1.Elemental analysis calcd(%)for C11H15N20O10(587.37):C 22.49,H 2.57,N 47.69;found: C 22.46,H 2.55,N 47.72.
2.4.NMR spectroscopy
The1H and13C NMR spectra of compounds2—8,10—17were given in the ESI (Figs.S1—S30) and the chemical shifts were given with respect to DMSO as external standard.For compound2—8,the carbon signals of the 1,2,4-triazole rings appear around δ=152 and 142 ppm while the methylene has the chemical shifts around 32 ppm.The two amino groups linked to the 1,2,4-triazole have single peaks around 5.90 and 8.30 ppm while the protonated amino group has the chemical shift around 8.50 ppm.Besides,the methylene shows the carbon signal around 4.20 ppm.It can be found that the1H chemical shifts of the fused-cyclic cations are similar to that of the monocyclic ones.In addition,the newly fused 1,2,4-triazole gives a new13C signal around 157 ppm.The trivalent salt17has a different1H spectrum for imino groups with chemical shifts of 10.94 ppm while its13C spectrogram has an obvious carbonyl signal at 166 ppm.
3.Results and discussion for the preparation
As shown in Fig.2,the investigation started with the cheap and readily available reactant glycine.Initially,the glycine underwent the cyclization reaction with diaminoguanidine hydrochloride(DAG.HCl) in the composite system of orthophosphoric acid and phosphorus pentoxide at 120°C for 12 h.As a result,polyamine compound 3-(aminomethyl)-4,5-diamine-1,2,4-triazole (1) was isolated from the alkalized solution.In order to further explosive modification,energetic acids 5-nitraminotetrazole,3-nitro-5-one-1,2,4-triazole,1,1′-diolate-5,5′-bistetrazole,3,4-di (nitramino)furazan,4,4′-bis(nitramino)azoxyfurazan,4,4′,5,5′-tetranitro-bisimidazole and 5,5′-dinitramino-2,2′-bi (1,3,4-oxadiazole) with good detonation performance were chosen as reactant for Brønsted acid-base reactions.The corresponding energetic salts2—8were isolated with the ratio (cation versus anion) of 1:1 (compounds4—8)or 1:2(compounds2,3).They were determined to be unusual divalent cationic salts with monocycle by single crystal X-ray diffraction.
Inspired by this,molecule 3-(aminomethyl)-4-amine-5-hydrazino-1,2,4-triazole (A) was designed and expected to be the precursor of a trivalent cation and fused cyclic compound.However,the pure product ofAcould not be obtained from the cyclization reaction with triaminoguanidine hydrochloride (TAG.HCl)even after trying a variety of reaction conditions.To find out the structure of the intermediate,the unknown product was treated with energetic acid 5,5′-dinitramino-2,2′-bi (1,3,4-oxadiazole) and recrystallized from hot mixed solvent (H2O and N,Ndimethylformamide) directly.To our delight,trivalent cationic salt17was obtained and its crystal structure was confirmed by single crystal analysis,which also proved the structure of intermediateBrather than originally envisaged compoundAindirectly.Although compoundAwas not obtained as expected,the predesigned compound9could also be synthesized from the fused-cyclization reaction of intermediateB.Under the condition of hydrochloric acid,intermediateBdecomposed at -NH-CO-bond and underwent hydrazine cyclization with cyanogen bromide to yield the fused cyclic compound 3,7-diamine-6-(aminomethyl)-[1,2,4]triazolo [4,3-b][1,2,4]triazole (9) as white solid.The explosive modification was also carried out on compound9with the seven energetic acids mentioned above.Similar to the corresponding monocyclic energetic ones,divalent cationic salts10—16with fused cycle were obtained in the 1:1 or 1:2(cation:anion)stoichiometric ratios.The short synthetic routes from readily available reactant and the simple post treatment endow these compounds with considerable potential for practical application.
4.Single crystal analysis
The single crystals2.H2O,3.H2O,5.H2O and13.H2O were obtained by evaporation of the corresponding saturated aqueous solution.The single crystal17.5H2O was recrystallized from the mixed solution of H2O and N,N-dimethylformamide(volume ratio=2:1).The detailed crystallographic data were listed in the Supporting Information.
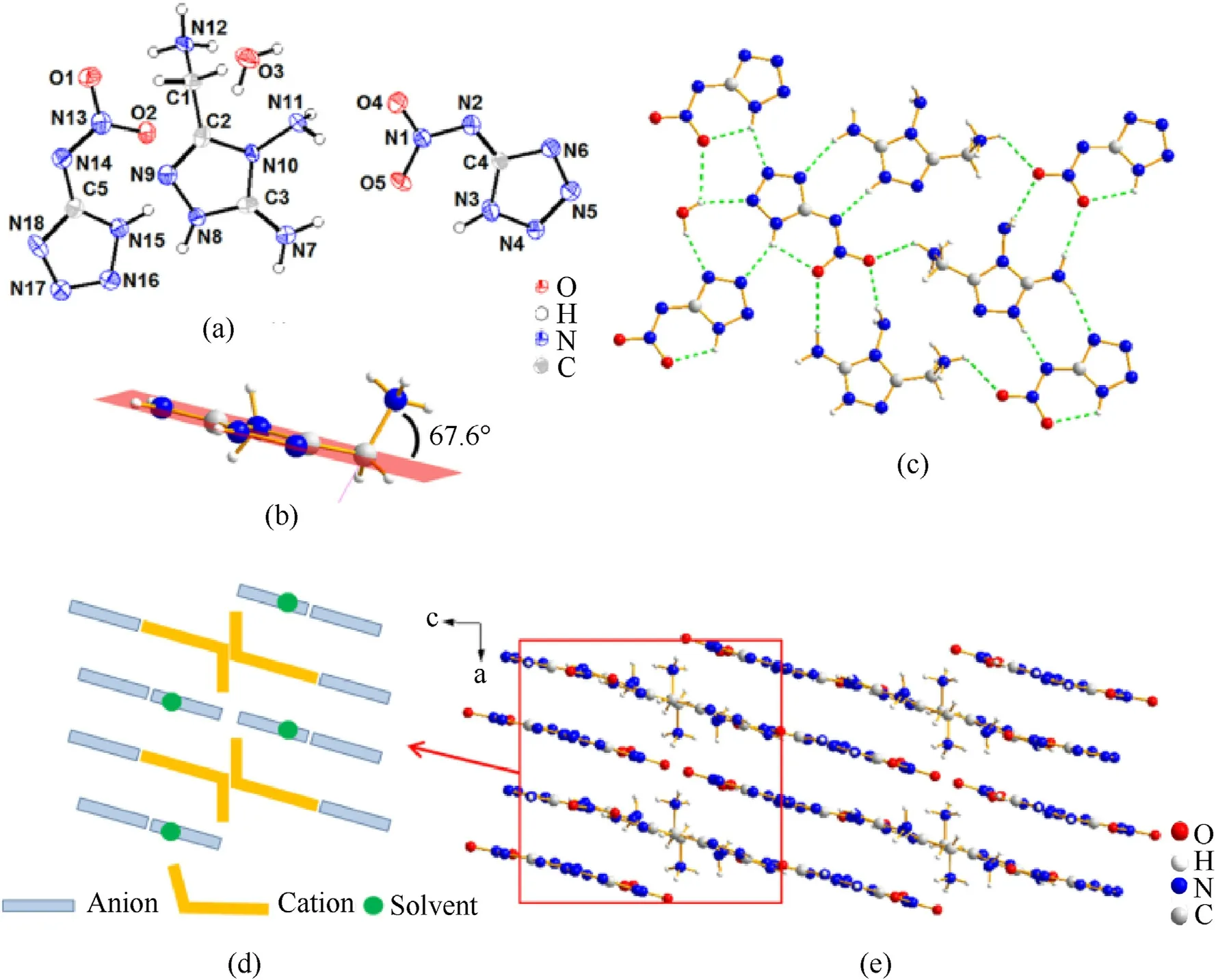
Fig.3.(a) The molecule structure of 2.H2O (50% thermal ellipsoid plot);(b) The included angle between the C—N single bond and the 1,2,4-triazole plane;(c) Partial hydrogen bonds (green dashed lines);(d) Stacking diagram of 2.H2O (b-axis);(e) Packing system of 2.H2O (b-axis).
The crystal2.H2O belongs to monoclinicP21/cspace group with 4 molecules per unit cell.Its crystal density is 1.747 g/cm3at 170.0 K.As is shown in Fig.3,the X-ray crystallography analysis clearly reveals the formation of divalent cations.This is a rare example in monocyclic cations.Hydrogen positive ions are located at N8 and N12 positions.Because of the protonation of N12-amino,the bond length of C1—N12 bond (1.500 (9) Å) is slightly longer than that of typical C—N single bond (1.47 Å).The included angle between the C—N single bond and the 1,2,4-triazole plane is 67.6°.This structural feature can also be observed in crystal3.H2O and5.H2O.When compared with protonated C1—N12 bond,the bond lengths of C—N and N—N bonds(from 1.308(8)Å to 1.388(8)Å)in 1,2,4-triazole are relatively shorter.These values lie between the typical carbon-nitrogen single and double bonds(C—N:1.47 Å,C═N: 1.22 Å,N—N: 1.48 Å,N═N: 1.20 Å) which indicate the aromaticity of the triazole ring system[38].There are massive inter-and intramolecular hydrogen bond interactions existing between H,N,O atoms in crystal2.H2O.With the influence of multiple interactions,the anions and cations form parallel layer arrangement.Then the crystal packing system presents a layer by layer stacking.The simplified diagram (Fig.3(e),viewed down theb-axis) was drawn to get a better understanding of the crystal stacking.The pale blue short rod represents for the anions and the yellow hook shape represent for the cations.The solvent molecules in each layer are represented by green dots.It is clear that the twisted amino groups of two adjacent cations are arranged at the opposite side of the layer.And through this intermediary,hydrogen bonds are formed between the upper and lower layers,which contribute to the stability of the crystal.
The crystal3.H2O was measured at 170.0 K with a density of 1.696 g/cm3.It crystalized in monoclinic system andP21/cspace group.There are four molecules in a single unit cell (Z=4).Like crystal2.H2O,the N10-amino group also twist out of the 1,2,4-triazole plane,which can be confirmed by the twist angle(N7—C4—C5—N10 59.5(3)o).As demonstrated in Fig.4,the included angle between the C5—N10 bond and triazole plane is 54.9°.The bond length of C5—N10 bond is 1.482(3)Å which is lightly shorter than the corresponding bong in crystal2.H2O.Fig.4(d)and(e)are the same crystal packing system viewed from different directions.It can be seen that the whole crystal is composed of several regularly arranged parts.Each part presents a layer by layer stacking.Hydrogen bonding exists between ions in the same plane(N5—H5B…O6,2.22 Å) and between upper and lower layers (N9—H9A∙∙∙O4,2.42(3)Å),too.In order to describe the crystal stacking pattern explicitly,two stacking diagrams were drawn with pale blue rob-like anions,yellow crowbar-like cations and green dot-like solvent,respectively.These two different stacking parts staggered in thec-axis direction and they are interacted by the hydrogen bonds interactions at the intersection (N10—H10C…N2,1.89 Å).
The crystal5.H2O possesses much higher crystal density(1.784 g/cm3at 170 K)than the previous two crystals.It belongs to monoclinic system andCcspace group with 4 molecules per unit cell(Z=4).The bond length of protonated C3—N1 bond is 1.484(6)Å,which is similar to that of crystal3.H2O.The C3—N1 bond line is an angle of 65.8°to the triazole plane.As can be seen in Fig.5,except for the protonated N1-amino group,the other parts of this molecule are basically in the same plane.Fig.5(d) exhibits the crystal packing system viewed fromb-axis.The stacking diagram was drawn to make the stacking clear.The simplified drawing“whether the yellow forked cation projection were blocked by the blue rob-like anion projection or not”are intended to describe the spatial arrangement of cations and anions in the b-axis direction.By checking the ions arrangements carefully,it is found that two different arrangements of molecule 5 compose a column of layers stacking with the form of “ABAB…ABAB”.Then these columns layers are staggered from each other and finally form the relatively regular spatial stacking system.There are also hydrogen bonding interactions existing between the edges of segregate layers(N2—H2A∙∙∙N10,2.30 (7) Å;N6—H6B…O5,2.19 Å;N2—H2B…O6,2.36 (8) Å,etc.).(see Fig.7)
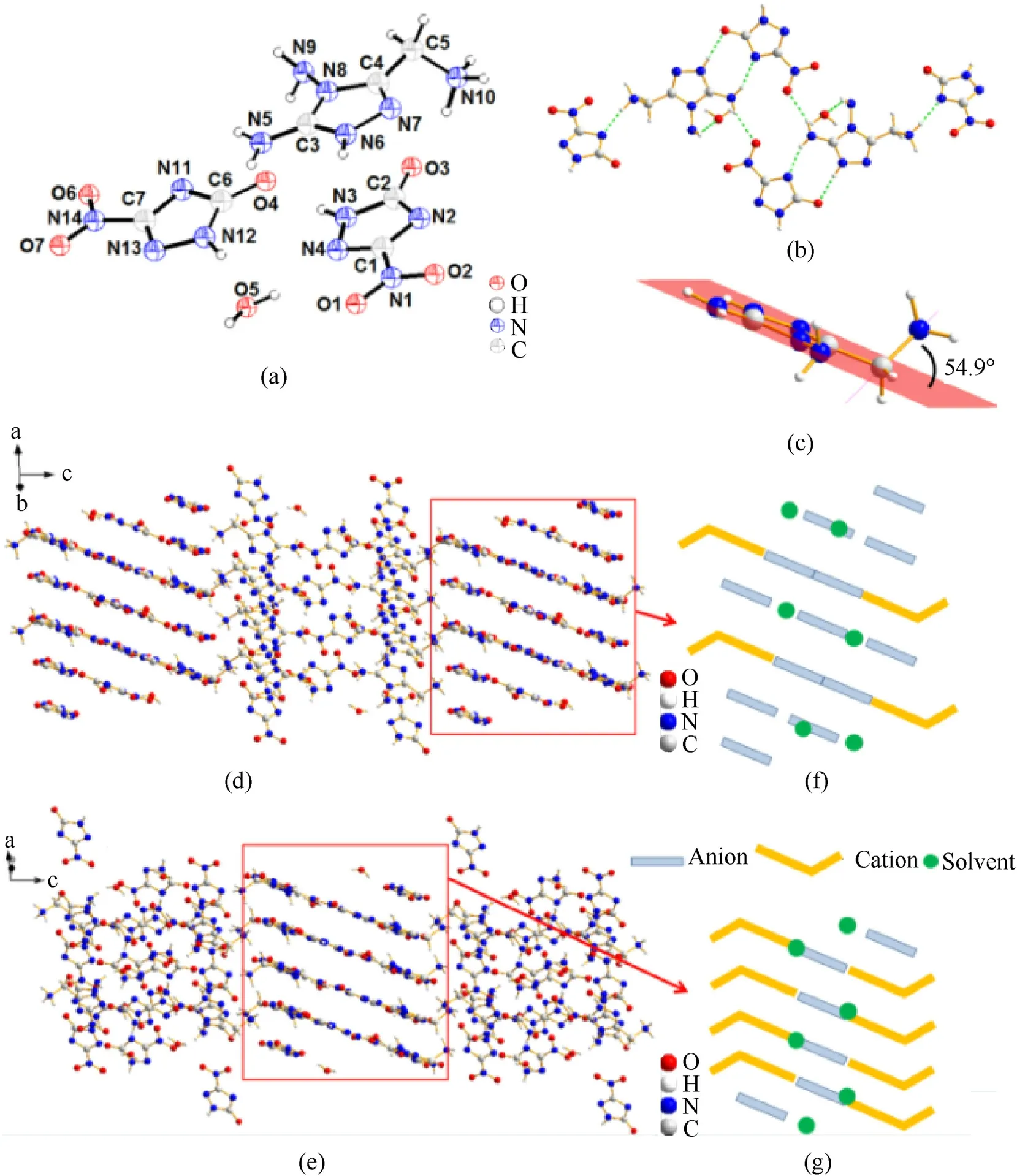
Fig.4.(a)The molecule structure of 3.H2O(50%thermal ellipsoid plot);(b)Partial hydrogen bonds(green dashed lines);(c)The included angle between the C—N single bond and the 1,2,4-triazole plane;(d) Stacking diagram of 3.H2O;(e) Rotated stacking diagram of 3.H2O;(f) Packing system of 3.H2O;(g) Rotated packing system of 3.H2O.
The fused cyclic energetic crystal13.H2O crystallizes in the monocilinicP21/cspace group and has a crystal density of 1.793 g/cm3at 170 K (Fig.6).Each crystal unit cell has four molecules(Z=4).The fused cyclic cation was also determined to be a divalent cation by the X-ray crystallography analysis.The two hydrogen positive ions are located at the similarly N14-amino position and the newly formed N7 position,respectively.Unlike monocyclic cations,the N14-amino group is nearly coplanar with the fused cyclic backbone.This can be confirmed by the torsion angle(N11—C5—C6—N14,-8.0 (2)o).The bond length of C6—N14 is 1.483(2)Å while the bond lengths of the fused cyclic skeleton range from 1.301(2)Å to 1.409(2)Å.The crystal stacking system was provided in Fig.6(c).Its projection along thea-axis was drawn with blue Vshape anions,yellow rob-like cations and green dot solvent.The spatial stacking system is influenced by abundant hydrogen bonds involving intermolecular and intramolecular interactions.It can be found in the hydrogen bonds data that the fused cyclic cation acts as a donor of hydrogen bond in the crystal structure.Both C-amino groups and the N—H on the fused ring participate in hydrogen bonding (N14—H14A∙∙∙O5,2.20 Å;N9—H9B…N5,2.32 Å;N7—H7…N4,1.86 (3) Å;etc.).
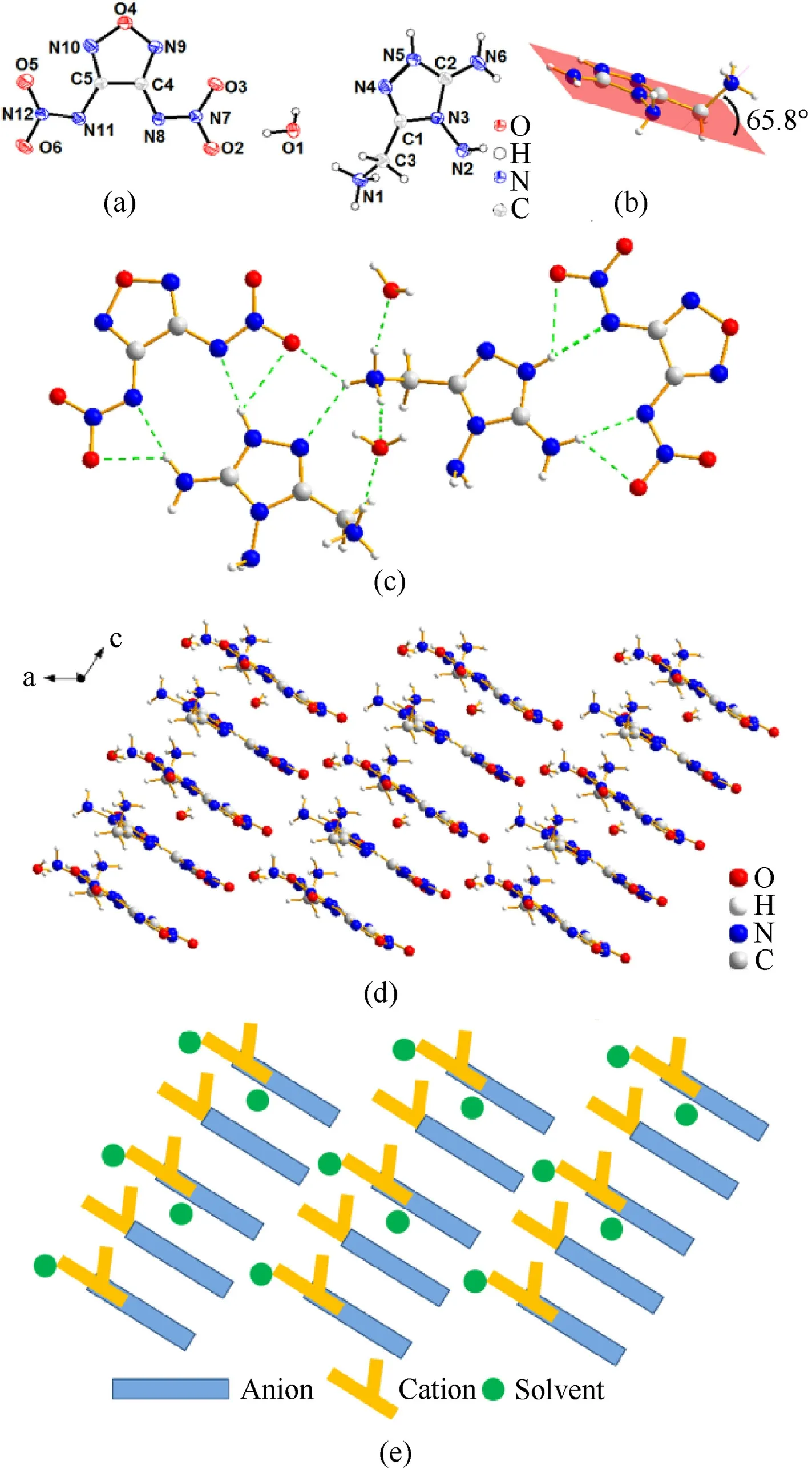
Fig.5.(a) The molecule structure of 5.H2O (50% thermal ellipsoid plot);(b) The included angle between the C—N single bond and the 1,2,4-triazole plane;(c) Partial hydrogen bonds (green dashed lines);(d) Stacking diagram of 5.H2O (b-axis);(e) Packing system of 5.H2O (b-axis).
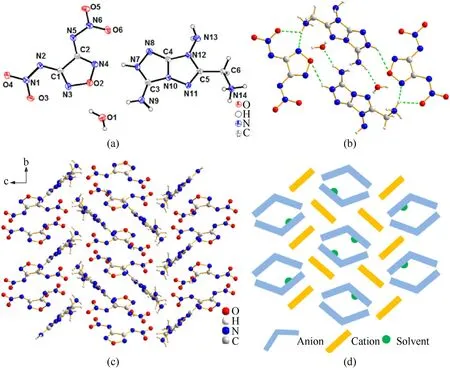
Fig.6.(a) The molecule structure of 13.H2O (50% thermal ellipsoid plot);(b) Partial hydrogen bonds (green dashed lines);(c) Stacking diagram of 13.H2O (b-axis);(d) Packing system of 13.H2O (b-axis).
The crystal17.5H2O belongs to triclinic andP-1 space group.It has a crystal density of 1.731 g/cm3at 170 K and two molecules in a single unit cell (Z=2).The cation was confirmed to be a trivalent cation by the analysis result.The torsion angle reveals that the carbonyl group twist out of the 1,2,4-triazole plane(C6—N10—N11—C7,77.7(5)o).It can be seen from the crystal space stacking that the anions are arranged in parallel in space while the cations parallel in the other direction.The oblique-crossing arrangement was stacked by the cations and anions along with H2O via hydrogen bonds.
5.Physicochemical and energetic properties
5.1.Thermal stability
The thermal stability of newly synthesized compounds2—8and10—17were measured by differential scanning calorimetry (DSC)under the flowing nitrogen atmosphere.Aluminium-standard pans were used in the experiments and the heating rate was 5°C/min.The decomposition temperatures were recorded at the onset point of the exotherm during incremental heating.All the results are listed in Table 1.The 5-nitraminotetrazolate salts2,10,4,4’-bis(-nitramino)-azoxyfurazanate salt 14 and trivalent cationic salt 17 decompose at 198,183,182,182°C,respectively.Similarly,the 3-nitro-1-one-1,2,4-triazolate salts 3 and 11 decomposes at nearly 200°C (3: 199°C,11: 197°C).The other energetic salts exhibit improved thermal stabilities which are higher than 200°C.The compounds 4,7,12,15 and 16 have superior thermal stabilities(≥212°C) when compared with 1,3,5-trinitro-1,3,5-triazacyclohexane (RDX) (210°C).The decomposition temperature of 1,1’-diolate-5,5’-bistetrazolate salt 4 (245°C) was the highest among all these energetic salts.The results of differential scanning calorimetry indicate that all of these energetic compounds have meet the demand of thermal stability for practical use(≥180°C) [49,50].
5.2.Detonation properties
The heats of formation (HOFs) of these energetic compounds2—8and10—16along with17were calculated by using theoretical methods through Gaussian 09 program[39].The results were listed in Table 1.All these energetic salts exhibit positiveHOFs which could be attributed to their high nitrogen content(44.33%—66.26%).The existence of massive C—N and N—N bonds gives all the compounds high HOFs (225.1—2207.5 kJ/mol),which are much greater than that of RDX (70.3 kJ/mol).In addition,it could be found that theHOFs of fused heterocyclic compounds were higher than that of the corresponding monocyclic ones.The density of energetic compound is another important parameter in calculating detonation properties.The densities of all the anhydrous salts ranged from 1.68 g/cm3to 1.82 g/cm3were further measured by a gas pycnometer (Table 1).
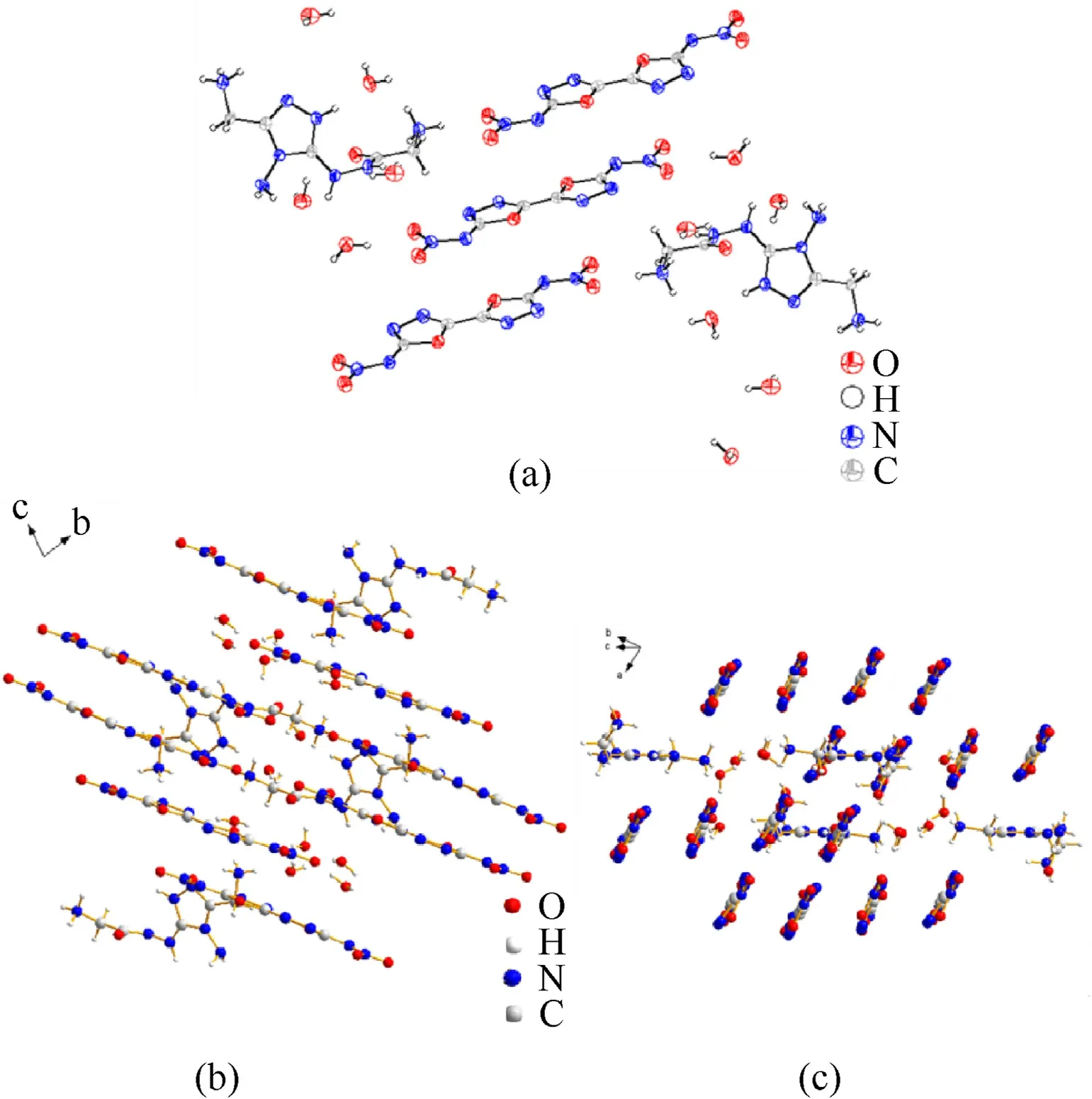
Fig.7.(a)The molecule structure of 2(17.5H2O)(50%thermal ellipsoid plot)(Because there are too many atoms,the atomic serial number is not marked);(b),(c)The crystal cell of 17.5H2O viewed from different axes.
The detonation properties of compounds2—8and10—16were further evaluated based on the calculated HOFs and the measured density by using EXPLO5(v6.05.02)[40].It is worth noting that the detonation velocity of compounds10(8843 m/s) surpasses that of RDX (8795 m/s) while the detonation velocities of compound2(8748 m/s) was comparable to RDX.Because of their poor oxygen balance,their detonation pressures(2:28.29 GPa;10:29.14 GPa)is relatively lower than RDX(34.9 GPa).The other energetic salts have moderate detonation velocities (7941—8685 m/s) which were superior to that of trinitrotoluene(TNT:D=6881 m/s).Besides,their detonation pressures (24.18—30.38 GPa) lie between TNT(P=19.5 GPa) and RDX.Compared with the monocyclic energetic salts(2—8),fused cyclic energetic salts(10—16)exhibit an increase of 83—159 m/s,0.67—1.41 GPa in detonation velocity and pressure,respectively.
5.3.Sensitivity
Apart from detonation property,sensitivity property is also an important index to directly evaluate whether energetic materials are safe or not in practical applications[51].The energetic salts2—8and10—17underwent mechanical sensitivity testing by using standard BAM procedures[41].As can be seen in Table 1,the impact and friction sensitivities of 5-nitraminotetrazolate salt2were 8 J and 144 N,respectively.The corresponding fused heterocyclic salt10was slightly better than compound2with the values of 10 J and 168 N,respectively.These two salts were relatively sensitive among these newldy synthesized compounds and their sensitivity performances were comparable to that of RDX (7.4 J,120 N).Besides,the 3,4-di (nitramino)furazanate salts (5,13) and 4,4’-bis(nitramino)azoxyfurazanate salts (6,14) were comparatively insensitive towards impact and friction.The outcomes showed that they were more insensitive than RDX.Compared with the energetic salts mentioned above,the other salts were relatively insensitive to both impact and friction stimuli.Their resistances towards friction stimuli(range from 324 N to 360 N)were comparable to that of TNT(353 N).Meanwhile,their impact sensitivities(ranged from 28 J to 40 J) were even better than that of TNT(15 J).
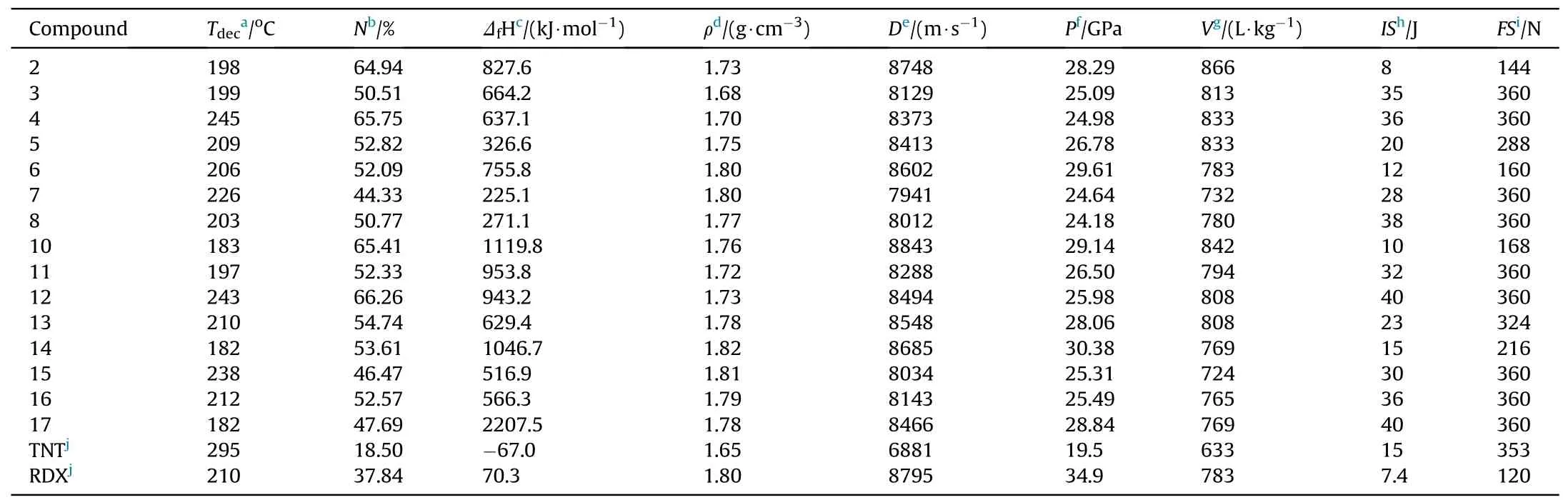
Table 1 Physical and detonation properties of the compounds.
5.4.Theoretical calculations
To gain intuitionistic insights on inter-and intramolecular effects and visualize the distribution of hydrogen-bonding interactions in crystal packing,the noncovalent interaction (NCI)plots of 2,3,5 and 13 were calculated by using the functional components of Multiwfn [42,43].The result was drawn on a redblue-green scale in Fig.8 which represents for the strong nonbonded overlap,weak interactions,and strong attractive interactions,respectively.These dark blue shapes between the N—H and electronegative acceptor (usually N/O atoms) represent for large accumulation of electron density and imply hydrogen bond interactions.The existence of abundant hydrogen bonds could be observed in the marked red circles,which contribute to their good thermal stability.Moreover,the π-π interaction between the parallel 3,4-di (nitramino) furazanate anions in13could be observed as larger green isosurfaces in the red rectangle,which could contribute to higher crystal density and better resistance to mechanical sensitivities than5.
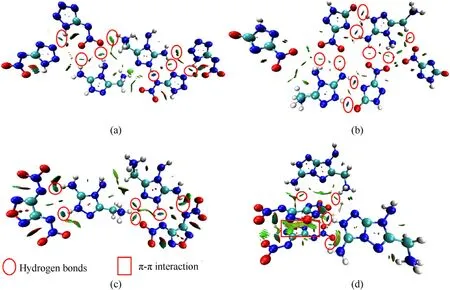
Fig.8.NCI plots of gradient isosurfaces for (a) 2,(b) 3,(c) 5 and (d) 13.
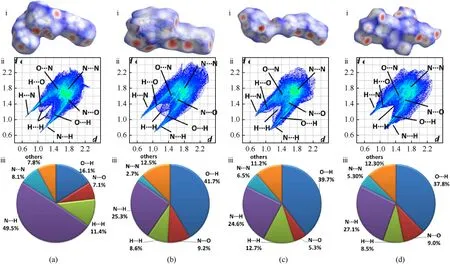
Fig.9.Hirsheld surfaces,fingerprint plots and individual atomic contact percentage contribution for (a) 2,(b) 3,(c) 5 and (d) 13.
The Hirshfeld surfaces and the associated two-dimensional(2D)fingerprint were employed to analyze the weak interactions in2.H2O,3.H2O,5.H2O and13.H2O[44—46].The results of the analysis along with the percentage of interactions were drawn in Fig.9.Generally speaking,the graphic appearance of Hirshfeld surfaces demonstrates the distribution of electron population of molecular structure and further visualizes the molecular interaction proportion.According to the strength of close contact interactions,the graphic appearance is colored by red,white and blue in turn from high to low.As is shown in Fig.9 (a(i)),the Hirshfeld surface of2presents the shape of two storey plane related to its bilayer molecular structure.The red dots mainly disperse on the surface edges which indicate the aggregation of its high electron density and the interactions of hydrogen bonds for molecules.Similarly,the Hirshfeld surface of3has a bilayer molecular structure with abundant red dots located on the surface edges.The Hirshfeld surface of5exhibits uneven blocks with red dots dispersed in many orientations while compound13exhibits irregular Hirshfeld surface related to its atypical layer stacking.In the two-dimensional(2D) fingerprint figure,the O…H close contacts appears inside the pair of sharp spikes at the bottom left of the plot while the N…H close contacts appears on the outside.According to the percentage pie chart,N…H contacts accounts for nearly half of all the interactions in2(49.5%)while O…H contacts possess the percentage of 16.1%.This suggests a large amount of hydrogen bond existing in2.Extensive hydrogen-bonding interactions dominated by O…H(41.7%)and N…H(25.3%)exist in3with strong O…H spikes at the bottom left of the plot.The value of N…H(24.6%)and O…H(39.7%)contacts demonstrated the extensive hydrogen-bonding interactions in5.The majority of the interactions in13are belong to O…H and N…H hydrogen bonds.It possesses high proportions of O…H contacts (37.8%) and moderate N…H proportion (27.1%).Overall,these abundant hydrogen bonding interactions are driving force for enhancing molecular stability and result in the thermostability.
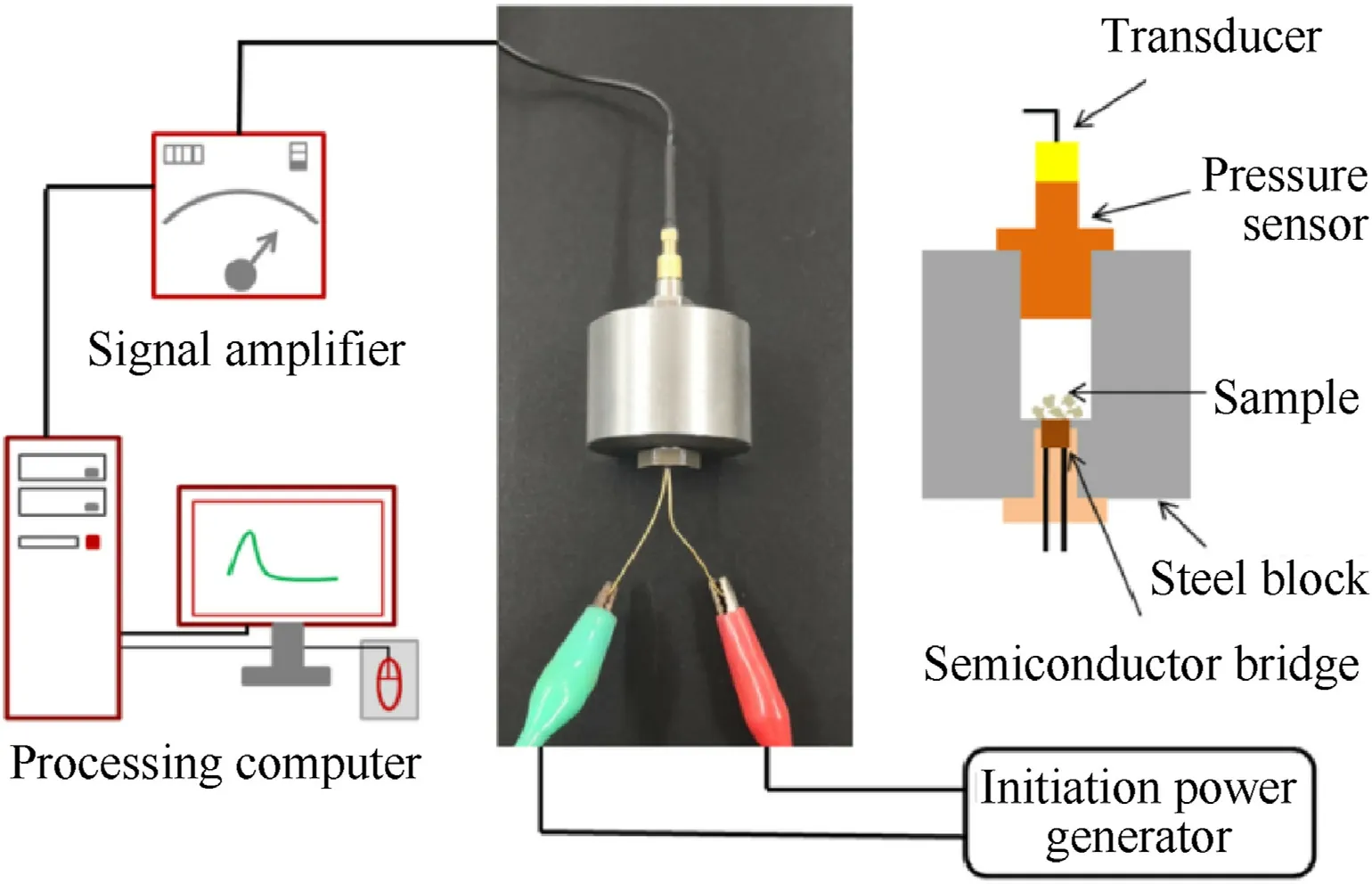
Fig.10.Schematic diagram of test equipment.
6.Combustion performance
The energetic compounds with high nitrogen content usually generate a large amount of gas products after combustion decomposition.The detonation gas volumes of these newly synthesized energetic salts were calculated by EXPLO5 (v 6.05.02).As can be seen from the results in Table 1,high nitrogen compounds2,4,10and12(N% ≥64.94%) exhibit great capacities of gas generation which were higher than 800 L/kg.It is worth noting that compound2possess the highest volumes of detonation gas up to 866 L/kg.In order to further estimate their potential as for gas generant component,the constant-volume combustion experiment was conducted on these four compounds.The experiment was carried in an assembled steel container with the ignition device at the bottom and the pressure sensor at the top (Fig.10) [47,48].The wiring circuit should be checked to avoid short circuit.The ground sample (6 mg) and ignition powder B/KNO3(0.5 mg) were placed directly above the semiconductor bridge in the hollow part of the container.After the sample is loaded,the threads were tightened to ensure air tightness.Through the instantaneous discharge of the power generator,the sample was lighted by the semiconductor bridge.With the production of a large amount of gas,the increased pressure was detected and transformed into an electrical signal.Then,the signal was processed by the amplifier and then analyzed by the computer.As shown in Table 2 and Fig.11,the 5-nitraminotetrazolate salts2and10show pretty fine performance with the maximum pressure of 10.08 and 9.16 MPa,respectively.The results were more than two times as much as that of commonly used reagent guanidine nitrate (GN,Pmax: 4.20 MPa).Besides,the max-pressure-rise rates of these two compounds (2;136.3 GPa/s,10: 117.6 GPa/s) were also better than GN (79.2 GPa/s).The rapid process of decomposition and gas releasing for10lasts 0.12 ms.It was close to that of GN(0.09 ms).By comparison,2toke a bit longer(0.20 ms) to reach its maximum pressure.The gas production capacity of 1,1’-diolate-5,5’-bistetrazolate salt 12 is similar to that of compound 10 in both pressure-rising time (0.13 ms) and maxpressure-rise rate (101.6 GPa/s).The maximum pressure of 12(8.17 MPa) is slightly inferior,but it is still twice that of GN.The other 1,1’-diolate-5,5’-bistetrazolate salt 4 surpass GN in maximum pressure (7.88 MPa) while its max-pressure-rise rate (82.2 GPa/s)was comparable to GN.The results of the constant-volume combustion experiments conducted on these compounds indicate their great gas production capacities and make them potential candidates for gas generant component.
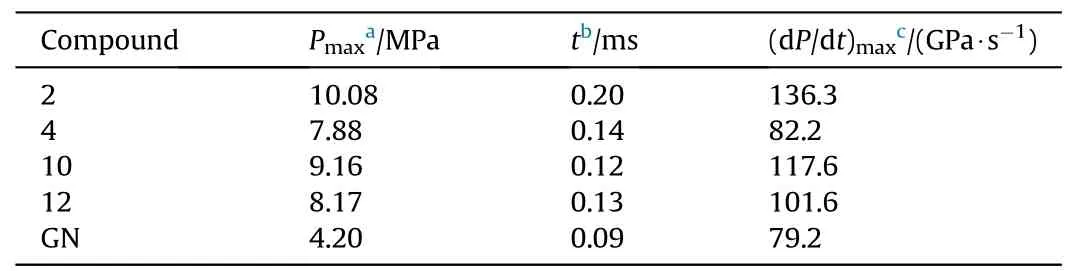
Table 2 Constant-volume combustion parameters of 2,4,10 and 12 and GN.
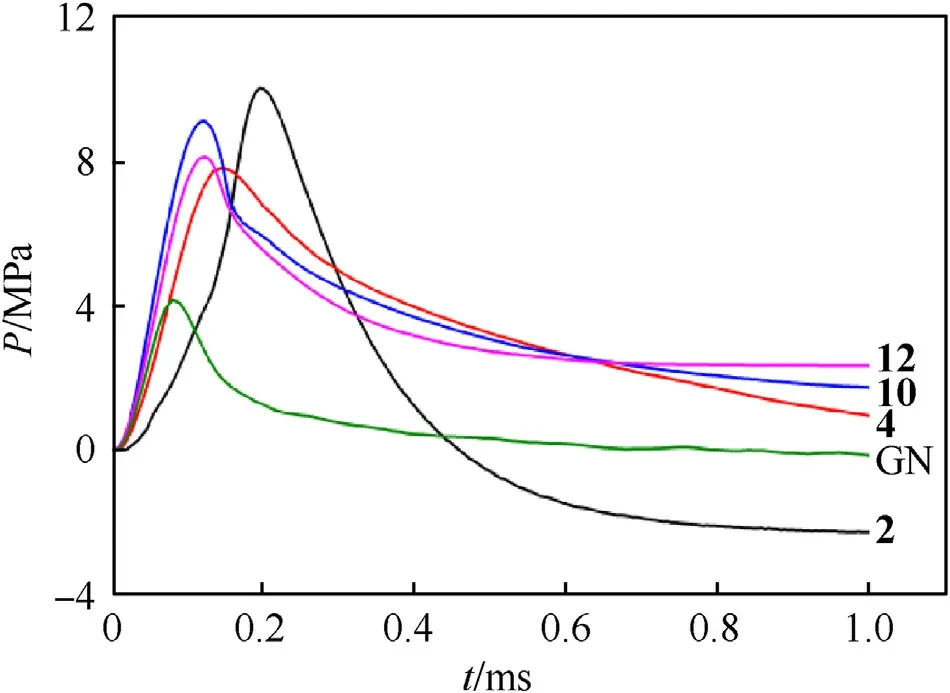
Fig.11.The P-t curves of 2,4,10,12 and GN.
7.Conclusions
In summary,monocyclic nitrogen-rich triazole compound1and its fused cyclic derivative9were synthesized from cheap and readily available reactant glycine through one/two steps cyclization reaction in this work,respectively.The structure of intermediateBwas proved indirectly by its trivalent cationic crystal17.5H2O.Two series of divalent cationic salts2—8and10—16were obtained with high nitrogen content (44.33%—66.26%) and positiveHOF(225.1—2207.5 kJ/mol).Their decomposition temperatures range from 182°C to 245°C and meet the demand of practical use.The molecular structures of2,3,5and13were also confirmed by single crystal X-ray diffraction analyses.It is worth noting that 5-nitraminotetrazolate salt10possesses the remarkable energetic performance(D=8843 m/s)which surpasses RDX.Meanwhile,its resistance to mechanical stimulation (IS: 10 J,FS: 168 N) was slightly better than RDX.The other energetic salts exhibit moderate detonation velocities (7941—8748 m/s) and detonation pressures(24.18—30.38 GPa).The sensitivity properties (IS: 8—15 J,FS:144—216 N)of2,6,10and14are between TNT and RDX while the others are even better (IS: 20—40 J,FS: 288—360 N).Besides,the hydrogen bonding interactions along with structure-property relationship were illustrated by two-dimensional fingerprint plots,Hirshfeld surfaces and NCI analysis.Moreover,compounds2,4,10and12with good gas production capacities(≥808 L/kg)were specifically tested by constant-volume combustion experiments.All of them have much better maximum pressure(7.88—10.08 MPa)than traditionally gas generant GN.These fine results indicate that 5-nitraminotetrazolate salts2and10have great application potential in the field of gas generant.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work is supported by the National Natural Science Foundation of China(No.21875110,22075143)and the Science Challenge Project (No.TZ2018004).H.Yang thanks the Qing Lan Project for the grant.
Appendix A.Supplementary data
Supplementary data to this article can be found online at https://doi.org/10.1016/j.dt.2021.11.016.
杂志排行
Defence Technology的其它文章
- Dual Attribute Adversarial Camouflage toward camouflaged object detection
- A bi-population immune algorithm for weapon transportation support scheduling problem with pickup and delivery on aircraft carrier deck
- One-step green method to prepare progressive burning gun propellant through gradient denitration strategy
- Benchmark calculations and error cancelations for bond dissociation enthalpies of X—NO2
- GO/HTPB composite liner for anti-migration of small molecules
- Resilient tightly coupled INS/UWB integration method for indoor UAV navigation under challenging scenarios
